Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Research Article - (2012) Volume 0, Issue 0
The present work focuses on treatment of glaucoma by formulating ocular inserts of different polymeric combination and Timolol maleate to enhance therapeutic effect through prolonging contact time with corneal surface, accurate, and sustain the release of the drug over a long period. The selected polymers for formulation of ocular inserts are Methyl Cellulose (MC), Hydroxypropyl cellulose (HPC), Eudragit RL100 (ERL100), Eudragit RS100 (ERS100), Ethylcellulose (EC), Polyvinylpyrrolidone (PVP). Films were plasticized using different plasticizers. The prepared ocular inserts were evaluated for their mechanical properties and physico-chemical properties. Accelerated stability studies were conducted to investigate the change in appearance, pH, and drug content after storage in drastic conditions. In vitro drug release and kinetics of drug release from different formulations were studied. In vitro permeation study was conducted on selected formulations showed better results in previous studies. In vivo release study was conducted on rabbits after sterilization of ocular inserts by gamma radiation. Intraocular pressure was measured at different time intervals using Schotz tonometer. The In vitro release data of Timolol maleate from the prepared formulations followed diffusion mechanism. The permeability studies data revealed that the permeability coefficient was found to be dependent on polymer type, the higher the solubility of the polymer the higher permeability coefficient. The reduction in IOP for F3 (HPC/ERL100 5:1), F7 (MC/ERL100 1:1), and F8 (MC/ERL100 1:3) was prolonged for 120 hours (5 days), and 96 hours (4 days) for F12 (HPC/EC 15:1).
Ocular drug delivery is one of the most fascinating and challenging tasks facing the Pharmaceutical researchers. The poor bioavailability and therapeutic response exhibited by conventional ophthalmic solutions due to rapid precorneal elimination of the drug, this include the blinking reflex, tear turnover and low corneal permeability [1]. The cul-de-sac of the eye (the corners) normally holds around 7-9 μl of tears [2], moreover, tear dynamics and nasolacrimal duct drainage is the major way of entry into the circulatory system of potent ocular drugs applied by topical administration. This may cause undesired and toxic systemic side effects [3-5]. The cornea is considered to be the main pathway for the permeation of drugs into the eye. The tight junctions and hydrophobic domains in this layer make it the most important barrier to drug delivery [6-8]. For a drug to cross the cornea effectively, it has to have both hydrophilic and lipophilic properties, and be sufficiently small to pass through tight junctions [9,10]. Conventional eye drops require frequent instillation, as less than 1% of the administered dose is ocularly absorbed [11], multiple applications increase ocular and systemic side effects [12-15].
The ocular inserts, ocular films, wafers, and rods are solid devices which are placed in the cornea, cul-de-sac. These are having advantages over liquid formulation of longer retention time, accurate dosing, increased stability, and shelf life [16].
Glaucoma is optic nerve damage (often, but not always, associated with increased eye pressure) that leads to progressive, irreversible loss of vision [17,18]. Elevated Intraocular pressure is a risk factor for glaucoma. Timolol maleate is widely used worldwide as a standard medication to lower intraocular pressure, and its efficacy and safety have been proven [19,20], it’s a beta adrenergic blocker which is nonselective between beta-1 and beta-2 adrenergic receptors [21]. It is capable of treating glaucoma by preventing the production of aqueous humor, thus, lowering the pressure inside the eye [20]. Side effects such as a reduction in heart rate during exercise, nocturnal hypotension, bradyarrhythmias and bronchospasm in patients with reactive airway or chronic obstructive pulmonary disease associated with the use of ophthalmic administration of timolol have been reported. This work aimed to prepare ocular inserts using different polymers containing timolol maleate, to reduce side effects observed during application of Timolol maleate eye drops.
Timolol maleate was kindly provided by EPICO Pharmaceuticals, Cairo, Egypt, Poly-ethylene glycol 600 was purchased from El-Nasr Chemical Co., Egypt. Hydroxypropylcellulose MF was purchased from Kolmar Company, California, and USA. Eudragit RL100 and RS100 were purchased from Rhom and Haas GmbH pharma Darmstadt, Germany. Polyvinyl pyrrolidone K90 was purchased from BASF Chemical Company, Germany. All other chemicals used were of reagent grade; albino rabbits, weighing from 1800 grams to 2000 grams.
Preparation of ocular inserts
Ocular inserts of Timolol maleate (300 μg/insert) were prepared by solvent casting method [22]. Required amount of polymer was dissolved in the corresponding solvent and plasticizer according to the composition in Table 1. The solution was poured over a poly-tetrafluoroethylene (PTFE) mold covered with an inverted funnel to allow slow and uniform evaporation at room temperature. The films obtained were punched with a sharp edged die (Surface area 0.785 cm2).
| Formula code | Polymer | Plasticizer | Solvent |
|---|---|---|---|
| F1 | Hydroxypropyl cellulose (HPC) (10%w/w) | Triethyl citrate(15% w/w) | Distilled water |
| F2 | Hydroxypropyl cellulose (HPC) (10%w/w) Eudragit RL100 (2%w/w) (15:1) | Triethyl citrate(15% w/w) | Distilled water Isopropyl alcohol |
| F3 | Hydroxypropyl cellulose (HPC) (10%w/w) Eudragit RL100 (2%w/w) (5:1) | Triethyl citrate(15% w/w) | Distilled water Isopropyl alcohol |
| F4 | Hydroxypropyl cellulose (HPC) (10%w/w) Eudragit RL100 (2%w/w) (2:1) | Triethyl citrate(15% w/w) | Distilled water Isopropyl alcohol |
| F5 | Methyl cellulose(5%w/w) | Triethyl citrate(20%w/w) | Distilled water |
| F6 | Methyl cellulose(5%w/w) Eudragit RL100 (5%w/w) (3:1) | Triethyl citrate(20%w/w) | Distilled water Isopropyl alcohol |
| F7 | Methyl cellulose(5%w/w) Eudragit RL100 (5%w/w) (1:1) | Triethyl citrate(20%w/w) | Distilled water Isopropyl alcohol |
| F8 | Methyl cellulose(5%w/w) Eudragit RL100 (5%w/w) (1:3) | Triethyl citrate(20%w/w) | Distilled water Isopropyl alcohol |
| F9 | Eudragit RL100 (2%w/w) | Triethyl citrate(10% w/w) | Isopropyl alcohol |
| F10 | Eudragit RL100 (5%w/w) | Triethyl citrate(10% w/w) | Isopropyl alcohol |
| F11 | Eudragit RS100 (5%w/w) | Polyethylene glycol 600 (10%w/w) | Acetone/Isopropylalcohol (4:1) |
| F12 | Hydroxypropyl cellulose (HPC) (10%w/w) Ethylcellulose(2%w/w) (15:1) | Triethyl citrate(30%w/w) | Methanol/Methylene chloride(1:1) Methanol/Methylene chloride(1:1) |
| F13 | Hydroxypropyl cellulose (HPC) (10%w/w) Ethylcellulose(2%w/w) (5:1) | Triethyl citrate(30%w/w) | Methanol/Methylene chloride(1:1) Methanol/Methylene chloride(1:1) |
| F14 | Ethylcellulose(2%w/w) | Glycerin (5% w/w) | Isopropyl alcohol |
| F15 | Polyvinyl pyrrolidone K90 (10%) | Polyethylene glycol 600 (25%w/w) | Ethyl alcohol |
| F16 | Polyvinyl pyrrolidone K90 (10%) Eudragit RS100 (5%w/w) (6:1) | Polyethylene glycol 600 (25%w/w) | Ethyl alcohol Acetone/Isopropyl alcohol (4:1) |
| F17 | Polyvinyl pyrrolidone K90 (10% w/w) Eudragit RS100 (5%w/w) (2:1) | Polyethylene glycol 600 (25%w/w) | Ethyl alcohol Acetone/Isopropyl alcohol (4:1) |
| F18 | Polyvinyl pyrrolidone K90 (10% w/w) Eudragit RL100 (5%w/w) (6:1) | Polyethylene glycol 600 (15%w/w) | Ethyl alcohol Isopropyl alcohol |
| F19 | Polyvinyl pyrrolidone K90 (10% w/w) Eudragit RL100 (5%w/w) (2:1) | Polyethylene glycol 600 (15%w/w) | Ethyl alcohol Isopropyl alcohol |
Table 1: Composition of ocular inserts containing Timolol maleate (300 μg).
Evaluation of ocular inserts
Determination of mechanical properties of prepared ocular inserts. Mechanical properties which are tensile strength, percent elongation at break and strain were determined according to the following calculation method using Zwick Roell Z100 device:
Tensile strength: Tensile strength of the prepared films was calculated according to the following equation [23-25].
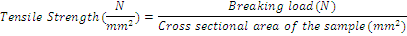 (1)
(1)
Percentage of elongation at break: Percentage elongation of the prepared films was calculated according to the following equation [23-25].
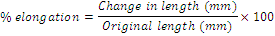 (2)
(2)
Strain: Strain of the films was calculated according to the following equation:
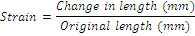 (3)
(3)
Determination of the physico-chemical properties of the films: Timolol maleate ocular inserts were evaluated by different means such as film thickness, moisture absorbtion, and drug content.
Determination of film thickness: Thickness was measured using a digital micrometer. Such determinations were carried out at five different places and the mean value was calculated for each formulation [26-29].
Percentage moisture absorption: Percentage moisture absorption test was carried out to check physical stability and integrity of the films withstanding high humidity. The films were placed in a dessicator containing silica gel for 24 hours to make sure that no moisture was absorbed by the film under normal conditions. The films were weighed individually. Then the films were placed in a dessicator which maintained at high Relative Humidity (RH) at about 75 ± 5% RH using saturated sodium chloride solution. During three days the films were taken each day out and reweighed [27]. The percentage moisture was calculated according to the following equation:
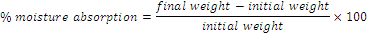 (4)
(4)
Drug content uniformity: Content uniformity of the drug in the circular films was determined using three inserts punched out from each film. Each insert was then dissolved in 10 ml of the solvent of choice. The absorbance of the solution was measured by Double Beam spectrophotometer at 294 nm against blank solution which was prepared by dissolving a placebo insert in the same solvent and the same volume used with medicated insert to prevent polymer or plasticizer interference [27].
Determination of pH of insert solution: pH of ocuserts was determined in order to investigate the possibility of any side effects in an eye [29]. An insert from each film was dissolved in 10 ml distilled water and then the pH was measured using pH meter.
Drug-polymer interaction: Infrared (IR) spectroscopy (using IR spectrophotometer FTIR-8300, Shimadzu [ Kyoto, Japan], by the KBr pellet method) was performed on Timolol maleate, each pure polymer and polymer containing Timolol maleate to investigate the interaction between Timolol maleate and various polymers used [30].
Stability studies: Stability studies were carried out on all formulations by storing triplicates of ocular inserts (packaged in aluminium foil) in a humidity chamber with a relative humidity of 75 ± 5% and a temperature of 40° ± 0.5°C. The sample was withdrawn after 1, 2, and 3 months and analyzed for physicochemical parameters (appearance, pH, and drug content ) [27].
In-vitro release studies: The inserts were placed on dialysis cell using cellophane membrane in contact with isotonic phosphate buffer pH 7.4 kept at 37 ± 1°C with constant stirring of 50rpm. 1 ml Sample was withdrawn at different time intervals analyzed for drug content spectrophotometrically at 294 nm. Blank experiments containing the same constituents as in the experiment, except the drug, were carried out to ensure the absence of polymer or plasticizer interference.
Permeability study: To measure corneal permeability, glass diffusion cells were constructed from 50 ml erlenmeyer flasks. The end of the side arm projection on each half-cell had a ground-glass finish with a circular opening in the middle. The cross-sectional surface area of this opening was 0.38 cm2. The study was conducted in male albino rabbits. Rabbits were sacrificed, the entire eye enucleated, and the corneas were removed, gently rinsed with saline. The cornea was positioned on the donor half-cell such that the epithelial surface facing the donor solution. The receptor half-cell was positioned symmetrically opposite the donor half-cell. The half-cells were secured together with a clamp. This procedure prevented any leaks. After the cornea was securely mounted, 25 ml isotonic buffered saline solution (pH=7.4) was first added to the receptor cell, likewise, 25 ml isotonic saline buffered solution (pH =7.4), an insert was added to the donor half-cell. Both cells were capped with aluminium foil to prevent evaporation. The entire apparatus was thermostated at 37 ± 5°C. The donor and receptor solutions were stirred (100 rpm) with magnetic stir bars. Samples (5 ml) were withdrawn at different time intervals from receptor side for analysis of drug content [31,32].
The mean cumulative amount of drug permeated per unit surface area of the cornea was plotted versus time. The slope of the linear portion of the plot was calculated [33,34]. The rate (slope of the linear portion of the plot) divided by the area available for diffusion (A) generates steady state flux [35] as shown in the following equation.
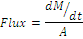 (5)
(5)
dM/dt is the rate (slope of the linear portion of the plot), A is the area available for diffusion.
Corneal permeabilities were calculated by dividing the steady state flux by the donor concentration (Cd) of Timolol maleate [35,36].
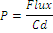 (6)
(6)
Sterilization of ocular insets: The selected ocular inserts were sterilized by gamma radiation before in vivo study using the Cobalt-60 source (Indian Gamma Cell 4000A) located at the national centre for radiation Research and Technology (NCCRT), Nasr City, Cairo, Egypt. Ocular inserts were packaged in amber glass vials. The package was exposed to a total dose of 2.5 mega rads [28,37]. After sterilization, the ocular inserts were also evaluated for sterility using the fluid thioglycolate medium and its alternative method (soybean-casein digest medium) were applied according to the USP guidelines for sterility testing to determine if the prepared samples comply with the requirements set forth in the individual monograph with respect to the test for sterility. For microbial contamination test fluid thioglycolate medium was used, while soybean- casein digest medium was used to test for fungi with incubation under aerobic conditions [38,39].
In vivo drug release studies: The protocol for in vivo studies in rabbit was designed and approved by German University in Cairo animal ethics committee. The rabbits were fed balanced diet pellets and maintained in a temperature-controlled room, at 20°C to 24°C before the experiment [40]. The intraocular pressure was measured in both eyes immediately prior to applying the drug (zero-time), and at predetermined intervals after inserting an insert containing 300 μg Timolol maleate into the conjunctival sac of albino rabbits. For each formulation three animals was used (5 eyes as experiment and one eye as control). The reduction in the intraocular pressure was measured as a mean (± S.D.) using Schiötz Tonometer.
Mechanical properties of ocular inserts
Results of tensile strength presented in Table 2 showed that, addition of ERL100 to HPC and MC (F1-F8) decreased tensile strength compared with that of HPC and MC alone. The decrease in the tensile strength may be due to addition of hydrophobic polymer (ERL100) to hydrophilic polymers (HPC & MC). This explanation was confirmed by the data obtained from F9 (ERL100 2%) and F10 (ERL100 5%). Also the results showed that the addition of ethylcellulose to hydroxypropylcellulose led to decrease in tensile strength of the prepared film compared to that of hydroxypropylcellulose alone. This also potentiates our previous explanation that the addition of hydrophobic polymer (EC) to hydrophilic polymer (HPC) led to decrease in tensile strength.
| Formula Code | Tensile Strength (N/mm2) | Elongation at Break (%) | Strain (mm) |
|---|---|---|---|
| F1 | 4.19(0.1) | 40(0.2) | 0.4(0.07) |
| F2 | 1.41(0.09) | 30(0.1) | 0.3(0.01) |
| F3 | 2.52(0.07) | 32(0.09) | 0.32(0.08) |
| F4 | 2.99(0.2) | 35(0.2) | 0.35(0.05) |
| F5 | 22.93(0.05) | 3.5(0.1) | 0.035(0.02) |
| F6 | 8.52(0.5) | 1.2(0.09) | 0.012(0.1) |
| F7 | 6.68(0.02) | 1.5(0.1) | 0.015(0.02) |
| F8 | 8.58(0.05) | 20(0.09) | 0.2(0.2) |
| F9 | 3.8(0.05) | 195(0.01) | 1.95(0.2) |
| F10 | 2.41(0.06) | 63(0.05) | 0.63(0.02) |
| F11 | 2.13(0.09) | 76(0.02) | 0.76(0.1) |
| F12 | 0.99(0.01) | 18(0.08) | 0.18(0.01) |
| F13 | 1.72(0.01) | 21(0.2) | 0.21(0.09) |
| F14 | 234.68(0.3) | 1.5(0.6) | 0.015(0.1) |
| F15 | 2.84(0.01) | 130(0.02) | 1.3(0.05) |
| F16 | 3.28(0.05) | 120(0.1) | 1.2(0.07) |
| F17 | 2.26(0.01) | 105(0.1) | 1.05(0.05) |
| F18 | 5.59(0.01) | 19(0.2) | 0.19(0.1) |
| F19 | 7.62(0.3) | 14.5(0.5) | 0.145(0.3) |
Table 2: Mechanical properties of the prepared films.
| Formula Code | Parameter | Time (month) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| F1 | Appearance pH Drug content (%) | Clear 5.4(0.11) 98.9(0.)22 | NC 5.07(0.25) 95.9(0.14) | NC 5.01(0.22) 95.88(0.39) | NC 5.00(0.11) 95.12(0.99) | ||
| F2 | Appearance pH Drug content (%) | Clear 5.1(0.55) 99.5(0.6) | NC 5.45(0.55) 97.23(0.22) | NC 6.38(0.2) 97.78(0.95) | NC 6.38(0.19) 96.55(0.99) | ||
| F3 | Appearance pH Drug content (%) | Clear 5.8(0.33) 97.99(0.39) | NC 4.94(75) 96.28(0.69) | NC 4.29(0.22) 97.22(0.99) | NC 4.3(0.44) 96.88(1) | ||
| F4 | Appearance pH Drug content (%) | Clear 4.86(0.26) 100.62(0.66) | NC 4.55(0.6) 98.99(0.25) | NC 4.55(0.25) 96.55(0.89) | NC 4.48(0.15) 96.2(1.5) | ||
| F5 | Appearance pH Drug content (%) | Clear 5.46(0.23) 110.66(0.21) | NC 5.67(0.1) 101.45(0.44) | NC 6.2(0.6) 100.669(1) | NC 6.15(0.23) 99.99(0.95) | ||
| F6 | Appearance pH Drug content (%) | Clear 5.28(0.65) 109.23(0.22) | NC 5.37(0.22) 107.13(0.64) | NC 6.41(0.5) 100.93(0.99) | NC 6.41(0.22) 100.55(1) | ||
| F7 | Appearance pH Drug content (%) | Clear 5.99(0.49) 99.98(0.22) | NC 5.66(0.11) 96.626(0.21) | NC 5.78(0.25) 96.626(1) | NC 5.55(0.5) 96.18(0.92) | ||
| F8 | Appearance pH Drug content (%) | Clear 5.51(0.99) 97.23(0.69) | NC 4.77(0.66) 96.4(0.22) | NC 4.22(0.45) 95.22(0.99) | NC 4.3(0.21) 95.21(1.5) | ||
| F9 | Appearance pH Drug content (%) | Clear 3.38(0.22) 99.96(0.36) | Brittle 5.00(0.55) 98.19(0.22) | Brittle 5.00(0.55) 98.5(0.25) | Brittle 4.88(0.23) 97.66(0.95) | ||
| F10 | Appearance pH Drug content (%) | Clear 3.58(0.22) 111.9(0.26) | Brittle 6.34(0.65) 103.5(0.66) | Brittle 6.34(0.1) 100.92(1) | Brittle 6.00(0.1) 99.98(1) | ||
| F11 | Appearance pH Drug content (%) | Clear 5.65(0.36) 99.7(0.59) | Brittle 6.53(0.25) 97.972(0.22) | Brittle 6.58(0.9) 97.19(0.99) | Brittle 6.2(0.44) 96.99(0.89) | ||
| F12 | Appearance pH Drug content (%) | Clear 4.6(0.12) 99.99(0.56) | Sticky 5.06(0.5) 98.04(1) | Sticky 6.43(0.12) 96.28(0.99) | Sticky 6.3(0.5) 96.12(0.99) | ||
| F13 | Appearance pH Drug content (%) | Clear 4.02(0.33) 128.21(0.98) | Sticky 4.12(0.33) 110.05(1.5) | Sticky 4.11(0.39) 99.55(1) | Sticky 4.11(0.66) 99.10(0.96) | ||
| F14 | Appearance pH Drug content (%) | Clear 4.98(0.22) 109.98(0.96) | Precipitation 5.33(0.12) 102.45(0.96) | Precipitation 5.28(0.42) 99.25(1.5) | Precipitation 5.25(0.12) 98.66(1) | ||
| F15 | Appearance pH Drug content (%) | Clear 5.89(0.21) 119.99(0.99) | melt - - | - - - | - - - | ||
| F16 | Appearance pH Drug content (%) | Clear 5.82(0.45) 100.99(0.99) | melt - - | - - - | - - - | ||
| F17 | Appearance pH Drug content (%) | Clear 5.81(0.23) 99.98(0.89) | melt - - | - - - | - - - | ||
| F18 | Appearance pH Drug content (%) | Clear 5.3(0.44) 120.96(0.5) | melt - - | - - - | - - - | ||
| F19 | Appearance pH Drug content (%) | Clear 5.82(0.43) 98.36(0.56) | melt - - | - - - | - - - | ||
Table 3: Stability of Timolol maleate ocular inserts (NC: Referred to no change in appearance).
Incorporation of Eudragit ERS100 into polyvinylpyrrolidone K90 had approximately no effect on tensile strength of the formed films. This finding may be attributed to high concentration of PEG-600 (25% based on polymer weight).
Elongation at break percentage
Elongation at break percentage of the formulations (F1-F4) prepared from HPC alone, and its combination with ERL100 at different ratios showed a moderate elongation at break percentage, while incorporation of ERL100 led to slight decrease in elongation at break percentage as shown in Table 2.
Strain
The results obtained from elongation at break percentage and strain indicated that HPC and HPC/ERL100 films are more flexible than MC and MC/ERL100 films. And films plasticized with PEG-600 were more flexible than the films plasticized with TEC.
Determination of the physico-chemical properties of ocular inserts
Determination of insert thickness: The mean thickness and standard deviation were calculated. The low standard deviation of all the nineteen formulations indicates uniform thickness of the prepared films. Thickness was found to be in the range 0.011 ± 0.001 mm (F14) to 0.351 ± 0.001 mm (F1). It was found that insert thickness increased by increase in the total polymer concentration.
Percentage moisture uptake: The percentage moisture uptake was calculated for all nineteen formulations and the mean of five replicates. According to the results obtained, the moisture absorption was more in the films composed of PVP polymer and plasticized with PEG-600 as plasticizer. Formulation F15 showed maximum moisture absorption (22.321 ± 0.002%), this may be due to that both PVP and PEG-600 are hydrophilic in nature and expected to absorb water. Also, it was shown that formulations prepared from HPC had higher moisture uptake than formulations prepared from MC although those prepared from MC had lower thickness than those prepared from HPC. Formulations which were prepared from hydrophobic polymers F9, F10 (ERL100 2%, and 5% respectively) had 0% moisture absorption.
Timolol maleate content in ocular inserts: The USP and IP specifications for assay are that the drug content should not be less than 90 % and not more than 110 % [41]. The results showed that Timolol maleate in all formulations were in the range from 96% to 110% of labelled claim.
Determination of pH of insert solution: The results showed that nearly all formulations were slightly acidic. This could be attributed to the presence of Timolol maleate. Generally, ophthalmic formulations must be in the pH range between 4.5 and 11.5 [29]. According to this range formulations, F9 (pH =3.38), F10 (pH =3.58) and F13 (pH = 4.02) are out of range.
Drug polymer interaction: The optimized formulations, placebo formulation and the pure drug were subjected to IR analysis. No major difference was observed in the IR spectra of pure drug and the medicated formulations, indicating that there was no chemical interaction between the drug and excipients in the ocular inserts.
Stability studies of prepared formulations: Formulations of HPC (F1), HPC/ERL100 (F2-F4), MC (F5) and MC/ERL100 (F6-F8) were stable physically (showed no significant difference in properties than fresh ones) through the whole three months. While formulations F9, F10 and F11 showed brittleness upon storage. Also, the stability results revealed that formulations F15-F19 showed complete melting after one month storage at these conditions indicating physical instability, therefore, formulations with PVP should be stored in moisture free environment.
In-vitro drug release study: The effect of Eudragit RL100 concentration on the release of Timolol maleate from hydroxypropyl cellulose presented in Figure 1. Formulation F1 showed a maximum cumulative percentage drug release of 94.8% at the end of 72 hours, followed by formulation F2 (85.6% ± 0.25%) and F3 (84.4% ± 0.5%) at the end of 96 hours. Formulation F4 released the whole drug content at the end of 72 hours (103.6% ± 0.39%). As the proportion of Eudragit RL100 in the combination with hydroxypropyl cellulose increased the cumulative percentage drug released decreased. This may be attributed to the increase in hydrophobicity of inserts and decrease in their solubility leading to sustained release of Timolol maleate from the inserts. A higher cumulative drug released in case of F4, this could be attributed to the difference in the thickness of inserts (0.35 to 0.11mm). As when the thickness of film decreased the surface area for drug release increased. Also the data showed that incorporation of hydroxypropylcellulose into Eudragit RL100 increased the release of Timolol maleate from the inserts in comparison with the inserts prepared from Eudragit ERL100 alone (F9).
The effect of ethylcellulose concentration on the release of Timolol maleate from hydroxypropyl cellulose films presented in Figure 2. Formulation F12 showed a maximum cumulative percentage drug release of 91.7% (± 0.44) at the end of 96 hours, followed by F13 (98.1% ± 0.55) at the end of 48 hours and F14 (98.9% ± 0.5) at the end of 72 hours. F13 showed faster release (at the end of 48 hours) compared with the other formulations (F12 & F14). Formulation F14, showed the highest cumulative percentage drug release compared with the formulations F12 and F13. This may be attributed to the solution diffusion mechanism that has been demonstrated for many polymer films prepared from organic solvent. Therefore, it is a likely mechanism for ethylcellulose films prepared from organic solvents. The drug molecules diffuse through molecular sized openings between the crosslinked polymer chains. This process is known as hindered molecular diffusion. When the ratio of ethyl cellulose increased, the openings in the film increased allowing more drug release [42].
The results in Figure 3 showed that incorporation of hydrophobic polymer (Eudragit RL100) into hydrophilic polymer (MC) retard the release of Timolol maleate. Formulation F5 (MC 5%) gave 100% (± 0.71) drug release at the end of 48 hours. Formulation F6 (MC/ ERL100 3:1) and F7 (MC/ERL100 1:1) gave 97.5% (± 0.36) at the same period of time. Increasing the ratio of Eudragit RL100 in the insert F8 (MC/ ERL100 1:3) leading to prolongation of Timolol maleate release up to 96 hours.
Regarding F10 (ERL100 5%) only 30% of the dose was released at the end of five hours which indicated that formulating an ocular insert needs a polymer of certain water solubility to allow water entrance into the matrix allowing swelling of the matrix opening channels to drug release.
The results in figures 4 and 5 showed that the total amount of Timolol maleate released from F16 (PVP/ERS100 6:1) was 97.89% (± 0.35%), while 83.4% (± 0.09%) drug released from F17 (PVP/ERS100 2:1). F18 (PVP/ERL100 6:1) showed 95.23% (± 0.15%) drug release and 98.19% (± 0.5%) drug release from F19 (PVP/ERL100 2:1). Increasing the ratio of Eudragit RL100 or RS100 led to prolongation of Timolol maleate release time. Also the obtained results showed that ERL100 had a more sustained effect on the release of Timolol maleate than ERS100 when incorporated in PVP films. Also the obtained results showed that release of Timolol maleate increased with the increase of the amount of ERL100 and decreased with increased ERS100 due to higher water permeability of ERL100 which contains 10% of functional quaternary ammonium groups and lower water permeability of ERS100 having only 5% of functional quaternary ammonium groups.
Mechanism and kinetics of drug release from ocular insert
The kinetic parameters of drug release for the different formulations are presented in table 4 according to Korsmeyer-Peppas model. Korsmeyer et al. used a simple empirical equation to describe the general solute release behavior from controlled release polymer matrices (Korsmeyer, Gurny et al. 1983; Ritger and Peppas 1987):
| Formula code | Release Exponent (n) | Determination Coefficient(r2) |
|---|---|---|
| F1 | 0.484 | 0.966 |
| F2 | 0.493 | 0.984 |
| F3 | 0.375 | 0.994 |
| F4 | 0.50 | 0.996 |
| F5 | 0.20 | 0.970 |
| F6 | 0.572 | 0.996 |
| F7 | 0.553 | 0.995 |
| F8 | 0.411 | 0.995 |
| F9 | 0.370 | 0.970 |
| F10 | 0.295 | 0.980 |
| F11 | 0.588 | 0.960 |
| F12 | 0.512 | 0.995 |
| F13 | 0.40 | 0.995 |
| F14 | 0.406 | 0.960 |
| F15 | 0.314 | 0.970 |
| F16 | 0.519 | 0.970 |
| F17 | 0.80 | 0.996 |
| F18 | 0.810 | 0.980 |
| F19 | 0.830 | 0.996 |
Table 4: In vitro slopes and regression values of Korsemyer models.
 (7)
(7)
Where mt/m∞ is the fraction of drug released, k is the kinetic constant, t is the release time and n is the diffusional exponent for drug release. Peppas stated that the above equation could adequately describe the release of solutes from slabs, spheres, cylinders and discs, regardless of the release mechanism.
The value of n gives an indication of the release mechanism: where n = 1, the release rate is independent of time (zero order) (case II transport), n= 0.5 stands for Fickian diffusion, when n < 0.5 indicating that the release rates exhibit a combined mechanism of diffusion partially through a swollen matrix and partially through water-filled pores. And when 0.5 < n < 1.0, diffusion and non-Fickian transport are implicated. When, n > 1.0, supper case II transport is apparent [43,44].
Results of kinetics indicated that the n < 0.5 for F1, F2, F3, F5, F8, F9, F10, F13, F14, F15 which means that the release rates exhibit a combined mechanism of diffusion partially through a swollen matrix and partially through water-filled pores [45,46]. The n value of F4 is perfectly equals 0.5 which stands for Fickian diffusion. Formulations F6, F7, F11, F12, F16, F17, F18, F19 showed a value 0.5 < n < 1 which indicated diffusion and non-Fickian transport mechanism.
In-vitro permeation studies
Formulations showed better physico-chemical parameters with prolonged drug release were selected for permeation studies. Out of nineteen formulations five formulations were selected. The selected formulations were: F3, F4, F7, F8 and F12. The permeation data presented in table 5 showed that all the tested formulations in terms of their steady-state flux are approximately the same except formulation F4 (HPC /ERL100 2:1). This may be attributed to the retardation effect of high concentration of Eudragit (HPC/ERL100 2:1) to the release profiles of the drug from the matrix. The low permeability coefficient of the formulation F4 can substantiate the previous explanation. The permeability coefficient data was shown to be dependent on the polymer type from which the ocular insert was formulated. The higher the solubility of the polymer, the higher permeability coefficient, increasing the ratio of Eudragit RL100 in the formulated film led to reduction in permeability coefficient of Timolol maleate from the insert (F3-F4). Hence, for sustaining the Timolol maleate release from the ocular inserts increased ratio of Eudragit ERL100 was required. These results were compared to the permeability coefficient of Timolol maleate eye drops (0.5%). The permeability coefficient and the flux observed for the eye drops were 68.19*10(-3) cm.hr(-1) and 20.458 μg.cm(-2).hr(-1) respectively. The high permeability coefficient and flux showed by eye drops indicated that the formulated ocular inserts delayed drug permeation through corneal membrane allowing less frequent dosing, and consequently decrease the dose administered, decrease ocular toxicity and less systemic absorption.
| Formulation Code | Permeability Coefficient (cm.hr-1) (*10-3) | Steady-State Flux (µg.cm-2.hr-1) |
|---|---|---|
| F3: HPC 10%, ERL100 2% at the ratio (5:1) | 7.8279 | 2.778 |
| F4: HPC 10%, ERL100 2% at the ratio (2:1) | 5.444 | 1.64 |
| F7: MC 5%, ERL100 5% at the ratio (1:1) | 10.4 | 2.865 |
| F8: MC5%, ERL100 5% at the ratio(1:3) | 9.30 | 3.041 |
| F12: HPC 10%, EC 2% at the ratio(15:1) | 10.06 | 3.0007 |
| Marketed eye drops | 68.19 | 20.458 |
Table 5: Permeability coefficient and steady-state fluxes of Timolol maleate through rabbits’ corneas from different ocular inserts.
Sterility testing results
After incubation of all the prepared broths, (negative control, positive control and test for each formula in each type of media) growth of bacteria and fungi was checked. The overall results of the sterility test showed that ophthalmic formulation prepared passes the sterility test as there was no evidence of the growth found in the negative control or the test tubes. These results proved the efficiency of gamma radiation as a method of sterilization at the given dose 2.5 mega rad.
In vivo study results
The potential of formulated Timolol maleate in ocular insert as delivery system in controlling the IOP was evaluated by using tonometric technique [47].
The effect of Timolol maleate formulated in the selected formulations on IOP of rabbit’s eye in comparison to the marketed product (eye drops 0.5%) as in figure 6. It that all the formulations exhibit a reduction in IOP over a period of 6 hours (eye drops), 96 hours (F12) and 120 hours (F3, F7, F8).
The maximum response for all the selected formulations was approximately the same (4.9-5.9 mmHg). These results indicate that the type of the polymer used, has no effect on maximum response of Timolol maleate on rabbit’s eye. Also, the time of maximum response of all the formulations was the same 6 hours, but it was 4 hours for the eye drops.
The reduction in IOP for the formulations F3, F7 and F8 extended for 120 hours duration, while it was extended for 96 hours in case of formulation F12. The time of maximum response of the prepared ocular inserts was 1.5 times more than eye drops. The duration of response from formulation F3, F7 and F8 was higher 20 times more than that obtained from eye drops.
An interesting observation of this study that upon administration of Timolol maleate ocular inserts, no effect on IOP observed in the control eye, this may be an indication that no systemic absorption occurred.
Area above the curve of the formulations F3 and F8 are the same, while AAC of F8 was 1.5 times more than that of F7 due to high ratio of ERL100 in the blend of the polymers used (MC/ERL100) in the preparation of ocular insert. This control in IOP for prolonged periods may be attributed to the increased corneal residence and sustained drug release of the formulated ophthalmic inserts compared with marketed eye drops.
The ideal anti-glaucoma agent should reduce IOP, by 25-30% [48]. The ocular inserts formulated with HPC and MC in combination with ERL100 (F3 and F7) were found to reduce the IOP by 27.5% and 29.4% respectively.
Our efforts to formulate and develop Timolol maleate ophthalmic inserts intended to sustain the release of the drug in order to prevent or minimize its systemic absorption which may lead to the feeling of patient with bronchial spasm especially patients suffer from bronchial asthma. Moreover, these inserts were very effective in controlling IOP for approximately 4 days as deduced from the in vivo animal studies.
I’d like to express my gratitude and thanks, to the National Centre for Radiation Research and Technology (NCCRT) for giving me the chance to sterilize formulations.