Journal of Sleep Disorders & Therapy
Open Access
ISSN: 2167-0277
ISSN: 2167-0277
Research Article - (2020)Volume 9, Issue 5
Study objectives: To evaluate, in a pilot fashion, efficacy and tolerability of electrical counter-stimulation using the Scrambler device in alleviating discomfort and urge to move in patients with medically refractory restless legs syndrome/Willis Ekbom Disease (RLS/WED).
Methods: Eligible patients had moderate to very severe RLS/WED symptoms for ≥ 3 months, based on the International Restless Legs Syndrome Study Group (IRLS) Rating Scale. Subjects were treated with Scrambler Therapy for up to ten daily 1-2 hour sessions. Symptoms were monitored using the IRLS as well as questionnaires regarding daily symptoms and global impression of change. Questionnaires were administered at baseline, during therapy, and one-week after the last Scrambler session. Subjects were also queried regarding any adverse effects.
Results: Eight subjects were enrolled (M=F). Subjects were on an average of 3 RLS drugs at time of enrollment. Two patients were asked to taper off of gabapentin or pregabalin prior to starting treatments with Scrambler. The IRLS score decreased from a baseline of 27.6 ± 6.2 (mean ± SD) to 19.5 ± 8.0 (p=0.03) following therapy, and to 20.3 ± 10.8 (p=0.07) one week after the last Scrambler treatment. No adverse effects were noted by subjects.
Conclusions: Scrambler therapy may be associated with symptom improvements in patients with severe, medically refractory RLS/WED. No adverse effects were associated with therapy. Further work is necessary to characterize this possible treatment option.
This study is registered in Clinicaltrials.gov as “Treatment of RLS/WED Symptoms through Sensory Counterstimulation.” ClinicalTrials.gov identifier: NCT03249779.
Restless Legs Syndrome; Willis Ekbom Disease; Electroanalgesia; Counter-stimulation therapy; Scrambler
RLS/WED: Restless Legs Syndrome/Willis Ekbom Disease; IRLS: International Restless Legs Syndrome Study Group; TENS: Transcutaneous Electrical Nerve Stimulation
Current Knowledge/Study Rationale
Restless legs syndrome/Willis Ekbom Disease (RLS/WED) is common, yet has limited therapeutic options. The pharmacologic treatments are associated with significant side effects and patients often experience refractory symptoms despite medications. Novel treatments are necessary. Preliminary data supports use of counter-stimulation therapies in managing RLS/WED symptoms. We report data from a small prospective interventional pilot evaluating a form of electroanalgesia therapy (Scrambler) in medically refractory, chronic RLS/WED patients with moderate to severe symptoms.
Study Impact
Our study demonstrates that Scrambler therapy may be associated with symptomatic improvements in medically refractory RLS patients. There were significant improvements in the IRLS score from baseline to end of therapy. Scrambler therapy may represent a novel nonpharmacologic modality of managing RLS symptoms, without adverse effects.
Restless legs syndrome/Willis Ekbom Disease
Restless legs syndrome/Willis Ekbom Disease (RLS/WED) is characterized by an unpleasant or uncomfortable urge to move the legs which comes on at rest and is at least partially alleviated by movement. Symptoms classically occur in the evening and nighttime hours [1]. It is estimated that 7.2% of the Western population has RLS/WED, with 2.7% of the population experiencing moderate to severe symptoms [2].
RLS/WED has a significant negative impact on quality of life and is associated with anxiety and depression. Patients often experience significant sleep fragmentation due to symptoms, which can lead to daytime sleepiness and fatigue, cognitive symptoms, and loss of productivity at work [3].
One-third of RLS/WED patients require daily pharmacologic therapy to manage symptoms [4]. Patients with refractory symptoms often require multiple drugs of different classes [5]. Adverse effects from the most common agents used in treating RLS/WED include: drowsiness, dizziness, unsteadiness, weight gain, depression, augmentation, and development of impulse control disorders [6-8].
Nonpharmacologic modalities are an important means of treating RLS/WED symptoms. Many nonpharmacologic strategies are based on counter-stimulation techniques to mask RLS/WED symptoms, such as warm baths and massage. Sequential compression devices have occasionally been shown to be helpful in alleviating symptoms [9]. Nonpharmacologic counter stimulation modalities may be an important means of reducing medication burden in patients with RLS/WED and complementing pharmacotherapies in medically refractory patients.
Electrical Stimulation in the management of centrally driven neurogenic pain
Electrical stimulation has been utilized as an intervention in treatment of various forms of neurogenic pain (including peripheral neuropathy, trigeminal neuralgia, and multiple sclerosis). Much of the published data involve use of transcutaneous electrical nerve stimulation (TENS). TENS appears to work to reduce pain through both central and peripheral mechanisms. Animal and clinical studies demonstrate that the electrical impulses have a local effect on peripheral nerves to reduce pain at area of application. There also appear to be effects on central nervous system pathways (periaqueductal gray, medulla, and spinal cord) thus reducing pain at sites outside of the area of stimulation [10,11]. The opiate and GABA receptors are thought to be implicated in the effects of TENS. These receptors are also targeted by pharmacologic therapies commonly used in RLS management. There are no systematic studies evaluating the efficacy of TENS for RLS.
Scrambler therapy is a specific form of electrical stimulation which has also been utilized in chronic neurogenic pain [12]. This modality of therapy differs from TENS in that the goal is to mediate the patient’s perception of pain, rather than masking the peripheral pain signal. The results of this modality of treatment may be longer-lasting than TENS, presumably via reduction in central signal generation. Scrambler therapy works through C fibers to retrain the peripheral sensation in the area being treated. Further description of this technology is available at: International Patent PCT/IT2007/000647 and U.S. Patent No. 8,380,317. A literature search did not yield prior studies regarding efficacy of Scrambler therapy in treating RLS.
Multiple clinical trials involving over 800 patients have shown that electrical stimulation using Scrambler therapy is an effective method of treating chronic neurogenic pain [12-19]. Treatment is generally well tolerated and without significant side effects.
Electrical stimulation as a potential treatment of RLS/WED
Most patients with RLS/WED experience discomfort in the lower limbs which is described using terminology including: crawling, tingling, restless, electric, tension, and itching [20]. The peripheral discomfort localized to the lower extremities in RLS/WED appears to be driven by a central mechanism. Functional neuroimaging studies have elucidated that RLS/WED stems from striatal dopaminergic dysfunction [21,22]. This is corroborated by the positive response obtained when treating patients with dopaminergic medications and would also suggest symptoms of RLS/WED arise from the central nervous system [23].
There appears to be overlap between peripheral neuropathy related discomfort and RLS; suggesting that both phenomenon involve the C-fibers. The Scrambler therapy device produces multiple different electrical currents organized into algorithms to simulate nerve action potentials. The net effect appears to provide analgesia via stimulating the C-fibers and substituting “pain” information with “non-pain” information [24]. The data from prior clinical trials as cited above demonstrate efficacy of electrical stimulation in other forms of centrally-mediated neurogenic pain and in particular peripheral neuropathy. This suggests Scrambler therapy may also be an effective treatment in RLS/WED. Additionally, within the Mayo Clinic Pain Medicine Center, it has been anecdotally noted that some patients who received Scrambler therapy for peripheral neuropathy incidentally noted improvement in RLS/WED symptoms.
Study Population
A total of eight eligible subjects were recruited from clinical practice. All subjects were diagnosed with chronic RLS/WED in the Mayo Clinic Center for Sleep Medicine. Subjects had daily symptoms, despite use of prescription medications, and were typically symptomatic during the timeframe in which Scrambler therapy was to be utilized (12-5 PM). All subjects had moderate to very severe RLS/WED symptoms at baseline, as indicated by the International Restless Legs Syndrome Rating Scale (IRLS), which assesses severity of RLS symptoms. This scale has been well-validated and has high levels of internal consistency, interexaminer reliability, and test-retest reliability [25,26]. All subjects indicated having discomfort or pain in the limbs as part of their RLS symptomatology.
Inclusion criteria included subjects at least age 18 who had been diagnosed with RLS/WED by a board certified sleep medicine physician within the Mayo Clinic Center for Sleep Medicine; moderate to severe RLS/WED symptoms which had been present for ≥ 3 months; patient endorsement that discomfort was part of their typical RLS/WED symptomatology; patient report that they experienced daily symptoms during afternoon hours (12-5 PM); and patients who were on alpha-2-delta ligands (pregabalin, gabapentin) were willing to be weaned off these medications two weeks prior to starting protocol treatments and remain off these medications throughout the study protocol. Exclusion criteria included prior use of Scrambler therapy; female subjects of child-bearing potential; patients with implantable drug delivery systems, heart stents, or metal implants (including pacemakers and defibrillators); a history of epilepsy or other medical conditions that in the opinion of the investigators should be excluded; and skin conditions in the lower extremities around the area of planned electrode application.
Scrambler Therapy
Subjects received Scrambler therapy on a daily basis for up to 10 consecutive weekdays. Electrodes were placed proximal to the area of RLS symptomatology, with gradual downward localization until the entire area of RLS symptoms had been treated. The initial treatment was performed in only one lower extremity. Subsequent treatments were performed in both extremities. Treatments were administered by a technician trained in using the Scrambler device. Each session lasted between 60-120 minutes. A study physician (with familiarity of Scrambler therapy) was available throughout each treatment session.
Questionnaires
The primary outcome measure of the study was the change in IRLS score from baseline to completion of Scrambler therapy. IRLS was also measured one week following the last treatment, to gauge duration of effect.
Subjects completed daily questionnaires before and after each Scrambler session (Appendix I). These questionnaires were developed by the authors to detect differences in RLS symptoms and global impressions of change correlating with each individual treatment session as well as the treatment as a whole. There are no similar validated questionnaires specifically for RLS currently available, but these global ratings of change scales were constructed using commonly accepted best practices [27]. Subjects were asked to numerically rate the degree of discomfort they currently had in the lower extremities and to rate the worst discomfort they had experienced in the prior 24 hours using a 0-10 point Likert scale. Subjects were also asked to rate severity of urge to move when resting they were currently experiencing and the worst urge to move experienced over the last 24 hours. Post-treatment, subjects were again asked to separately rate discomfort and urge to move in the lower extremities. After each session, subjects were also asked to rate changes in RLS/WED symptoms since beginning study treatment, and to rate their overall quality of life. After each treatment, subjects were verbally queried by the technician regarding any adverse effects they attributed to Scrambler therapy. The protocol was reviewed and approved by the Mayo Clinic Institutional Review Committee.
Statistical Analyses
All continuous data distributions were evaluated for normality using the Shapiro-Wilk test. Data are summarized as mean ± SD when normally distributed, or as median and range [median (range)] when non-normally distributed. Paired t-tests were used for parametric comparisons of pre-and post-therapy outcomes. A p-value of <0.05 was considered statistically significant. Tests were performed using Wizard (Version 1.9.42 (267), ©2016) and JMP Version 14.1 (14.0), SAS Institute, Cary, NC.).
A total of 8 consecutive subjects (4 males and 4 females) were enrolled between 4/2/2017 and 1/28/2018. The median subject age was 72.5 (51-87). Mean ferritin level at baseline was 94.5 ± 39.4 mcg/L. No subjects had previously received any form of electro analgesia for RLS/WED.
All subjects were on prescription medications for RLS/WED, using a mean of 3 drugs at baseline (range 1-5) for this problem. Two patients were tapered off of gabapentin and/or pregabalin prior to initiating Scrambler therapy by the study physician (with approval from the treating sleep physician). Subjects were asked to discontinue these medications a minimum of two weeks prior to starting Scrambler. This was to exclude any potential residual withdrawal symptoms at time of starting Scrambler. Subjects were to remain off of these drugs during the period of Scrambler treatments. No other medication changes were requested as part of the study. No side effects associated with medication discontinuation were reported.
Two subjects self-discontinued all of their RLS medications during the trial (one due to complete resolution of RLS symptoms associated with Scrambler therapy, and another due to improvement in symptoms associated with Scrambler therapy). One subject discontinued Scrambler treatments after receiving three sessions as his daytime symptoms had resolved, although he continued to endorse nocturnal RLS/WED symptoms.
The mean baseline IRLS score was 27.6 ± 6.2, with a baseline IRLS range of 20-36, correlating with the highest end of moderate to very severe symptoms. The mean pre-treatment IRLS score on the last day of Scrambler treatment was 19.5 ± 8.0, (mean change in IRLS severity score from baseline to last treatment was -8.1, p=0.03).
The mean IRLS score one week following the last Scrambler treatment was 20.3 ± 10.8. No adverse effects were noted by subjects. The mean change in IRLS severity score from baseline to 1 week post treatment was -7.4 ± (p 0.07).
Table 1 provides baseline and end of therapy data from daily symptom assessments during Scrambler therapy.
Table 1: Changes in RLS symptom severity from baseline to final session of Scrambler therapy. Subjects were asked to rate discomfort and urge to move at rest using a 0-10 scale (0=no symptoms; 10=as bad as can be).
| RLS Symptom | Baseline symptom scores Mean (range) | Day 10 symptom scores Mean (range) | Improvement in mean symptom score (%) | P (paired t-test) | ||||
|---|---|---|---|---|---|---|---|---|
| Current | Worst over last 24 h | Current | Worst over last 24 h | Current | Worst over last 24 h | Current | Worst over last 24 h | |
| Discomfort | 2.3 (0-8) | 6.4 (1-10) | 0.9 (0-3) | 4.7 (0-9) | 62.5% improvement | 26.6% improvement | 0.12 | 0.2 |
| Urge to Move | 3.4 (0-9) | 7.3 (2-10) | 1.0 (0-3) | 5.0 (0-3) | 70.6% improvement | 31.5% improvement | 0.06 | 0.14 |
Figure 1 illustrates mean worst symptom scores (over the last 24 hours) prior to each session of Scrambler therapy. Figure 2a illustrates average discomfort just prior to and just after therapy, while Figure 2b illustrates data regarding the average urge to move.
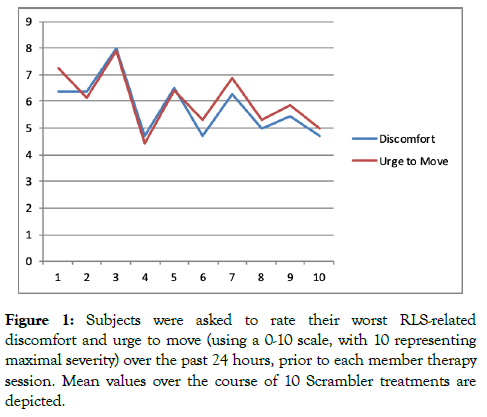
Figure 1: Subjects were asked to rate their worst RLS-related discomfort and urge to move (using a 0-10 scale, with 10 representing maximal severity) over the past 24 hours, prior to each member therapy session. Mean values over the course of 10 Scrambler treatments are depicted.
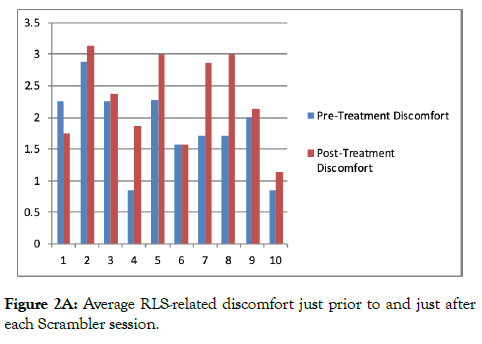
Figure 2A: Average RLS-related discomfort just prior to and just after each Scrambler session.
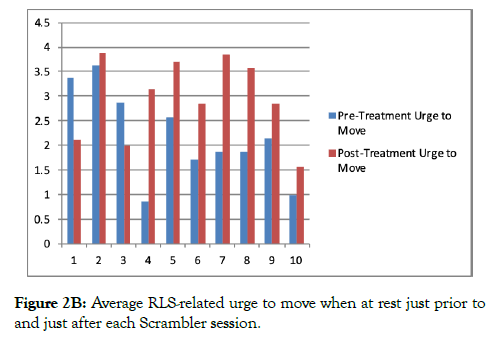
Figure 2B: Average RLS-related urge to move when at rest just prior to and just after each Scrambler session.
Following each Scrambler treatment session, subjects were asked to rate changes in RLS/WED symptoms since starting Scrambler (using a -3 (very much worse) to +3 (very much better) scale. Subjects were also asked after each session to rate any changes in overall quality of life using a similar scale. Following the last Scrambler session, mean improvement in RLS symptoms was rated as +1.5 ± 0.8 (+1=a little better and +2=moderately better), and the mean improvement in quality of life rating was +1.3 ± 1.04.
Individual subjects appeared to have a varying response to treatment. Some reported only modest changes in symptoms during the trial. One subject found complete relief of symptoms following treatments with Scrambler and had self-discontinued all prescription medications for RLS during the trial.
The results of this pilot study support that Scrambler therapy does appear to be beneficial in some patients with refractory RLS symptoms and that Scrambler therapy is well tolerated. The mean IRLS score one week following last treatment remained lower than baseline, suggesting that benefits from Scrambler therapy may last for a period of time following completion of such. This is similar to what has been observed with patients receiving Scrambler therapy for other painful situations, where the benefit appears to last for weeks to months. In such patients, subsequent booster Scrambler treatments appear to provide more sustained improvements [12].
It is noteworthy that the ratings of discomfort and urge to move were commonly increased immediately post-therapy, in comparison to the pre-treatment ratings (Figure 2). A possible explanation may be that in order to accommodate Scrambler therapy, subjects generally remained still and seated or in the recumbent position during each 1-2 hour Scrambler session, a condition known to worsen RLS/WED symptom severity.
There are multiple expected limitations with this exploratory pilot study which include the small sample size and inclusion of only patients with disease refractory to multiple medications. The apparently beneficial results from this trial could conceivably be due to a placebo effect, at least in some part. Additionally, due to logistical reasons, Scrambler therapy was administered during daytime hours (12-5 PM), while characteristically RLS symptoms are worse in the evening.
Future studies to evaluate efficacy of Scrambler therapy as a treatment for chronic RLS are warranted. We suggest a randomized controlled trial with a larger sample size (including subjects with milder symptoms and those who are not on medications). Ideally, further work would also assess the delivery of Scrambler therapy during the evening/nighttime hours (during hours when subjects experience peak symptoms).
Finally, we recognize that a multitude of factors may impact RLS severity, including those related to stress and co-morbid mood disorder symptomatology. Formal mood assessments at baseline and post-therapy should also be included in future work.
Scrambler therapy may be associated with improvements in RLS symptomatology in medically refractory patients with moderate to severe disease. This therapy appears to be safe and welltolerated. A randomized controlled clinical trial may better determine the clinical efficacy and duration of action of Scrambler therapy for the management of chronic RLS.
This report was supported by Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
None
We would like to acknowledge the Sleep Medicine providers in the Mayo Clinic Center for Sleep Medicine for their support of this pilot study and referral of RLS patients for enrollment.
Citation: Lipford MC, O’Neill C, Eibner K, Donlinger S, Herold DL, Morgenthaler TI (2020) Electrical Stimulation via the Scrambler Device as a Treatment for Refractory Restless Legs Syndrome/Willis Ekbom Disease. J Sleep Disord Ther 9:318. doi: 10.35248/2167-0277.20.9.318
Received: 30-Jun-2020 Accepted: 18-Aug-2020 Published: 25-Aug-2020 , DOI: 10.35248/2167-0277.20.9.318
Copyright: © 2020 Lipford MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.