Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2015) Volume 6, Issue 7
Detailed gas phase fragmentation behavior of a furo-furan lactone 1 with a novel skeleton of three fused furan rings isolated from medicinally important Heliotropium eichwaldi plant was studied. Elucidation of the major fragmentation routes was performed using CID-MS/MS analysis, insource-MSn fragmentation, parent ion scan analysis and exact mass measurements using electrospray ionization quadropole time-of-flight mass spectrometry (ESI-QqTOF-MS/ MS) hybrid instrument in negative and positive ion modes. Analysis showed the presence of [M+H]+ and [M-H]- at m/z 275 and m/z 273, respectively. Low-energy collision induced dissociation tandem mass spectrometric analysis (CIDMS/ MS) in both ion modes showed several characteristics losses of carbon monoxide and water molecules along with characteristic losses of acetaldehyde, formaldehyde, formic acid and carbondioxide. Moreover, compound 1 showed significant insource-MSn behavior during in-source fragmentation studies. LC-MS identification of 1 was performed in crude extract of H. eichwaldi. The knowledge of the fragmentation pattern and key fragment ions of furo-furan lactone, will be useful for further exploration of new members of this class of compounds in the H. eichwaldi and related species of family Boraginaceae utilizing very limited plant material.
Keywords: Furo-furan lactone; LC-MS/MS; Mass spectrometry; Heliotropium eichwaldi
Plants have been used for the treatment of various diseases and are the source of unprecedented lead molecules since several decades. In the search for novel natural products in plants, particularly those possessing potential bioactivity, it is vitally important to efficiently distinguish between previously reported compounds and the novel natural products, a process termed as dereplication. Techniques capable of quick and accurate identification of previously reported compounds are therefore of great value [1] thus saving time and money spent on the re-isolation, purification and spectrometric identification of previously known natural products.
Mass spectrometry (MS) has emerged as a complementary method with high sensitivity and selectivity for the high-throughput analysis of natural products coupled with various chromatographic techniques [2,3]. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) using low-energy collision-induced dissociation (CID) has been the technique of choice for such studies [4] owing to its ability to analyze compounds of medium to high polarities [5]. Several natural products have been investigated through ESI-MS/MS and this approach in combination with liquid chromatography (LC) offers a rapid dereplication of plant extract. In order to characterize a compound from its MS/MS data, previous knowledge of the fragmentation pathway of homologous compounds exhibiting a conserved structural core is required for confident identification [6] Such information is not only helpful in identifying characteristic fragments which provide useful information about the structures of compounds but also can be projected to infer the new and similar chemical structures [4]. Moreover, mass spectral database search has now turn out to be the well-accepted approach for the identification of unknown chemicals thus minimizing the need for authentic reference material [7].
The genus Helitropium belongs to the family Boraginaceae, is known to possess a number of medicinal properties which are predominantly attributed to pyrrolizidine alkaloids [8]. Helitropium eichwadi is herbaceous weed, widely distributed in the state of Punjab, Haryana and Rajasthan of India [9] In Pakistan this annual herb grows in cultivated fields, gardens and shallow lands of the province of Sind. The plant is emetic, antidote, antiseptic and is used for the treatment of scorpion stings, bee stings, mad dog bite, snake bite, and ear ache. It is also known to be used for cleaning and healing of wounds, warts and ulcers [10,11]. The plant has demonstrated hypotensive effect, [12] antimicrobial activity and nephrotoxicity [13].
In continuation of our studies on the gas phase fragmentation behavior and the identification of diagnostic fragments ions produced during CID-MS/MS analysis of various unique naturally occurring structures and elucidation of their fragmentation pathways, [14-17] this paper describe the ESI-QqTOF-MS (+ve ion and -ve ion mode) and the CID-MS/MS analysis of the novel furo-furan lactone (1) isolated from Helitropium eichwadi [18] The elucidation of the gas phase fragmentation pathways and the rapid characterization of this complex structure will be helpful for the identification of its congeners in other plant species. Present study is the first ESI-MS/MS report of this novel class of compound.
Standard and reagents
Standard and reagents Deionized water (Milli-Q) was used in the study. Isolation and characterization details of standard furo-furan lactone (4a,6b- Dihydroxy-5-(hydroxymethyl)-2a-isopropyl-2-methylhexahydro- 4H-1,3,6-trioxa-cyclopenta[cd] pentalen-4-one) 1 has already been reported [18].
ESI-QqTOF-MS analysis
Compound (1) was dissolved in methanol (0.5 mg/mL) and analyzed by electrospray ionization (ESI) and collision-induced dissociation (CID) on QqTOF-MS/MS instrument (QSTAR XL mass spectrometer Applied Biosystems/ MDS Sciex, Darmstadt, Germany) at laboratory conditions. Working dilutions (10 μg/mL) were prepared in 1:1 acetonitrile-water containing 0.1% formic acid and 1:1 acetonitrilewater containing 4 mM ammonium acetate for the analysis in positive and negative ion modes, respectively. High-purity nitrogen gas was used as the curtain gas and collision gas delivered from Peak Scientific nitrogen generator. The ESI interface conditions for positive ion modes were as follows: Ion spray capillary voltage of 5500 V, curtain gas flow rate 20 L min-1, nebulizer gas flow rate 30 L min-1, DP1 60 V, DP2 15 V, and FP 265 V. Collision energy was swept from 05 to 45 eV for MS/MS analysis in positive modes. Calibration was performed by using 10 pM reserpine solution as internal calibrant. For the generation of product ions in-source, DP1 was optimized at 90 while curtain gas flow was set at 35 L min-1. Sample was introduced into the mass spectrometer using a Harvard syringe pump (Holliston, MA) at a flow rate of 5 μL/ min during all analysis.
ESI interface conditions for the ESI-MS and ESI-MS/MS analysis in negative ion mode were optimized as follows: Ion spray voltage -4200 V, curtain gas flow rate 20 L, nebulizer gas flow rate 20 L min-1 DP1 -55, DP2 -15 V, and FP 25V. Collisional energy, during CID-MS/MS analysis, was swept from 5 to 40 eV and instrument was calibrated by using 0.2 ng/uL taurocholic acid solution as internal calibrant.
Plant sample preparation and LC-MS analysis
The plant material of H. eichwaldi was collected and identified according to already published report. The shade-dried and chopped whole plant of H. eichwaldi (5 g) was extracted by sonication with MeOH (15 mL) for 30 min at laboratory temperature and then centrifuged. The supernatant was then partitioned with EtOAc (5 mL × 3). EtOAc extract was evaporated to gummy mass (0.6 g). MeOH was then used to reconstitute the gummy mass to 1 mg/mL solution. After necessary dilution resulting solution was then directly injected to mass spectrometer for the ESI-MS/MS analysis of H. eichwaldi plant extract.
Chromatographic separation and mass spectrometric detection was achieved using UPLC-QqQ-MS system (Series 1260, Agilent Technologies, Wilmington, DE, USA), and on Agilent ZORBAX SBC18 column (2.1 × 50 mm, 1.8 μm). Injection volumes were 2 μL and the mobile phases were as follows: eluent A, H2O (0.1% formic acid) and eluent B, MeOH (0.1% formic acid). The flow rate was 0.6 mL min-1, and a gradient elution program was used. The chromatographic procedure was initialized and maintained at 10% B till 2 min, followed by linear increase to 90% B in 8 min. This composition was hold for 1 min and at 9.1 min system returns to initial conditions.
Compound 1 possesses three fused furan rings and has highly functionalized molecular structure. The structure comprises of seven oxygen functionalities present within the frame of twelve carbon atoms which include primary and tertiary hydroxyls, lactone and ether functions. Characteristic fragments in this novel furo furan lactone (1) are formed due to the subsequent losses of water and carbon monoxide molecules. Other characteristic fragments include losses of acetaldehyde, formaldehyde, and formic acid.
Optimization of collision energy and in-source parameters
ESI-QqTOF-MS analysis of 1 showed [M+H]+ at m/z 275.1307 corresponding to the protonated molecular formula C12H19O7 (calcd 275.1125). MS/MS analysis of [M+H]+ ion exhibiting interesting fragmentation pattern and the product ion abundance were found to be significantly influenced by the variation of collision energy after the optimization of all major parameters. MS/MS spectra of furo-furan lactone were screened against laboratory collision energies ranging from 5 to 40 eV in both negative and positive ion modes due to the significant changes in fragmentation pattern (Figure 1). It was observed that the fragment ions resulted due to the sequential losses were best appeared at collision energy 20 eV in positive ion mode (Figure 2A) whereas the collision energy of 25 eV was found to be the optimum energy for the fragment ions formed in the negative ion mode in order to observe intensity of all major fragment ions (Figure 2B). By optimizing declustering potential (DP) at 90 and curtain gas flow (CUR) at 35 Lmin-1 we were able to yield all major product ions via in-source with considerable intensity (Figure 3). These product ions were then successfully fragmented further using CID-MS/MS option and were utilized in proposing fragmentation routes (Supplementary Figures 1-6).
Figure 1: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
Characteristics fragmentation in the ESI-MS/MS
Major characteristic peaks were observed by the sequential removals of CO and water molecules from the [M+H]+ in positive ion mode. A characteristic simultaneous removal of methanol and carbon monoxide followed by the loss of two molecules of water i.e., [M+HMeOH- 2H2O -CO]+ appeared as a base peak at m/z 179 with collision energy of 25 eV. Other characteristic peaks were observed due to the loss of multiple hydroxyls as water and carboxylic group as carbon monoxide molecules at m/z 257 [M+H-H2O]+, 239 [M+H-2H2O]+, 197 [M+H-H2O-MeOH-CO]+, 179 [M+H-2H2O-MeOH-CO]+, 133 [M+H-MeOH-3H2O-2CO]+, and 105 [M+H-MeOH-3H2O-3CO]+.
The lactone and hydroxyl moieties have a significant influence on the fragmentation pattern of this new class of compound. In the second pathway, fragments were observed at m/z 229 [M+H-H2O-CO]+, 169 [M+H-2H2O-CO-CH2CO]+, 151 [M+H-3H2O-CO-CH2CO]+, 141 [M+H-2H2O-2CO-CH2CO]+, 123 [M+H-2H2O-2CO-CH2CO]+, 95 [M+H-3H2O-3CO-CH2CO]+. In another pathway, loss of acetaldehyde was observed preceding removal of water molecule at m/z 213 [M+HH 2O-CH3CHO]+ and 195 [M+H-2H2O-CH3CHO]+ (Figure 3A).
In negative ion mode, parent ion was observed at m/z 273 [M-H]-. Few characteristic fragments were observed including m/z 243 [M-HCH 2O]-, 133 [M-H-CH2O-C7H7O]-, 103 [M-H-2CH2O-C7H7O]- and 59 [M-H-2CH2O-C7H7O-CO2]- (Figure 2B). A second series of product ions were observed at m/z 199 [M-H-CH2O-CO2]-, 155 [M-H-CH2OCO 2-CH3CHO]-, 127[M-H-CH2O-CO2-CH3CHO-CO]- and 99 [M-H-CH 2O-CO2-CH3CHO-2CO]- (Figure 3B).
Evaluation of the mechanistic pathways in positive ion mode
Mechanistic pathways for the furo-furan lactone (1) fragmentation was proposed using different techniques including exact mass measurement for predicting elemental composition, in silico studies to predict possible protonation sites from where fragmentation possibly have initiated, MS/MS, in-source fragmentation and parent ion scan to elucidate fragmentation pathway. The HR-ESI-MS data of characteristic fragments in both positive and negative modes is summarized in Supplementary Tables 1 and 2, respectively.
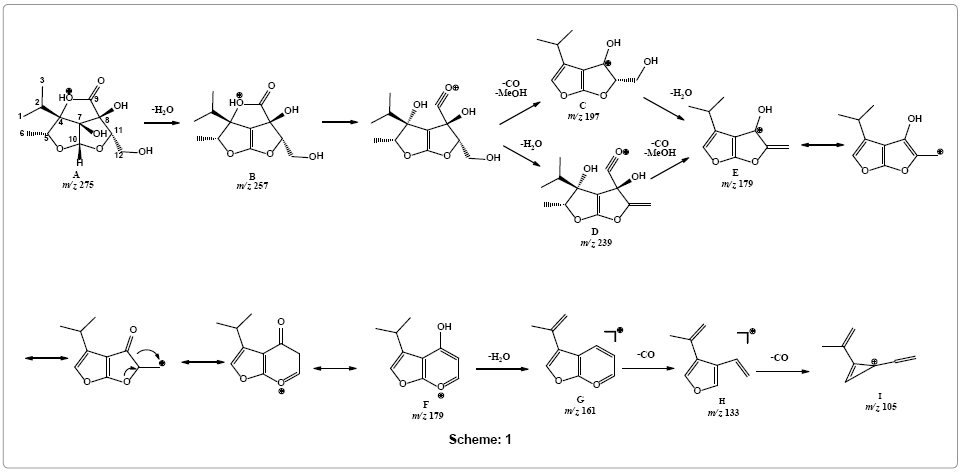
In silico calculation of HOMO by DFT at 6-31G* basis set using Spartan 08 (Wavefunction, CA, USA) suggested carbonyl oxygen to be the most favorable protonation site (Figure 4) that may tautomarize through a proton transfer to lactone oxygen forming specie A. Loss of water molecule was observed forming product ion B at m/z 257. Parent ion scan of product ion m/z 179 showed its formation from two ions i.e., m/z 239 and m/z 197 (Supplementary Figure 7). Therefore, simultaneous loss of carbon monoxide and methanol was suggested to produce fragment C (m/z 197) a highly stabilize aromatic ion. Alternatively, loss of water molecule is also preceded by simultaneous loss of methanol and carbon monoxide forming fragment ion E (m/z 179) via fragment D at m/z 239. Rearrangement of fragment E to F is proposed through ring expansion that may tautomarize to phenol-like form. Ion form F on releasing water molecule may forms fragment G (m/z 161), structure that readily undergo removal of carbon monoxide to produce fragment H at m/z 133. Loss of another carbon monoxide from fragment I resulted in the formation of fragment ion I with m/z 105 (Scheme 1).
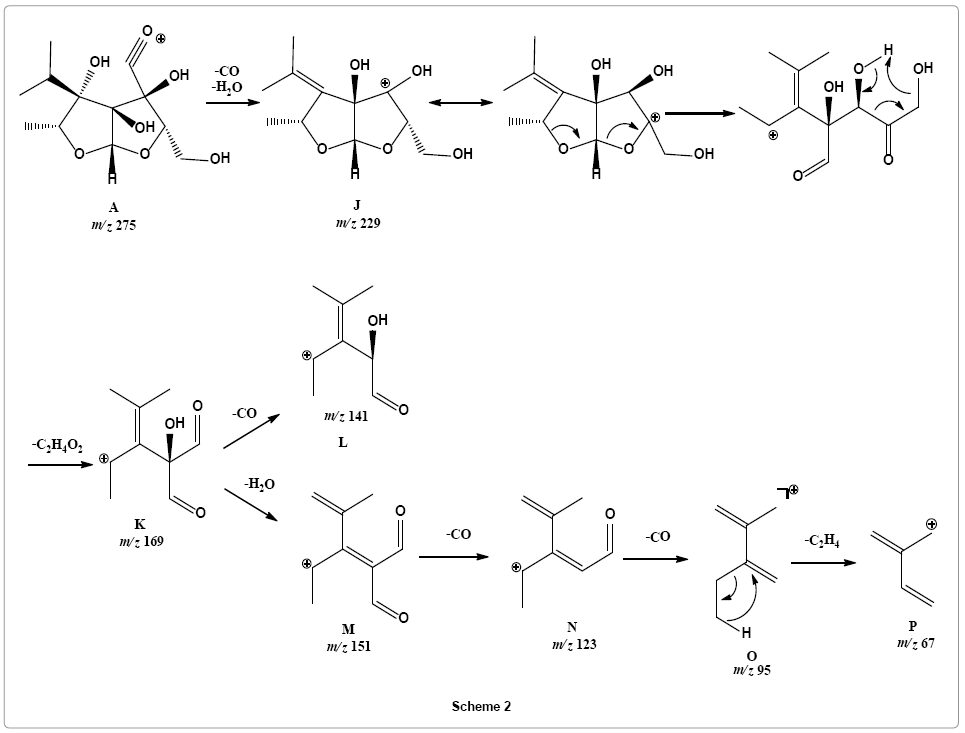
Parent ion scan of product ion at m/z 229 suggested that it was formed directly from parent ion at m/z 275 (Supplementary Figure 8). It revealed that another pathway is initiated with simultaneous loss of water and carbon monoxide molecule from parent ion providing fragment ion J at m/z 229 that may undergo rearrangement via six member transition state to stable vinyl cation K with m/z 169 by simultaneously losing ketene and water molecules, such reactions are entropically favored. Fragment ion K on further loss carbon monoxide molecule form fragment L with m/z 141. Loss of a water molecule from product ion at m/z 169 produces fragment ion M, a conjugated ion with m/z 151. Product ion at m/z 151 sequentially losses two carbon monoxide molecules to give rise to product ions N and O at m/z 123 and 95, respectively. Loss of ethene molecule is observed from product ion at m/z 95 to form product ion at m/z 67 (Scheme 2).
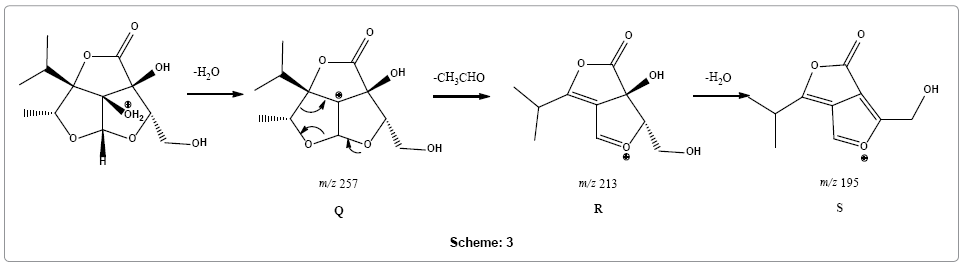
Parent ion scan of product ion at m/z 213 confirms its formation from product ion at m/z 257 (Supplementary Figure 9). Therefore, a different pathway is suggested, instead of multiple carbon monoxide losses, loss of an acetaldehyde molecule from fragment ion Q (m/z 257) was observed followed by loss of water molecule forming fragment ions R and S with m/z 213 and 195, respectively (Scheme 3).
Evaluation of mechanistic pathway in negative ion mode
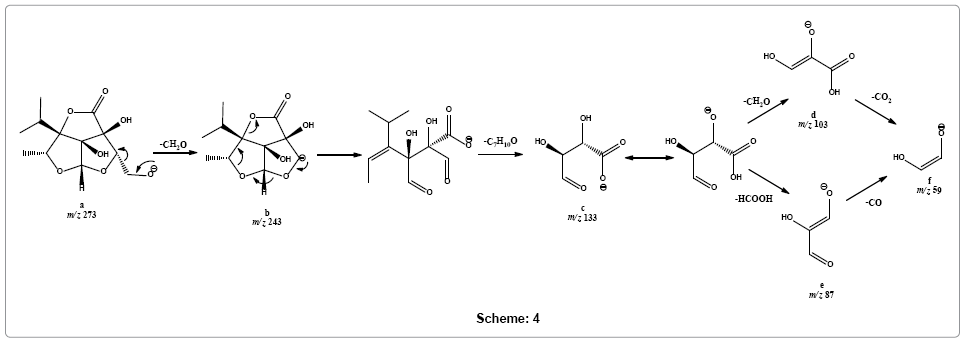
It was suggested that fragmentation in the negative ion mode initiated from deprotonation of primary alcohol forming [M-H]- ion a at m/z 273. The other two possible sites of deprotonation are the two relatively more sterically hindered hydroxyls at C-7 and C-8. Parent ion scans of product ions at m/z 103 and 133 shows that these ions are formed from parent [M-H]- ion (m/z 273) via fragment ion m/z 243 (Supplementary Figure 10). On this basis it is suggested that ion a readily losses formaldehyde molecule to give rise to a weak signal of fragment ion b at m/z 243. Fragment ion b simultaneously loses alkenyl part and carbon monoxide molecule to form base peak at m/z 133 of fragment ion c (for CE 25-35 eV). Fragment c may dissociate into fragment f at m/z 59 via two possible routes, either via product ion d (m/z 103) by sequentially losing formaldehyde and carbon dioxide molecules, respectively or via product ion e (m/z 87) through sequentially giving out formic acid and carbon monoxide molecules, respectively (Scheme 4).
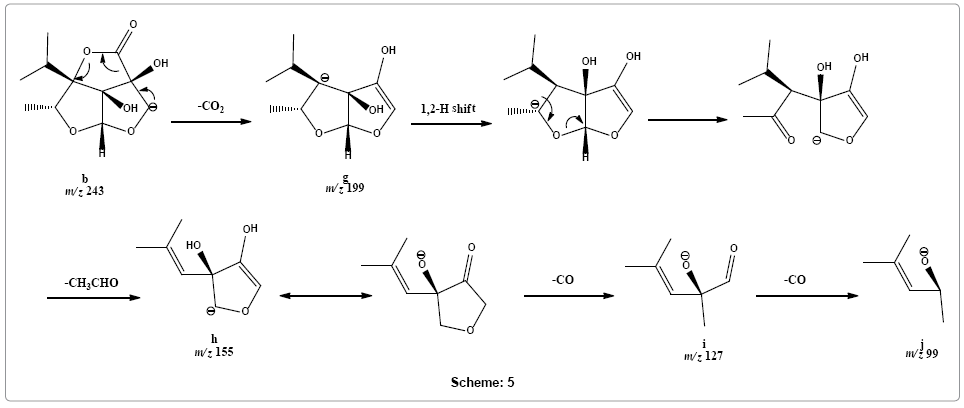
Following alternative dissociation pathway, fragment ion b may rapidly release carbon dioxide molecule to form fragment ion g at m/z 199. This product ion g may rearrange into an open structure that loses acetaldehyde molecule to give rise peak at m/z 155 of the product ion h. Product ion h upon sequential removal of two carbon monoxide molecules form product ions i and j at m/z 127 and 99, respectively (Scheme 5).
ESI-MS/MS analysis of Heliotropium eichwaldi extract
The furo-furan lactone in the extract of Heliotropium eichwaldi was investigated by LC-ESI-MS/MS. The full LC-MS scan in positive mode showed the presence of [M+H]+ and [M+Na]+ions of novel furo furan lactone (m/z 275) at Rt 5.1 min (Figure 5). The Rt and MS/MS data of this protonated ion matches the standard furo furan data, which showed several neutral losses of water and carbon monoxide molecules giving rise to characteristic peaks such as at m/z 257, 195, 179, 161, 133, and 95 etc. (Figure 6). Therefore, the compound was identified as novel furo furan lactone (1).
This study showed that CID-MS/MS fragmentation pattern of furo-furan lactone (1) is highly dominated by neutral losses of several carbon monoxide molecules in positive ion mode and losses of formaldehyde and carbon monoxide is predominant in negative ion spectra. It was found that due to highly functionalized nature of compound (1) fragmentation pattern is very complicated and several parallel pathways guide the dissociation of parent ion. Fragmentation mechanism has been proposed using several tools including exact masses and linked scanning data such as MS/MS and parent ion scanning data etc. The information acquired from MS/MS data and parent ion scanning of compound 1 standard was successfully applied to identify it in Heliotropium eichwaldi crude extract without rigorous sample preparation method. Such studies will be potentially useful to rapidly identify this class of compounds in complex mixtures such as plant extracts of other species and herbal product based on them.
The authors acknowledge the OPCW (Project No. L/ICA/ICB/173673/12) for providing the partial funding for this study. The authors are also thankful to Mr. Junaid ul Haq for kind assistance in LC-MS analysis.