
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495
+44 1478 350008

ISSN: 2161-0495
+44 1478 350008
Research Article - (2021)
Background: Povidone Iodine (PVP-I) nasal solutions are effective against the SARS-CoV-2 virus, but are cleared rapidly from the nasal cavity, limiting its use. PVP-I gel forming solutions can circumvent this problem due to their higher viscosity and prolonged clearing time.
Objective: Characterize the in vitro virucidal activity of long-acting PVP-I compositions developed using an in situ gel forming technology against the SARS-CoV-2 virus and test its safety using a rat model.
Methods: We tested different dilutions of the PVP-I gel forming solution– full concentration, 90%, 50%, 28% and 9% of the original formulation concentration – at varying exposure times to assess virucidal activity against SARSCoV- 2 in VERO76 cells infected. Virucidal activity was recorded as the reduction of virus in formulation-treated test wells compared to virus controls as a log reduction value. We conducted a 28-day toxicity study using Sprague Dawley CD® IGS rats to determine the potential delayed toxicity of a PVP-I formulation.
Results: The PVP-I gel-forming nasal spray rapidly inactivated SARS-CoV-2, inhibiting the viral infection of VERO76 cells. No toxicity was observed for the PVP-I formulations. Significant inactivation was noted with preincubation of the virus with this PVP-I formulation at the lowest concentrations tested. No delayed toxicity was observed in our animal model.
Conclusions: PVP-I gel forming formulations inactivate SARS-CoV-2 in vitro within 30 seconds of exposure, with increasing effects seen at higher exposure times. These formulations could prove useful in a clinical setting for managing SARS-CoV-2 infected patients.
Sinusitis; Nasal sprays; Chronic rhinosinusitis; Povidone-iodine; Povidone; COVID-19; SARS-CoV-2; Antiviral agents; Anti-infective agents; In vitro
The ongoing coronavirus pandemic caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) has taken countless lives and caused significant financial distress across the world. Public health measures can help control the spread of the virus [1]. However, there are no treatments available that reduce viral load or shorten the infectivity period in COVID-19 patients.
Nasal cells are identified as the key entry point for SARS-CoV-2 [2]. Goblet and ciliated cells in the nose have high levels of the entry proteins ACE-2 and TMPRSS2 that SARS-CoV-2 uses to get into human cells. Nasal carriage is likely to be a key feature of transmission, therefore drugs administered intranasally could be highly effective in limiting spread. Povidone-iodine (PVP-I) is a complex of polyvinylpyrrolidone and iodine effective against viruses, bacteria, fungi, and mold spores. PVP-I formulations are effective against both enveloped and non-enveloped viruses [3-5]. An in vitro study using three formulations of PVP-I (4% PVP-I skin cleanser, 7.5% PVP-I surgical scrub, and 1% PVP-I gargle/ mouthwash) showed at least a 4 Log reduction in MVA and MERSCoV viral loads under clean and dirty conditions after 15 seconds of exposure with either of the three formulations [6]. Similar studies have shown in vitro PVP-I’s virucidal activity against SARSCoV, MERS-CoV influenza virus A (H1N1), rotavirus, and murine norovirus (MNV) [7-9].
There are numerous studies demonstrating the safety of PVP-I in a variety of topical applications in ophthalmology, otology, rhinology and dermatology [10-15]. Nasal PVP-I usage is well documented [12,13,16]. However, these solutions are cleared off rapidly from the nasal cavity, limiting their usefulness and making them impractical in a clinical setting; patients would need to constantly administer these sprays to ensure adequate PVP-I concentrations throughout the day. In addition, frequent dosing can lead to irritation and potential toxicity. Therefore, a safe, non-toxic and long-acting PVP-I nasal gel would overcome these limitations and potentially help treat respiratory viruses.
In the current study, we investigated the in vitro virucidal efficacy of a PVP-I in situ gel forming formulations (IVIEW-1503) against the SARS-CoV-2 virus as well as a safety evaluation using a rat model.
A sustained release povidone-iodine in situ gel forming formulation (IVIEW-1503) [17] consisting of 0.6% of Povidone-iodine was prepared for the study. The formulation is a brownish aqueous gel packaged in amber glass bottle equipped with Aptar nasal spray pump for intranasal application. The effective concentration of PVP-I is maintained by the equilibrium between solution PVP-I and the gel bound components resulting in a long-lasting, less toxic pharmacological effect in the nasal cavity. The in situ gel forming PVP-I composition is formulated with ion-sensitive in situ gel forming materials such as Deacetylated Gellan Gum (Gelrite®) to increase the residence time of the dosage on the nasal mucosa. Preparations of PVP-I with gellan gum are sprayed into nasal cavity; gel formation takes place, induced by the electrolytes (Na+, K+, Ca2+, etc.) of the nasal fluid [17].
Virus strains and cell culture
SARS-CoV-2 (strain USA_WA1/2020, prepared by Natalie Thornburg, CDC and provided by WRCEVA, University of Texas Medical Branch) virus stocks were prepared by growing virus in VERO 76 cells (ATCC®, CRL-1587, ATCC, Manassas, Virginia). Test media used was MEM supplemented with 2% fetal bovine serum (FBS, GE Healthcare Hyclone, Marlborough, MA) and 50 μ g/mL gentamicin (Sigma-Aldrich, St. Louis, Missouri).
Virucidal assay
The 0.6% PVP-I formulation was tested for virucidal activity at the following concentrations: full strength (90% sample and 10% virus solution), 1/1.8, 1/3.2, and 1/10 diluted in simulated nasal fluid. SARS-CoV-2 virus stock was added to triplicate tubes of each prepared concentration at 1/10, so the final concentrations of solution tested were 90%, 50%, 28% and 9% of the original formulation concentration. Thus, the final PVP-I concentrations were 0.54%, 0.30%, 0.17%, and 0.05%. Nasal fluid only was added to one tube of each prepared concentration in the presence of virus to serve as the toxicity control. Simulated nasal fluid consisted of NaCl, CaCl2, KCl and water. Ethanol (45%) was tested in parallel as the positive control and water only to serve as the virus control. Solution and virus were incubated at 37˚C for three contact times of 30 seconds, 2 minutes, and 10 minutes. Following the contact period, the solutions were neutralized by a 1/10 dilution in test media containing 10% FBS and 0.5% sodium thiosulfate.
Virus quantification
Neutralized samples were serially diluted using eight half-log dilutions in the test medium. Each dilution was added to 4 wells of a 96-well plate with 80-100% confluent VERO 76 cells. The toxicity controls were added to an additional 4 wells and 2 of these wells were infected with virus to serve as neutralization controls, ensuring that the neutralized samples did not continue to inhibit growth and detection of surviving virus. All plates were incubated at 37˚C, 5% CO2.
On day 6, the post-infection plates were scored for presence or absence of viral Cyto Pathic Effect (CPE). The Reed-Muench method was used to determine end-point titers (50% cell culture infectious dose, CCID50) of the samples, and the Log Reduction Value (LRV) of the compound compared to the negative (water) control was calculated.
The reduction of virus in formulation-treated test wells compared to virus controls was calculated as the Log Reduction Value (LRV). Toxicity controls were tested with media not containing virus to see if the samples were toxic to cells. Neutralization controls were tested to ensure that virus inactivation did not continue after the specified contact time, and that residual sample in the titer assay plates did not inhibit growth and detection of surviving virus. This was done by adding toxicity samples to titer test plates then spiking each well with a low amount of virus (~60 CCID50) that would produce an observable amount of CPE during the incubation period.
Intranasal administration toxicity study
We conducted a 28-day toxicity study using Sprague Dawley CD® IGS rats to determine the potential delayed toxicity of a PVP-I formulation (0.8% PVP-I/0.064% Budesonide gel forming nasal spray formulation). The study was reviewed and approved by the PSL Institutional Animal Care and Use Committee (IACUC) and given the approval number P531.02 IVW. Sixty healthy rats were selected for the test and equally distributed into four test groups and two recovery groups (control and high dose). Intranasal administration of the formulation at dose levels of 25, 50 and 75 μl and saline control at dose levels of 75 μl were evaluated. The saline control or the test substance was administered into the right nostril via a 200 μl pipette twice daily (approximately 12 hours apart). The animals were observed at least once daily for viability, signs of gross toxicity, and behavioral changes, and weekly for a battery of detailed observations. All main study animals were subjected to a necropsy of the upper respiratory tract and related sinuses at study termination (Day 29). Thyroids and lungs were collected and weighed. The tested formulation has higher PVP-I concentration compared to the standard formulation (0.8% vs. 0.6%). Given that no detectable safety concerns were found using the high concentration formulation, we found it unnecessary to perform a similar experiment for the standard formulation.
Virucidal assay results
Virus LRV (Log Reduction Value) against SARS-CoV-2 virus is shown (Table 1). PVP-I formulation toxicity was not observed at any concentration. Ethanol (45%) had some observable toxicity at the 30 second and 2 minute time points. As a result of this toxicity, the presence of virus could not be ruled out in those wells therefore the limit of detection was 1.7 log10 CCID50 of virus per 0.1 mL.
| Sample | Drug Concentration (%) | PVP-I Concentration (%) | Contact Time (min) | Viral Titera | LRVb |
|---|---|---|---|---|---|
| IVIEW-1503 | 90 | 0.54 | 0.5 | 1.1 ± 0.1 | 3.1 |
| IVIEW-1503 | 50 | 0.3 | 0.5 | 1.1 ± 0.3 | 3.1 |
| IVIEW-1503 | 28 | 0.17 | 0.5 | 1.2 ± 0,4 | 2.9 |
| IVIEW-1503 | 9 | 0.05 | 0.5 | 1.9 ± 1.0 | 2.3 |
| Ethanol | 0.5 | <1.7 ± 0.0 | 3.5 | ||
| Virus Control | 0.5 | 4.2 ± 0.4 | 0 | ||
| IVIEW-1503 | 90 | 0.54 | 2 | <0.67 ± 0.0 | 2.9 |
| IVIEW-1503 | 50 | 0.3 | 2 | <0.67 ± 0.0 | 2.9 |
| IVIEW-1503 | 28 | 0.17 | 2 | 0.8 ± 0.2 | 2.8 |
| IVIEW-1503 | 9 | 0.05 | 2 | 1.7 ± 0.6 | 1.9 |
| Ethanol | 2 | <1.7 ± 0.0 | 1.9 | ||
| Virus Control | 2 | 3.6 ± 0.1 | 0 | ||
| IVIEW-1503 | 90 | 0.54 | 10 | <0.67 ± 0.0 | 3.3 |
| IVIEW-1503 | 50 | 0.3 | 10 | <0.67 ± 0.0 | 3.3 |
| IVIEW-1503 | 28 | 0.17 | 10 | <0.67 ± 0.0 | 3.3 |
| IVIEW-1503 | 9 | 0.05 | 10 | 2.4 ± 0.1 | 1.6 |
| Ethanol | 10 | <0.67 ± 0.0 | 3.3 | ||
| Virus Control | 10 | 4.0 ± 0.3 | 0 |
a. Log10 CCID50 of virus per mL, mean of 3 replicates ± standard deviation
b. LRV (log reduction value) is the reduction of virus compared to the virus control
Table 1: Virucidal efficacy against covid-19 virus after incubation with virus at 37˚C.
In antiviral kinetics studies, a dose response was observed after treatment (Figure 1). PVP-I formulations produced greater reduction in virus with increasing concentration and time of contact with the virus. Higher concentrations (0.54%) completely inactivated SARS-CoV-2 virus, reducing titers below the level of detection. This was similar for the half concentration (0.3%), which also reduced virus to near or below the level of detection. Lower concentrations (0.17%) also reduced the virus substantially. The lowest concentration (0.05%) of the formulations did not reduce virus significantly with increased contact time.
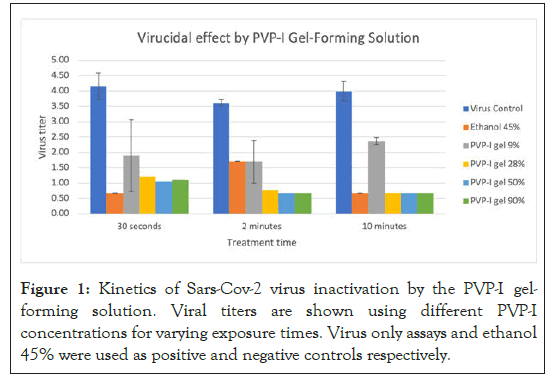
Figure 1: Kinetics of Sars-Cov-2 virus inactivation by the PVP-I gelforming solution. Viral titers are shown using different PVP-I concentrations for varying exposure times. Virus only assays and ethanol 45% were used as positive and negative controls respectively.
Neutralization controls demonstrated that residual samples did not inhibit virus growth and detection in the endpoint titer assays in wells that did not have cytotoxicity. Virus controls and positive controls performed as expected.
Intranasal administration toxicity study results
There were no mortalities during the course of the study and no test substance-related changes in body weight, body weight gain, food consumption, thyroid weights and lung weights for the duration of the study. There were no macroscopic observations at terminal sacrifice considered attributable to the administration of the formulation intranasally. Following a gross necropsy of the upper respiratory tract and related sinuses, there were no signs of irritation and no abnormalities were detected.
PVP-I is a low-cost topical medication which could significantly reduce the burden on the existing health care system if it is proven to be effective in reducing viral load. Previous studies have shown that PVP-I formulations are effective against SARS-CoV-2 in vitro [18,19]. It showed that an oral preparation of PVP-I reduced viral titers within 15 seconds [18]. A similar study showed that nasal preparation containing PVP-I in concentrations as low as 0.5% inactivated SARS-CoV-2 within 15 seconds of exposure [19]. In our study, viral titers were significantly reduced within 30 seconds of exposure for high PVP-I concentrations (0.54%), and lower concentrations significantly reduced viral titers with increasing exposure times.
The current PVP-I formulation is in the Pre-Investigational New Drug application phase for the treatment of chronic rhinosinusitis. Nonetheless, it might prove effective for the prevention and treatment of COVID-19, given its efficacy against SARS-CoV-2 in vitro. This formulation has the added benefit of a prolonged contact time in the nose and sinuses, given its mucoadhesive in situ gel formulation. Because of this property, it might be more effective in eradicating the SARS-CoV-2 virus from the nose and nasopharynx compared to standard rinses or other topical formulations containing PVP-I. Our study demonstrates that these formulations are safe for use in an animal model, and previous studies have shown that nasal epithelial cell cultures are resistant to similar concentrations of PVP-I [15]. Moreover, PVP-I has been used extensively in surgical procedures without any significant adverse reactions.
Given the encouraging results from our own data and previous in vitro investigations, it is reasonable to test PVP-I formulations in randomized controlled trials. The accessibility and low cost of PVP-I makes it ideal for quick implementation and usage during the current pandemic. Since it can be administered intranasally, it could help in the early stages of disease and could potentially limit the spread to other people. In addition, it would be important to determine whether there is a significant symptomatic improvement in the quality-of-life scores with the use of povidone-iodine nasal spray or irrigation. Further development of a long-acting PVP-I gel forming nasal spray, or a PVP-I nasal irrigation formulation, to treat patients infected with COVID-19 in the early stages to lessen the severity of the infection and possibly prevent it from progressing into a severe stage would be important.
In this study, PVP-I formulations were shown to inactivate SARSCoV- 2 virus efficiently in both dose and time-dependent manner. This suggests that PVP-I could potentially be used as a disinfectant for SARS-CoV-2 virus. More importantly, this formulation may potentially be used to inactivate SARS-CoV-2 virus in the nasal cavity thereby preventing infection of the airways.
We thank the Institute for Antiviral Research at Utah State University for performing the in vitro virucidal study, IUVO Biosciences in New York and Product Safety Labs in New Jersey for performing in vivo animal toxicology studies for intranasal administrations. We thank the University of British Columbia, St. Paul’s Sinus Centre colleagues for help with discussing COVID-19 trials.
Bo Liang and John Baldwin are co-founders of IVIEW Therapeutics Inc. and have financial interest in the company. Other authors have no financial interest in the company.
Funding from the NIH NEI SBIR Phase I Grant 1R43EY027238-01 and NIH NIAID Phase I Grant 5R43AI138660 was used to support this project. Supplemental funding was provided by IVIEW Therapeutics Inc.
Citation: Liang B, Yuan X, Wei G, Wang W, Zhang M, Peng H, et al. (2021)In vitro Inactivation of SARS-Cov-2 by Povidone-Iodine In situ Gel Forming Solution. J Clin Toxicol. S18:002.
Received: 15-Jun-2021 Accepted: 29-Jun-2021 Published: 06-Jul-2021 , DOI: 10.35248/2161-0495.21.s18.002
Copyright: © 2021 Liang B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.