Research Article - (2021)Volume 6, Issue 1
Objective: We aimed to investigate the effect of serum Creatine Kinase (CK) levels on disease progression and prognosis in coronavirus disease 2019 (COVID-19).
Methods: This was a retrospective study of 1751 COVID-19 patients at Leishenshan hospital in Wuhan, China. All patients were grouped to normal and elevated CK groups. Univariate and multivariate Cox regression analyses were performed to explore the relationship between mortality and CK levels. Univariate and multivariate logistic regression analyses were performed to explore the relationship between disease severity and CK levels. Survival curves were generated for normal and elevated CK groups. Fitting curves were performed to investigate the relationships between the number of days in hospital and Computed Tomography (CT) score.
Results: Elevated CK patients had higher incidences of critical disease severity (P<0.001), death, and higher CT score. There was an association between elevated CK levels and mortality on multivariate Cox regression analysis (HR=7.31; 95% CI, 1.09-48.96; P=0.04). Elevated CK patients were more likely to have critical disease severity on multivariate logistic regression analysis (OR=4.38; 95% CI, 1.16-16.49; P=0.029). Kaplan-Meier curves demonstrated poor prognosis with elevated CK levels (P<0.001).
Conclusion: Elevated CK level was an independent risk factor of mortality in COVID-19 patients. Inpatients with elevated CK had a higher risk for mortality, as well as critical severity condition compared with normal CK inpatients. This may help clinicians make more targeted drug choices to treat COVID-19 patients.
COVID-19; Creatine kinase; Prognosis; Risk factor; Severity
Coronavirus disease 2019 (COVID-19) first emerged in Wuhan, China, in December 2019 and was identified as a highly infectious disease. COVID-19 is caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). COVID-19 is now a declared pandemic that has affected most countries on the globe. As of 24:00 CEST, May 6, 2020, 82,885 people have been diagnosed with COVID-19 in China, with 4,633 cumulative COVID-19– related deaths. The World Health Organization (WHO) website reported a cumulative global COVID-19 diagnosis in excess of 3.5 million people by 10:00 CEST, May 6, 2020. The current global mortality rate of COVID-19 is listed as 6.89% and is predicted to rise even further, as no effective medication or guidelines for treatment are available.
Most published studies regarding the prognosis of COVID-19 are mainly descriptive, and the related risk factors are still under exploration. Cardiovascular complications are known to be associated with poor outcomes in COVID-19 patients [1]. One study also found that cardiac injury contributed to more severe disease progression and greater risk of mortality in COVID-19 patients [2]. Cardiac injury is determined by measuring serum myocardial necrosis indices, such as Creatine Kinase-Muscle/Brain (CK-MB), troponin I, and Lactate Dehydrogenase (LDH) [3].
The Leishenshan hospital is a temporary specialist establishment dedicated to the treatment of COVID-19 patients. It is equipped with professional medical equipment, medical management teams, and medical staff. This hospital is equipped for electrocardiographic diagnosis, ultrasound imaging, radiological imaging, and medical laboratory analysis to meet the diagnostic and treatment needs of COVID-19 patients. The clinical experiences of this hospital have already provided immense guidance to the rest of the world regarding the treatment of COVID-19 patients. Although several early studies [1,4-6] reported certain risk factors associated with poor prognosis in COVID-19 patients, the risk factors associated with the severity and mortality of COVID-19 are yet to be described in detail. Moreover, previous reports were not comprehensive and were derived from small patient samples. Currently, there is no specific drug for COVID-19 in the world. Understanding the risk factors related to the prognosis of COVID-19 can help clinicians make better treatment to choose drug, alleviate the disease as soon as possible and reduce the risk of death. In this large, retrospective study, we attempted to identify further risk factors for mortality and to investigate the relationship between CK levels and the prognosis of COVID-19 patients.
Study design and participants
Data used in this study are available from the corresponding author upon reasonable request. This retrospective, single-center study included 1,880 patients with COVID-19 infection admitted to Leishenshan Hospital in Wuhan, China, who had laboratory testing performed for CK levels. All information regarding COVID-19 patients was obtained from their medical records and was independently reviewed by a minimum of two physicians. Patients with unclear or unknown serum CK levels were excluded from the study cohort. Finally, a total of 1,751 COVID-19 patients with valid CK levels were included in the analysis. Patient information was reviewed to extract demographic characteristics, comorbidity, symptoms, laboratory findings, treatment, Computed Tomography (CT) images, disease progression, and number of days in hospital. Patients were admitted between February 8, 2020, and March 18, 2020. The final date of follow up was April 15, 2020.
Primary outcomes in this study
The primary patient outcomes for the study were patient survival (alive or dead) and the worst disease severity condition of COVID-19 during hospitalization. The disease severity condition was classified as general, mild, severe, or critical based on the seventh interim guidance of diagnosis and treatment of COVID-19 published by the Chinese National Health Commission. The number of days in hospital and CT findings was two other important outcomes of our study. All CT images were independently interpreted by two experienced radiologists; findings from inconsistent images were finalized by discussion. Based on previous studies and the CT image characteristics of COVID-19, we used an objective and optimized semi-quantitative scoring system to assess pulmonary inflammation. Score 1 was based on the number of the following categories of findings identified on pulmonary CT images: Ground-Glass Opacities (GGO), reticulation or cords change, consolidation, and pleural effusions [7-9]. One point was awarded for each category identified, and score 1 was calculated as the sum of all such points. Score 2 was calculated based on the area of involvement of the lung lobes: no involvement, 0 points;<25% involvement, 1 point; 26-%50% involvement, 2 points; 51%-75% involvement, 3 points; and 76-100% involvement, 4 points [10]. The total pulmonary inflammation score was the sum of score 1 and score 2.
Statistical analysis
Continuous variables are expressed as the median and Interquartile Range (IQR), and categorical variables are expressed as numbers and percentages (%). Statistical differences between the normal and elevated CK groups were analyzed using the Mann-Whitney U test for continuous variables and Fisher’s exact test or χ2 test for categorical variables. Survival analysis for the prognosis of COVID-19 patients was performed using univariate and multivariate Cox regression analyses to calculate the Hazard Ratio (HR) and 95% Confidence Interval (CI) in comparing the normal and elevated CK groups. Multiple variables were adjusted, including age, the history of cardiovascular disease, interleukin 6 (IL-6), platelets (PLT), Procalcitonin (PCT), Total Bilirubin (TBIl), and D-dimer. Univariate and multivariate logistic regression analyses to calculate the odds ratio (OR) and 95% CI were used to compare the relationship between the disease severity condition of COVID-19 patients during hospitalization and their CK levels. Multiple variables were adjusted, including age, the history of cardiovascular disease, IL-6, PLT, PCT, TBIl, and D-dimer. Survival curves and the cumulative hazard function for COVID-19 progression in both CK groups were analyzed using Kaplan-Meier analysis with log-rank tests. In addition, CT pulmonary scores as a function of time were quantitively assessed using the SPSS curve estimation module. All data were analyzed in SPSS Statistics (version 23.0 for windows, IBM, Armonk, NY, USA). A two-side P-value less than 0.05 were considered statistically significant.
Demographics, clinical information, and laboratory findings
There were 1,668 patients in the normal CK group and 83 patients in the elevated CK group, with mean ages of 59 years and 63 years, respectively. Table 1 showed that the difference between the two groups was statistically significant for age (P=0.037) and sex (P=0.003). Among both groups, cardiovascular disease was the most common comorbidity (normal CK: 40.9%, elevated CK: 46.8%), followed by endocrine diseases (normal CK: 15.6%, elevated CK: 19.1%). Patients in the elevated CK group had a higher proportion of pulmonary diseases (12.8%) than those in the normal CK group (10.6%). The most common presenting complaints in the two groups were respiratory symptoms (elevated CK: 81.1%, normal CK: 73.2%) (Table 1).
| Levels | All patients (n=1751), Median (IQR)/n (%) | Normal CK (n=1668), Median (IQR)/n (%) | Elevated CK (n=83), Median (IQR)/n (%) | P-value | |
|---|---|---|---|---|---|
| Age (year), median (IQR) | 59(49-68) | 59(49-68) | 63(53-71) | 0.037* | |
| Gender | Female | 916 (52.3) | 886 (53.1) | 30 (36.1) | 0.003* |
| Male | 835 (47.7) | 782 (46.9) | 53 (63.9) | ||
| Any comorbidity | 523 (61) | 489 (60.4) | 34 (72.3) | 0.124 | |
| Cardiovascular diseases | 353 (41.2) | 331 (40.9) | 22 (46.8) | 0.448 | |
| Pulmonary diseases | 87 (10.7) | 82 (10.6) | 5 (12.8) | 0.599 | |
| Neurological diseases | 54 (6.3) | 45 (5.6) | 9 (19.1) | 0.002* | |
| Endocrine diseases | 135 (15.8) | 126 (15.6) | 9 (19.1) | 0.536 | |
| Malignancy | 59 (6.9) | 56 (6.9) | 3 (6.4) | 1 | |
| Digestive diseases | 45 (5.3) | 41 (5.1) | 4 (8.5) | 0.302 | |
| Initial symptoms | |||||
| Fever or fatigue | 610 (79) | 580 (79.3) | 30 (73.2) | 0.329 | |
| Respiratory symptoms | 623 (80.7) | 593 (81.1) | 30 (73.2) | 0.222 | |
| Digestive symptoms | 80 (10.4) | 74 (10.1) | 6 (14.6) | 0.424 | |
| Neurological symptoms | 26 (3.4) | 24 (3.3) | 2 (4.9) | 0.643 | |
| Other | 25 (3.2) | 24 (3.3) | 1 (2.4) | 1 |
Note: *represent the P value<0.05
Table 1: Demographic characteristics and symptoms of 1751 patients with COVID-19.
The number of patients with SARS-CoV-2 and positive IgG was significantly higher in COVID-19 patients with elevated CK levels than in those with normal CK levels (P=0.016) (Table 2). There were significant differences between the two groups in terms of the severity at admission, the worst disease severity during hospitalization, and the need for oxygen support (all variables, P<0.001) (Table 3). A majority of patients in the two groups had high CT scores (59.8% and 80.0%, respectively). Mortality was higher in the elevated CK group (4.9%). Median survival days in the normal and elevated CK groups were 18 and 16 days, respectively (Table 3).
| Covariate | All patients (n=1751), Median (IQR)/n (%) | Normal CK (n=1668), Median (IQR)/n (%) | Elevated CK (n=83), Median (IQR)/n (%) | P value | Reference range |
|---|---|---|---|---|---|
| Leucocyte count, × 109/L | 5.69 (4.71-6.92) | 5.68(4.7-6.89) | 6.38(5-7.21) | 0.033* | 3.5-9.5 |
| 3.5-9.5 | 1559 (89.1) | 1486(89.2) | 73(88.0) | 0.268 | |
| <3.5 | 101 (5.8) | 98(5.9) | 3(3.6) | ||
| >9.5 | 89 (5.1) | 82(4.9) | 7(8.4) | ||
| Neutrophil count, × 109/L | 3.28 (2.54-4.28) | 3.27(2.53-4.25) | 3.53(2.82-5.07) | 0.013* | 1.8-6.3 |
| 1.8-6.3 | 1526 (87.2) | 1457(87.5) | 69(83.1) | 0.017* | |
| <1.8 | 114 (6.5) | 111(6.7) | 3(3.6) | ||
| >6.3 | 109 (6.2) | 98(5.9) | 11(13.3) | ||
| Lymphocyte count, × 109/L | 1.6 (1.24-1.99) | 1.60(1.25-1.99) | 1.60(1.06-1.91) | 0.195 | 1.1-3.2 |
| 1.1-3.2 | 1432 (81.8) | 1373(82.4) | 59(71.1) | 0.020* | |
| <1.1 | 291 (16.6) | 270(16.2) | 21(25.3) | ||
| >3.2 | 26 (1.5) | 23(1.4) | 3(3.6) | ||
| Erythrocyte count, × 1012/L | 4.12 (3.76-4.49) | 4.12 (3.76-4.48) | 4.20(3.74-4.66) | 0.392 | 4.3-5.8 |
| 4.3-5.8 | 625 (35.7) | 591(35.5) | 34(41) | 0.452 | |
| <4.3 | 1113 (63.6) | 1065(63.9) | 48(57.8) | ||
| >5.8 | 11 (0.6) | 10(0.6) | 1(1.2) | ||
| Monocyte count, × 109/L | 0.5 (0.4-0.63) | 0.5 (0.4-0.63) | 0.51(0.41-0.63) | 0.991 | 0.1-0.6 |
| 0.1-0.6 | 1233 (70.5) | 1173 (70.4) | 60(72.3) | 0.837 | |
| <0.1 | 5 (0.3) | 5 (0.3) | 0 (0) | ||
| >0.6 | 511 (29.2) | 488 (29.3) | 23 (27.7) | ||
| Hemoglobin, g/L | 126 (115-137) | 126(115-136) | 131(118-140) | 0.019* | 130.0-175.0 |
| 130.0-175.0 | 699 (40.0) | 653(39.2) | 46(55.4) | 0.003* | |
| <130.0 | 1045 (59.7) | 1009(60.6) | 36(43.4) | ||
| >175.0 | 5 (0.3) | 4(0.2) | 1(1.2) | ||
| Platelet count, × 109/L | 228 (187-277) | 230(187.75-278) | 208(153-267) | 0.003* | 125.0-350.0 |
| 125.0-350.0 | 1524 (87.1) | 1456(87.4) | 68(81.9) | 0.000* | |
| <125.0 | 73 (4.2) | 62(3.7) | 11(13.3) | ||
| >350.0 | 152 (8.7) | 148(8.9) | 4(4.8) | ||
| Albumin, g/L | 37.7 (35-4) | 37.7(35.0-39.9) | 38.2(33.6-40.9) | 0.753 | 40-55 |
| 40-55 | 438 (25.1) | 411(24.7) | 27(32.5) | 0.119 | |
| <40 | 1308 (74.9) | 1252(75.3) | 56(67.5) | ||
| Alanine aminotransferase, U/L | 23 (15-37) | 23(15-37) | 25(16-44) | 0.083 | 9-50 |
| 9-50 | 1403 (80.4) | 1338(80.5) | 65(78.3) | 0.501 | |
| <9 | 93 (5.3) | 90(5.4) | 3(3.6) | ||
| >50 | 250 (14.3) | 235(14.1) | 15(18.1) | ||
| Aspartate aminotransferase, U/L | 20 (16-27) | 19(16-26) | 25(21-35) | 0.000* | 15-40 |
| 15-40 | 1285 (73.6) | 1222(73.5) | 63(75.9) | 0.000* | |
| <15 | 313 (17.9) | 310(18.6) | 3(3.6) | ||
| >40 | 148 (8.5) | 131(7.9) | 17(20.5) | ||
| Total bilirubin, μmol/L | 9.1 (7-12) | 9.1(6.9-11.9) | 10(7.3-13.5) | 0.087 | 5.0-21.0 |
| 5.0-21.0 | 1557 (89.2) | 1488(89.5) | 69(83.1) | 0.072 | |
| <5.0 | 121 (6.9) | 114(6.9) | 7(8.4) | ||
| >21.0 | 68 (3.9) | 61(3.7) | 7(8.4) | ||
| Creatinine, μmol/L | 64.3 (54.50-76.2) | 63.8(54.3-75.7) | 71(60.8-86.3) | 0.001* | 64.0-104.0 |
| 64.0-104.0 | 795 (45.40) | 754(45.2) | 41(49.4) | 0.000* | |
| <64.0 | 864 (49.4) | 837(50.2) | 27(32.5) | ||
| >104.0 | 91 (5.2) | 76(4.6) | 15(18.1) | ||
| Ureanitrogen, mmol/L | 4.8 (3.9-5.8) | 4.8 (3.9-5.8) | 5.2 (3.9-7.4) | 0.033* | 2.8-7.6 |
| 2.8-7.6 | 1543 (88.2) | 1482(88.9) | 61(73.5) | 0.000* | |
| <2.8 | 68 (3.9) | 65(3.9) | 3(3.6) | ||
| >7.6 | 139 (7.9) | 120(7.2) | 19(22.9) | ||
| Procalcitonin, ng/mL | 0.04 (0.03-0.05) | 0.04(0.03-0.05) | 0.04(0.03-0.12) | 0.003* | <0.05 |
| <0.05 | 982 (66.3) | 944(67.0) | 38(51.4) | 0.008* | |
| >=0.05 | 500 (33.7) | 464(33.0) | 36(48.6) | ||
| Interleukin-6, pg/mL | 1.5 (1.50-4.04) | 1.5(1.5-3.98) | 1.5(1.5-4.72) | 0.784 | 0-7.0 |
| 0-7.0 | 579 (83.5) | 551(83.9) | 28(77.8) | 0.354 | |
| >7.0 | 114 (16.5) | 106(16.1) | 8(22.2) | ||
| Lactate Dehydrogenase, U/L | 184 (160-216) | 182(159-214) | 219(185-290) | 0.000* | 125-343 |
| 125-343 | 1653 (94.4) | 1587(95.1) | 66(79.5) | 0.000* | |
| <125 | 43 (2.5) | 43(2.6) | 0(0) | ||
| >343 | 55 (3.1) | 38(2.3) | 17(20.5) | ||
| Prothrombin time, s | 11.3 (10.9-11.8) | 11.3(10.9-11.7) | 11.5(10.9-12.25) | 0.014* | 9.4-12.5 |
| 9.4-12.5 | 1438 (92.1) | 1377(92.7) | 61(80.3) | 0.000* | |
| <9.4 | 1 (0.1) | 1(0.1) | 0(0) | ||
| >12.5 | 123 (7.9) | 108(7.3) | 15(19.7) | ||
| International Normalized Ratio | 0.97 (0.93-1.02) | 0.97(0.93-1.01) | 0.99(0.93-1.07) | 0.016* | 0.8-1.3 |
| 0.8-1.3 | 1484 (95.0) | 1418(95.4) | 66(86.8) | 0.000* | |
| <0.8 | 19 (1.2) | 19(1.3) | 0(0) | ||
| >1.3 | 59 (3.8) | 49(3.3) | 10(13.2) | ||
| Activated partial thromboplastin time, s | 27.2 (24.6-30.43) | 27.2(24.55-30.4) | 28.7(24.83-31.58) | 0.132 | 25.1-36.5 |
| 25.1-36.5 | 1016 (65) | 967(65.1) | 49(64.5) | 0.275 | |
| <25.1 | 463 (29.6) | 443(29.8) | 20(26.3) | ||
| >36.5 | 83 (5.3) | 76(5.1) | 7 (9.2) | ||
| Fibrinogen, (g/L) | 2.95 (2.51-3.73) | 2.95(2.51-3.73) | 2.83(2.4-3.84) | 0.333 | 2.38-4.98 |
| 2.38-4.98 | 1159 (74.2) | 1106(74.4) | 53(69.7) | 0.621 | |
| <2.38 | 304 (19.5) | 286(19.2) | 18(23.7) | ||
| >4.98 | 99 (6.3) | 94(6.3) | 5(6.6) | ||
| Thrombin time, s | 17.7 (17-18.5) | 17.65(17-18.4) | 17.85(16.93-18.6) | 0.424 | 10.3-16.6 |
| 10.3-16.6 | 238 (15.2) | 225(15.1) | 13(17.1) | 0.624 | |
| >16.6 | 1324 (84.8) | 1261(84.9) | 63(82.9) | ||
| D-dimer, g/L | 0.38 (0.21-0.9) | 0.38(0.21-0.90) | 0.48(0.23-1.08) | 0.319 | 0-0.5 |
| 0-0.5 | 915 (58.6) | 876(59.0) | 39(51.3) | 0.191 | |
| >0.5 | 647 (41.4) | 610(41.0) | 37(48.7) | ||
| SARS-CoV-19 IgM | - | ||||
| NO | 379 (64.6) | 360 (64.1) | 19 (76.0) | 0.287 | |
| YES | 208 (35.4) | 202 (35.9) | 6 (24.0) | ||
| SARS-CoV-19 IgG | - | ||||
| NO | 49 (2.8) | 43 (2.6) | 6 (7.2) | 0.016* | |
| YES | 523 (29.9) | 505(30.3) | 18 (21.7) | ||
| Note: * represent the P value<0.05 |
80 (10.4) | 80 (10.4) | 80 (10.4) | 80 (10.4) | 80 (10.4) |
Table 2: Laboratory test results of 1751 patients with COVID-19.
| Covariate | All patients (n=1751), Median (IQR)/n (%) |
Normal CK (n=1668), Median (IQR)/n (%) |
Elevated CK (n=83), Median (IQR)/n (%) |
P-value |
|---|---|---|---|---|
| Severity on admission | ||||
| General | 784 (44.8) | 751 (45) | 33 (39.8) | 0.000* |
| Mild | 663 (37.9) | 637 (38.2) | 26 (31.3) | |
| Severe | 280 (16.) | 262 (15.7) | 18 (21.7) | |
| Critical | 24 (1.4) | 18 (1.1) | 6 (7.2) | |
| Severity at worst | ||||
| General and Mild | 908 (52) | 872 (52.4) | 36 (43.9) | 0.000* |
| Severe | 792 (45.4) | 754 (45.3) | 38 (46.3) | |
| Critical | 46 (2.6) | 38 (2.3) | 8 (9.8) | |
| Oxygen support | ||||
| Low flow oxygen therapy | 256 (83.1) | 243 (83.8) | 13 (72.2) | 0.000* |
| High flow oxygen therapy/ Non-invasive ventilation | 46 (14.9) | 44 (15.2) | 2 (11.1) | |
| Invasive mechanical ventilation | 5 (1.6) | 2 (0.7) | 3 (16.7) | |
| ECMO | 1 (0.3) | 1 (0.3) | 0 (0) | |
| Drugs | ||||
| Antiviral therapy | 852 (99.2) | 809 (99.5) | 43 (93.5) | 0.004* |
| Antibiotic therapy | 518 (99.4) | 491 (99.4) | 27 (100) | 1.000 |
| The appliance of Vitamin C | 242 (100) | 229 (100) | 13 (100) | - |
| Traditional Chinese medicine therapy | 1508 (100) | 1440 (100) | 68 (100) | - |
| Anticoagulation treatment | 124 (7.1) | 108 (6.5) | 16 (19.3) | 0.000* |
| Use of corticosteroid | 107 (6.1) | 99 (5.9) | 8 (9.6) | 0.160 |
| Antimalarial drugs | 134(74.9) | 126(77.3) | 8(50) | 0.030* |
| ICU care | 35(92.1) | 27(90) | 8(100) | 1.000 |
| CT score | ||||
| 1-4 | 73(38.6) | 70(40.2) | 3(20.0) | 0.169 |
| 5-7 | 116(61.4) | 104(59.8) | 12(80.0) | |
| Disease Progression | ||||
| Stableness / Hospitalisation | 15(0.9) | 14(0.8) | 1(1.2) | 0.000* |
| Improvement / Recover | 1699(98.3) | 1623(98.5) | 76(93.8) | |
| Death | 15 (0.9) | 11 (0.7) | 4 (4.9) | |
| Hospital days, median (IQR) | 18(13-24) | 18(13-24) | 16(11-22) | 0.057 |
| <=18 | 895 (51.6) | 846 (51.1) | 49 (60.5) | 0.111 |
| >19 | 841 (48.4) | 809 (48.9) | 32 (39.5) |
Note: * represent the P value<0.05
Table 3: Clinical treatment and outcomes of 1751 patients with COVID-19.
On univariate Cox regression analysis, CK level (HR, 8.19; 95% CI, 2.61-25.73; P<0.001) was positively correlated with the risk of inhospital death. After adjusting for age, the history of cardiovascular disease, IL-6, PCT, PLT, TBIL, and D-dimer, the same trend was seen on multivariate Cox regression analysis (HR, 7.31; 95% CI, 1.09-48.96; P, 0.04) (Table 4). On univariate logistic regression analysis, CK level (OR, 4.63; 95% CI, 2.08-10.27; P<0.001) was positively correlated with the risk of critical severity. After adjusting for age, the history of cardiovascular disease, IL-6, PCT, PLT, TBIL, and D-dimer results, the same trend was seen on multivariate logistic regression analysis (OR, 4.38; 95% CI, 1.16-16.49; P=0.029) (Table 5).
| Cox Regression Analysis | |||||
|---|---|---|---|---|---|
| Cox Regression Analysis | Group | HR | 95% CI | P value | 18(13-24) |
| Univariate Analysis | Normal CK | ref | |||
| Elevated CK | 8.19 | 2.61 | 25.73 | 0.000* | |
| Multivariate Analysis# | Normal CK | ref | |||
| Elevated CK | 7.31 | 1.09 | 48.96 | 0.040* | |
Note: # Adjust for age, the history of cardiovascular disease, IL-6, PLT, PCT, TBIL, D-dimer
* represent the P value <0.05
Table 4: The risk of CK levels for the disease mortality of COVID-19 patients.
| Cox Regression Analysis | |||||
|---|---|---|---|---|---|
| Logistic Regression Analysis |
Group | OR | 95% CI | P value | |
| Univariate Analysis | Normal CK | ref | |||
| Elevated CK | 4.63 | 2.08 | 10.27 | 0.000* | |
| Multivariate Analysis# | Normal CK | ref | |||
| Elevated CK | 4.38 | 1.16 | 16.49 | 0.029* | |
Note: # Adjust for age, the history of cardiovascular disease, IL-6, PLT, PCT, TBIL, D-dimer
* represent the P value<0.05
Table 5: The risk of CK levels for the disease severity condition of COVID-19 patients.
Kaplan-Meier analysis of mortality in COVID-19 patients
The Kaplan-Meier curves demonstrated a significantly poorer prognosis for patients in the elevated CK group than in those in the normal CK group (P<0.001, Figure 1).
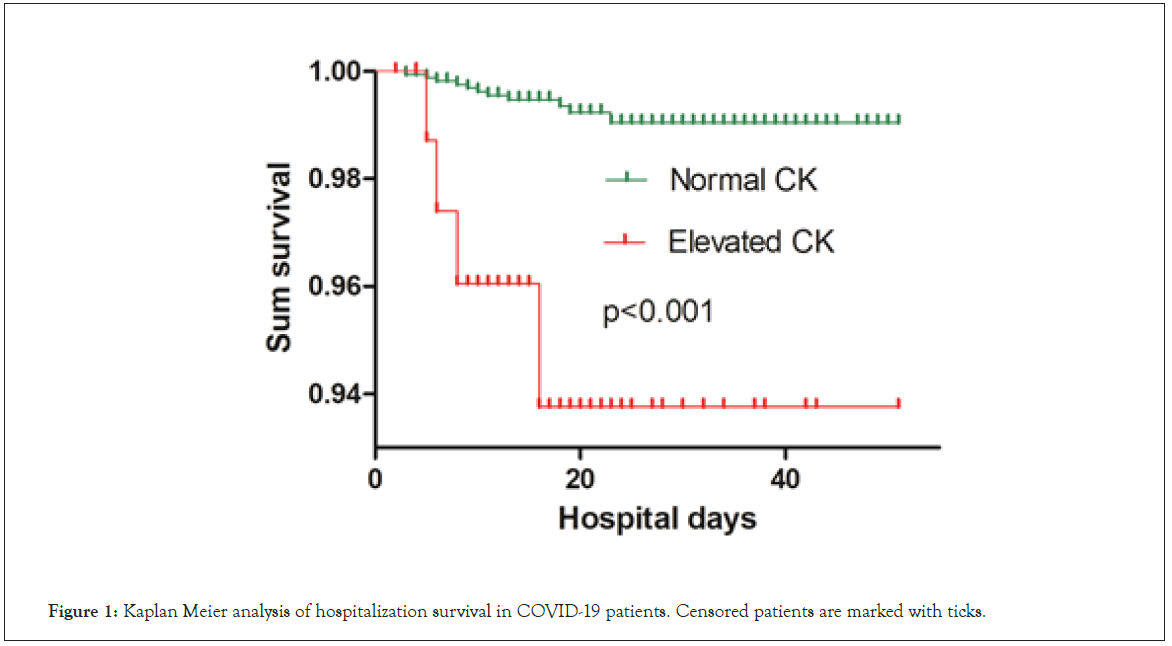
Figure 1: Kaplan Meier analysis of hospitalization survival in COVID-19 patients. Censored patients are marked with ticks.
Evaluation of chest CT images
Overall, on the fitting curves for all patients, score 1 for pulmonary inflammation had decreased by approximately 10 days after the onset of symptoms (Figure 2a), although it always fluctuated along with score 2. The peak values for score 2 and for the total score were 2.35 (day 12) and 4.35 (day 14), respectively. All score values fluctuated mildly from day 14 to day 28 after the onset of symptoms (Figures 2b and 2c). In the normal CK group, score 1 increased mildly within 18 days of admission, and then remained unchanged (Figure 2d) except for fluctuating along with score 2. The peak values for score 2 and for the total score were 2.36 (day 11) and 4.35 (day 14), respectively. These scores remained unchanged over the next 25 days, and then increased sharply after day 40 (Figures 2e and 2f). In the elevated CK group, the peak values for score 1, score 2, and the total score were 2.38 (day 40), 2.97 (day 25), and 5.15 (day 28), respectively. Subsequently, all of these scores decreased (Figures 2g, 2h and 2i).
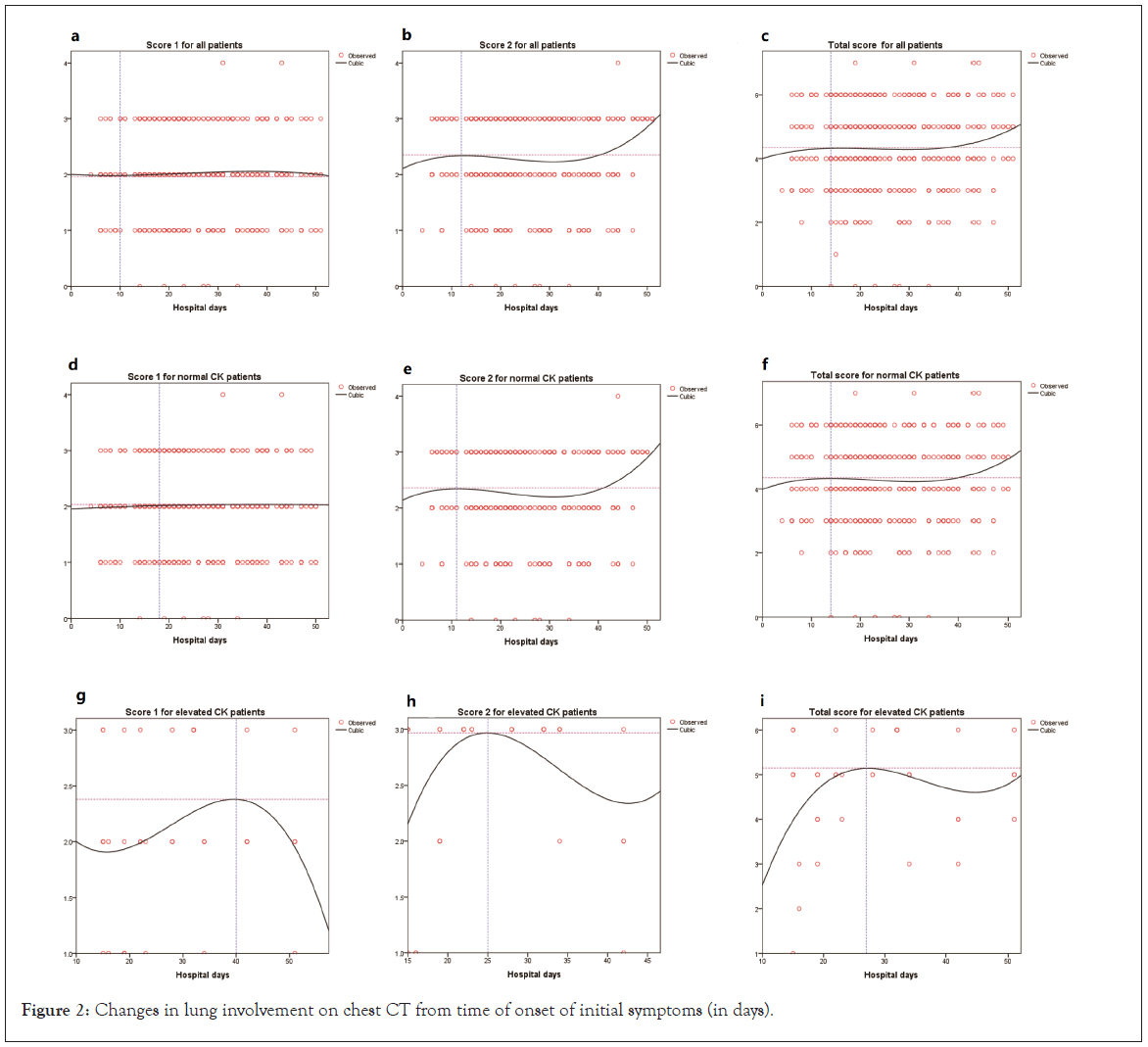
Figure 2: Changes in lung involvement on chest CT from time of onset of initial symptoms (in days).
In this retrospective study, elevated CK levels were significantly associated with a higher risk of overall mortality among COVID-19 patients. This association remained significant even after adjusting for age, the history of cardiovascular disease, IL-6, PCT, PLT, TBIL, and D-dimer as potential confounding factors. Although the HR in the multiple Cox regression models was lower than on univariate analysis, the overall risk of mortality in COVID-19 patients with elevated CK levels was more than 7 times higher than that in patients with normal CK levels. Moreover, CK level was also an important predictive factor of COVID-19 severity.
Our study also showed that more than 70% of patients presented with symptoms of fever or fatigue and respiratory symptoms. These symptoms were characteristic of viremia and might be related to the virus receptor Angiotensin-Converting Enzyme 2 (ACE2) [11,12] reported that ACE2 was identified as the receptor responsible for acute lung injury caused by SARS-CoV-2 and that the up-regulated expression of ACE2 was protective against lu ng injury. Zhou et al. [13] reported that SARS-CoV invaded human alveolar epithelial cells primarily through ACE2. In an animal model, SARS-CoV– infected mice exhibited significantly reduced ACE2 expression in their lungs [14]. Viruses of the same family have a similar pathogenesis of pneumonia. Hence, we deduced that SARS-CoV-2 infection resulted in lung injury by down regulating the expression of ACE2, causing severe respiratory symptoms, similar to SARSCoV.
Although the clinical manifestations of the overwhelming majority of COVID-19 patients were dominated by respiratory symptoms, some patients also had cardiovascular disease and these patients with cardiovascular disease had an increased risk of mortality [15]. CK level is an important representative biochemical index of myocardial injury. Several studies found evidence supporting the association between cardiovascular complications and poor prognosis in COVID-19 patients. A recent study on COVID-19 patients reported that older age, high Sepsis-Related Organ Failure Assessment (SOFA) score, cardiac complications, and a D-dimer greater than 1 µg/mL were associated with higher mortality [1]. The study also identified an association between elevated CK levels and death in COVID-19 patients. However, they did not investigate the role of CK levels in the prognosis of COVID-19 patients in detail. Yuan et al. [16] found that the decline of CK levels was significantly correlated with the elimination of viral mRNA with COVID-19, which may predict a favorable prognosis. Our study identified elevated CK levels as an independent risk factor for mortality in COVID-19 patients. Research pertaining to cardiac involvement in COVID-19 patients identified an association between cardiac risk factors and susceptibility to COVID-19 infection, disease severity, and poor prognosis Li et al. [17] and Gao et al. [18] found that COVID-19 patients with chronic cardiac diseases or cardiac involvement had higher mortality than those without cardiac disease. In our study, among the COVID-19 patients with elevated CK levels, nearly half had cardiovascular disease. Therefore, the poor prognosis of COVID-19 patients with elevated CK levels may also be related to cardiac involvement. The mechanism of myocardial injury induced by SARS-CoV-2 may be closely related to ACE2. ACE2 is widely distributed in the lungs, heart, kidneys, and testes [19]. Signaling pathways associated with ACE2 might be involved in myocardial injury in COVID-19 patients [20]. In our study, not all patients with elevated CK levels had associated cardiovascular disease, which may imply that the poor prognosis of patients with elevated CK levels without cardiovascular disease may be influenced by other non-cardiac factors. Further prospective studies should be conducted to investigate these potential factors. We also found that elevated CK levels were significantly correlated with critical severity of the disease. Despite adjusting for age, the history of cardiovascular disease, IL-6, PCT, PLT, TBIL, and D-dimer as potential confounding factors, the risk of critical disease severity in COVID-19 patients with elevated CK levels remained more than four times higher than that in patients with normal CK levels on multiple logistic regression analysis. A study on children with SARS-CoV-2 infection reported that two patients with elevated CK levels had severe disease [21]. Consistent with our findings, a retrospective study involving 161 COVID-19 patients in Changsha also showed that CK level may predict disease severity [22]. The sample size of our study is 10-fold larger than the former study.
Chest CT examination was performed to screen, confirm, and assess the severity condition and disease progression of COVID-19 [23]. Common CT manifestations of most viral pneumonias include GGO, focal lesions (patches, stripes, or nodules), and bilateral patches. GGO are the most typical manifestations of pulmonary CT in COVID-19 patients, in addition to the other findings mentioned previously. Researchers believe that lesions of varying nature and extent of lung tissue may be related to the duration of COVID-19 [24]. In our study, we analyzed the CT score trends over patients’ hospital days, and we concur with that observation. We found that among the patients with elevated CK levels, CT scores rose rapidly following the onset of symptoms. The peak value for score 1 and 2 were 2.38 on day 40 and 2.97 on day 25, respectively. The peak value for the total CT score was 5.15 on day 28. The interpretation for these findings is as follows: following admission to Leishenshan hospital, the disease severity condition of patients progressively worsened, as represented by their rising CT scores; however, the lung affected area of patients reduced faster than the pulmonary image features. This may indicate response to the relevant treatment measures.
This study has several limitations. First, this was a retrospective study, and inpatient data may not be complete. The missing values may have contributed to research bias. Second, as all subjects in our study belong to Wuhan, Hubei, China, our findings may not be generalizable globally. Third, we only considered a few potential confounders and may have ignored many more.
In conclusion, elevated CK level was an independent risk factor for in-hospital mortality among COVID-19 patients. In-patients with elevated CK levels had a higher risk of overall mortality than those with normal CK levels. COVID-19 patients with elevated CK levels were more likely to have a critical severity condition. Monitoring serum CK levels during hospitalization is important to reduce the risk of cardiovascular complications and mortality in COVID-19 patients. This may provide a reference for the later clinical treatment of such patients, and the drug selection is more targeted.
None.
Citation: Wang X, Wu X, Guo L, Liu Z, Yang Y, Zeng G, et al. (2020) Elevated Serum Creatine Kinase as an Independent Prognostic Factor for Mortality in Hospitalized Patients with COVID-19. Immunogenet Open Access. 6:135.
Received: 02-Dec-2020 Accepted: 16-Dec-2020 Published: 24-Dec-2020 , DOI: 10.35248/igoa.21.6.135
Copyright: © 2020 Wang X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.