Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2016) Volume 5, Issue 4
Aquilaria crassna (agarwood) has been used in traditional Asian medicine to treat vomiting, rheumatism, asthma, cough and inflammation. Despite the wide range of ethno-pharmacological use of agarwood, its anti-angiogenic effect has not previously been evaluated through systematic studies. Therefore, the aim of this study was to prepare essential oils extracts from the stem bark of A. crassna native to Malaysia, and determine their anti-proliferative and anti-angiogenic activities. Stem bark samples were extracted with 80% ethanol and hydrodistillation methods. The resulting essential oils from both extraction approaches were subjected for cytotoxic activity using four cancer cell lines and one normal cell line. Hydrodistilled essential oils displayed significant cytotoxic effect against HCT 116 cells with the lowest IC50 value calculated (28.0 ± 1.5 μg/mL). Furthermore, we have investigated the effect of hydrodistilled essential oils (active extract) on angiogenesis in vitro and ex vivo, and found that essential oils directly inhibited tube formation after plating endothelial cells on matrigel. In addition, essential oils caused significant inhibition of microvessels outgrowth of rat aortic ring assay in a dose-dependent manner (P<0.05) with an IC50 (43.13 μg/ml). The GC-MS analysis of the most active extract showed the presence of several potent phytochemicals such as β-caryophyllene, 1-phenanthrenecarboxylic acid, α-caryophyllene, benzenedicarboxylic acid and azulene. It can be concluded that the anti-cancer and anti-angiogenic effects of the essential oils could be due to the synergistic effect of the biologically active phytoconstituents.
Keywords: Aquilaria crassna; Tube formation; Angiogenic; Essential oils
Cancer is a complex multifaceted group of more than 100 types of diseases characterized by uncontrolled cell growth, local tissue invasion and metastasis. Despite much advancement in understanding and treating cancer in the recent decades, it has remained a rampant killer across the globe. According to recent statistics, cancer accounts for about 23% of all disease-related deaths in the USA. It is the second most common cause of death after heart disease. However, while deaths due to heart disease decreased steeply in both older and younger populations in the USA from 1975 through 2002, no appreciable decline in cancer-caused deaths has been observed so far [1]. Increasing complexity and treatment-associated toxicity with nonspecific target of chemotherapy have aggravated the risk of side effects among the cancer patients [2].Thus,new agents that are safe, available and effective are urgently needed. Recently, there is a great interest in angiogenesis modulators as therapeutics of several angiogenesis related disorders. In this context, several natural products that inhibit angiogenesis also demonstrate anticancer activities. The plants that are traditionally used for anticancer treatment often have anti-angiogenic properties through multiple interdependent processes these include Artemisia annua (Chinese wormwood), Viscum album (European mistletoe), Curcuma longa (curcumin), Scutellariabaicalensis (Chinese skullcap), resveratrol and proanthocyanidin (grape seed extract), Magnolia officinalis (Chinese magnolia tree), Camellia sinensis(green tea), Ginkgo biloba, quercetin, Poriacocos, Zingiber officinalis (ginger), Panax ginseng, Rabdosiarubescenshora (Rabdosia), and Chinese destagnation herbs [3,4].
The use of medicinal plants has a strong track record of contributing to drug development, and there is abroad consensus that the potential for new natural products from plants is not exhausted and still represent an important source for the lead in drug discovery [5].Among plant-derived components, essential oils (EOs) fromaromatic plantshave been reported to possess anticancer activities and also enhance the quality of life of the cancer patients by reducing the level of their agony [6].
Aquilaria crassna (Agarwood) has been identified as promising source of bioactive components that is rich in phenolics, flavonoids and benzophenones, xanthones and sesquiterpenes. These constituents can target multiple cancer cell signaling pathways. They also have good safety profile with potentially lower side effects than standard chemotherapeutic compounds [7,8].Aquilariacrassna is an important traditional medicinal plant, which has been widely used by Arabs and Japanese to treat digestive and sedative disorders.Different parts of A. crassna have been studied and explored dynamic biological effects such as anti-ischemic [9],antifungal [10] and antibacterial effects [11]. Previous communications from this laboratory reported the extraction and purification of the essential oils of Aquilaria crassna and its active principle [12,13].The biological investigation revealed potent antioxidant, anti-inflammatory and anticancer properties against colorectal carcinoma cells (HCT 116) and pancreatic cancer cells (MIA PaCa-2) which mediated via apoptotic mechanism [14,15].In view of the potential beneficial effect of A. crassna products, the present study was designto meet the following objectives: (i) to study and compare the cytotoxicity effect of ethanol and hydrodistillation extracts from Aquilaria crassna stem bark against panel of cancer cell lines; (ii) to study the antiangiogenic effects of Aquilariacrassna most active extract.
Chemicals and reagents
All cell cultures and their reagents were purchased from Gibco (USA). Phosphate buffered saline, trypsin, HIFBS, PS, fibrinogen, aprotinin, thrombin, suramin, aprotinin, 6-aminocaproic acid, L-glutamine, thrombin, and gentamicin were purchased from Sigma (Germany). MTT (3-(4,5-dimethylthiazol-2-yl)- 2,5diphenyl tetrazolium bromide) were procured from Sigma-Aldrich (USA). Dimethyl sulfoxide (DMSO) was purchased from Fluka (USA).
Cell lines and culture conditions
The HUVEC cell line (catalogue number, C2517A), human colorectal carcinoma cell line HCT-116 (catalogue number CCL- 247), human hormone sensitive invasive breast cancer cell line MCF-7 (catalogue number CRL-1469), Human pancreatic carcinoma cell line (PANC-1) catalogue number (HTB- 14) and human prostate cancer cell line PC-3 (catalog number CRL-1435) were purchased from ScienCell (USA). HUVECs were maintained in ECM (ScienCell) supplemented with endothelial cell growth supplements (ECGS), 5% HIFBS, and 1% PS. HCT-116 cells and PANC-1 were maintained in RPMI, whereas MCF-7 and PC-3 cells were maintained in DMEM and F12K medium, respectively. The media were supplemented with 5% HIFBS and 1% PS. Cells were cultured in a humidified incubator at 37°C supplied by 5% CO2. Cell culture work was conducted in sterile conditions using a Class II biosafety cabinet (ESCO, USA).
Plant collection and authentication
The stem bark of Aquilaria crassna was collected from a local farm in Kajang, Selangor, Malaysia in the year 2015. Floral characteristics and taxonomical identification was confirmed by a senior taxonomist Mr. V. Shanmogan. A herbarium sample (Ref. No. USM/122083) was submitted at the Department of Botany, School of Biological Sciences, Universiti Sains Malaysia, Pulau Pinang, Malaysia.
Plant extraction
80% ethanolic extract: Fresh stem bark of Aquilaria crassna was sliced into small pieces and ground to a fine powder. Initially, 100 g of material was subjected to maceration method of extraction using 1 L of 80% ethanol. The extraction was carried out for 72 hours at 25 °C with continuous shaking on a magnetic stirrer. After filtration, ethanol was evaporated at 60°C by rotavapor and the liquid residue was kept at 2-8°C in normal fridge for 24 h. A yellow precipitate material was formed with yield of 0.6%.
Hydrodistillation extract: The heartwood of Aquilaria crassna was sliced into small pieces and grinded mechanically. The ground material of bark (500 g) was soaked in round-bottom flask with 5 L of water at room temperature (25 ± 2°C) for 72 hours. Then, hydrodistillation was carried out at boiling temperature of water for 48 hours. The essential oils were obtained by modified Clevenger-type apparatus and collected into hexane, yielding a pale-yellow liquid of about 12.6 g.
The transmission electron microscopy
TEM study was carried out in order to confirm the presence of essential oils bodies in Aquilaria crassna stem bark, samples were collected before and after hydrodistillation extraction and fixed in 0.1M McDowell– Trump and stained with 1% osmium tetroxide for 4 h at 4°C. They were then rinsed with 100 mM sodium phosphate buffer at 4°C. The samples were washed and dehydrated for 15 min with each series of ethanol (70, 80, 90 and 100%) followed by acetone. The samples were embedded in resin and infiltrated five times in Suprr’s mixture at 60°C for overnight. Thin sections (70 nm) were cut by a Leica Reichert Ultracut and collected on copper grids and stained with uranyl acetate and lead citrate and the samples were viewed on a Philips CM200 transmission electron microscope.
Gas Chromatography-Mass Spectrometry (GC/MS) analysis
Quantitative chemical analysis of the most active extract was carried out using GC/MS (Agilent Technologies – Hewlett Packard Model, Santa, CA, USA). The assay condition was as follows: HP-5MS capillary column (30 m × 0.25 mm ID × 0.25 μm, film thickness); held at 70°C for 2 min, raised to 285°C at a rate of 20°C/min and held for 20 min; 285°C for MSD transfer line heater; carrier gas helium at a flow rate of 1.2 mL/min; 2:1 split ratio. About 1 lL solution of SF-1 in chloroform (10 mg/mL) was injected automatically. Scan parameter low mass: 35 and higher mass: 550. The constituents were identified by comparison with standards using NIST 2002. A total ion chromatogram (TIC) was used to compute the percentage of the identified constitutes.
Cytotoxicity assay
The MTT cytotoxicity assay was performed according to the method previously described [16]. Cells were seeded at 1.5 × 104 cells in each well of a 96 well plate in 100 μL of fresh culture medium and were allowed to attach overnight. For screening, the cells (70–80% confluence) were treated with A. crassna both extracts at 3.125, 12.25, 25, 25, 50 and 100 μg/mL concentration to get the IC50. After 48 h of treatment, the medium was aspirated and the cells were exposed to MTT solution prepared at 5 mg/mL in sterile PBS. The solution was added to each well at 10% v/v and incubated at 37°C in 5% CO2 for 3 h. The water-insoluble formazan salts were solubilized with 200 μL DSMO/ well. Absorbance was measured using the TECAN microplate reader at the primary wave length of 570 nm and the reference wavelength of 620 nm. Each plate contained the samples, negative control and blank. DMSO (1% v/v) was used as the negative control. The assay was repeated four times, and the results were presented as a mean percent inhibition to the negative control ± SD (n=4).
Tube formation assay
The ability of HUVECs to form capillary-like structures on matrigel matrix using angiogenesis endothelial cell tube formation plate system was investigated according to the previously reported protocol [17]. In brief, the matrigel matrix was allowed to polymerize for 45 min at 37°C and 5% CO2. HUVECs were trypsinized and seeded (2 × 105 cells per well) in 100 μl of ECM containing various concentrations of A. crassna extract in triplicates. After 6 h, the tubular networks in the wells were formed and photographed by a digital camera (Leica, DC300, USA) connected to a light inverted microscope at 4X magnification. The quantitative assessment of tube formation inhibition was achieved by measuring the area occupied by tubular structures using the Scion Image analysis program. The results were expressed as the percentage of inhibition mean ± SD. It was calculated according to the formula:
% of inhibition of tube formation=(1-(Area T/Area C)) × 100
Where: Area T: the area of tubules in the treated wells
Area C: the area of tubules in the untreated wells
Rat aortic ring assay
This assay was carried out following previous method described [18], with slight modification. Thoracic aortas were removed from euthanized male Sprague-Dawley rats (12–14 weeks old), rinsed with serum-free medium, and cleared of fibro-adipose tissues. The aortas were cross-sectioned into small rings (approximately 1 mm thickness) and seeded individually in 48-wells plate in 300 μL of serum-Absorbance was measured by infinite® Pro200 TECAN Group Ltd., (Switzerland) free M199 medium containing 3 mg/mL fibrinogen and 5 mg/mL aprotinin. 10 microliters of thrombin (50 NIH U/mL in 1% bovine serum albumin in 0.15 M NaCl) were added to each well and incubated at 37°C for 90 min to solidify. A second layer (M199 medium supplemented with 20% HIFBS, 0.1% aminocaproic acid, 1% L-glutamine, 2.5 μg/mL amphotericin B, and 60 μg/mL gentamicin) was added into each well (300 μL/well). A. crassna active extracts were added at a final concentration of 100 μg/mL. Suramin and DMSO (1%) were used as positive and negative controls, respectively. After five day, aortic rings were photographed using the EVOS f1 digital microscope at 40 × magnification. Subsequently, the length of blood vessel outgrowth from the primary tissue explants was measured using Leica Quin software. The inhibition of blood vessel formation was calculated using the following formula:
% blood vessel inhibition= [1–(A0/A)] × 100,
Where, A0 is distance of blood vessel growth in treated rings in μm and A is distance of blood vessel growth in the control in μm. The results are presented as mean ± SD (n=6).
Statistical analysis
Results are expressed as mean ± SD. Differences between groups were compared by a one-way analysis of variance (ANOVA) using GraphPad Prism6 and considered significant at p<0.05.
Plant extraction
Two exacts were prepared from the stem bark of A. crassna, using ethanol solvent and hydrodistillation technique. The yield of each extract was calculated as w/w percent yield. Hydrodistillation extract gave pale yellow oil with a strong pleasant aromatic odor (yield of 2.52%), while ethanol extract sample showed lower yield (0.6%) and less aromatic odor.
Characterization of A. crassna essential oils using TEM and GC-MS
The majority of subcellular components in A. crassna stem bark were examined in transmission electron microscopy. Figure 1A and 1B shows the essential oils bodies before the hydrodistillation extraction were predominantly filled with clear osmium stained as dark entities. Whereas, no essential oils bodies were detected after extraction Figure 1C. Indicating that the hydrodistillation method is more reliable approach than solvent extraction. Therefore, hydrodistillation technique gives high yield of oil (2.52%) and potent cytotoxic effect against penal of cancer cell lines. Moreover, hydrodistilled EOs of A. crassna were subjected to GC–MS analysis to quantify the major chemical constituents and determine their molecular weights. Data obtained from GC–MS, including the retention times, peak areas, molecular formulas and molecular weights of the major constituents is shown in Table 1. The chemical composition of the essential oil extract was determined using NIST library. GC-MS analysis revealed that the essential oil mixture comprised various polyphenolic and aromatic compounds. Quantitative analysis of the essential oils showed the major peaks to correspond to β-Caryophyllene (8.111%), Octamethyl (7.103%), 2-Naphthalenemethanol (6.193%), alpha.-Caryophyllene (4.755%), benzenedicarboxylic acid (4.642%), azulene (3.925%), naphthalene (2.694%) and cyclodecene (2.583%). A detailed description of the peaks identified by GC–MS (Figure 2) can be observed in Table 1.
| Peak | Retention time (min) | Area % | Phytoconstituents | Molecular Formula | MolecularWeight | Structure |
|---|---|---|---|---|---|---|
| a | 7.26 | 3.925 | Azulene | C15H24 | 204 | 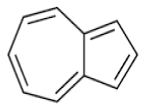 |
| b | 7.38 | 8.111 | β-Caryophyllene | C15H24 | 204 | 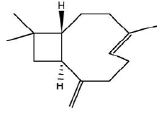 |
| c | 7.47 | 2.694 | Cyclopropa[a] Naphthalene | C15H24 | 204 | 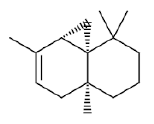 |
| d | 7.64 | 4.755 | α-Caryophyllene | C15H24 | 204 | 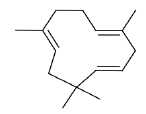 |
| e | 8.78 | 1.72 | Caryophyllene oxide | C15H24O | 220 | 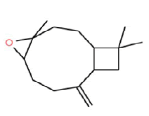 |
| f | 8.96 | 6.193 | 2-Naphthalenemethanol | C15H26O | 222 | 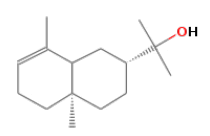 |
| g | 9.68 | 1.866 | 3-Methyl-1-phenyl -2,5-Pyrrolidinedione | C11H11NO2 | 189 | 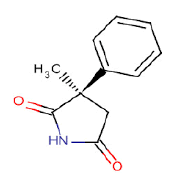 |
| h | 9.98 | 1.377 | Diallyl-cyclohexanone | C13H22O2 | 210 | 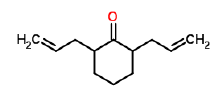 |
| i | 1.83 | 1.924 | Isobornyl propionate | C15H22O | 218 | 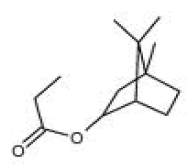 |
| j | 12.16 | 1.685 | 9-H-Cycloisolongifolene | C10H17Br | 216 | 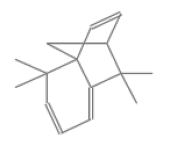 |
| k | 12.26 | 2.583 | Cyclodecene, 3-brormo | C21H34O2 | 318 | 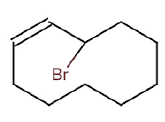 |
| l | 12.46 | 7.103 | 1-Phenanthrenecarboxylic acid | C16H22O4 | 278 | 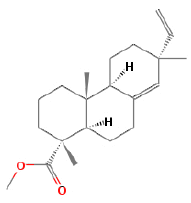 |
| m | 13.01 | 4.642 | Benzenedicarboxylic acid | C15H24 | 204 | 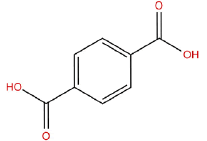 |
Table 1: GC-MS quantitative analysis of phytochemicals of A. crassna essential oils.
Anti-proliferative effect of A. crassna extracts
The antiproliferative effect of A. crassna extracts were tested against four tumor and one normal cell lines using the MTT assay. The median inhibitory concentration (IC50) values were calculated for each cell line and the values are presented in Table 2. Among the tested cancer cells, the hydrodistillation extract demonstrated pronounce anti-proliferative effect against two cancer cell lines, namely HCT 116 (colon cancer, IC50=28.0 ± 1.5 μg/ml), PANC-1 (pancreatic cancer, IC50=32.0 ± 3.2 μg/ ml). Whereas, it displayed either moderate or poor cytotoxic activity against breast (MCF-7) or prostate (PC3) cell lines with IC50 110.2 ± 3.5 and 79.0 ± 4.2 μg/ml, respectively. In addition, the hydrodistillation extract also displayed moderate cytotoxicity against human endothelial cells (HUVEC) with IC50 53.0 ± 2.7 μg/ml. Similarly, the ethanol extract has also demonstrated selective anti-proliferative effect against colorectal (HCT 116) and pancreatic (PANC-1) carcinoma cell lines with IC50 38.0 ± 0.9 and 72.0 ± 1.8 μg/ml, respectively. Whereas, it exhibited either moderate or poor cytotoxic activity against breast (MCF-7) or prostate (PC3) cell lines with IC50 140.5 ± 2.5 and 119.0 ± 1.6 μg/ml, respectively. The results were compared with the respective standard reference drugs, tamoxifen, betulinic acid and 5-fluorouracil (Table 2). Figure 3A and 3B shows the graphical illustration of the dosedependent antiproliferative effect of A. crassna extracts against tested cell lines.
| Samples | IC50 µg/ml HCT 116PANC-1PC3 MCF-7HUVEC | ||||
|---|---|---|---|---|---|
| Hydrodistillation | 28.0±1.5** | 32.0 ±3.2** | 79.0 ± 4.2* | 110.2 ± 3.5 | 53.0 ±2.7* |
| Ethanol | 38.0 ±0.9** | 72.0 ±1.8* | 119.0 ± 1.6 | 140.5 ± 2.5 | 48.0 ±1.6* |
| 5-fluorouracil | 12.7 ±0.5** | - | - | - | - |
| Tamoxifen | - | - | - | 9.5 ±0.9** | - |
| Betulinic acid | - | 19.4 ±1.2** | 8.4 ±0.6** | - | - |
| Suramin | - | - | - | - | 8.5 ±1.4** |
Results are presented as means ± SD (n=3), *P<0.05, **P<0.01
Table 2: IC50 values of A.crassna extracts and standard controls on various human cancer cell lines.
Antiangiogenic effects of A. crassna active extract
Inhibition of Tube Formation: In general, endothelial cells forms tube-like structure networks in a matrigel matrix within 6 h. In angiogenesis mechanism, tube formation is an important process in neovascularization. Antiangiogenic effect of A. crassna essential oils extracts were confirmed by studying the effect on differentiation of HUVECs on matrigel matrix. Treatment of HUVECs with essential oils extracts inhibited the growth factors induced differentiation in a dose-dependent manner (Figure 4). Extracts with (25 and 50 μg/ml) reduced the area occupied by the network structures with inhibitory effect of 28.07 ± 3.68 and 49.21 ± 2.9%, respectively. Whereas, the high concentration of essential oils extract at 100 μg/ml was completely abolishing capillary tubules formation in HUVEC cells. Similarly, the positive control of Suramin at 100 μg/mlcompletely abrogated endothelial tube formation. In addition, untreated HUVEC cells on matrigel matrix shows an obvious tubes formation in negative control.
Figure 4: Images of HUVECs Matrigel tube formation assay. A.crassna extracts inhibits matrigel tube formation. HUEVCs were treated with (A) 0.5% DMSO (B) 25 μg/ml (C) 50 μg/ml (D) 75 μg/ml (E) 100 μg/ml of EOs and (F) Suramin 100 μg/ml (G) The dose response relationship of EOs extracts on tube formation assay.
Antiangiogenic effect in rat aortic model
The 3D rat aortic ring assay was used to quantify antiangiogenic potential of the essential oils extract of A. crassna stem bark by measuring micro vessels sprouted from rat aortic rings. We carried out the experiment with five concentrations (6.25, 12.5, 25, 50 and 100) to obtain the medium inhibitory effect (IC50), which was 43.13 μg/ml. The inhibition of micro vessels from explants rat aorta treated with essential oils (EOs) extract was significantly compared to untreated micro vessels. Figure 5A shows the microvessels outgrowth from the untreated aortic rings (0.5% DMSO) which did not display any inhibitory effect. On the contrary, treatment with A. crassna extracts (Figure 5B-5E) caused significant inhibition of microvessels outgrowth in a dose-dependent manner (P<0.05) (Figure 6A). At 50 μg/ml, A. crassna extracts inhibited vascularisation by 50% and doubling the dose to 100 μg/ml led to a complete inhibition of microvessels outgrowth by 100%. Likewise, suramin, which was used as a positive control showed almost 100% inhibition of microvessels outgrowth at 100 μg/ml (Figure 5F). Figure 6B shows the dose-response curves of A. crassna extracts. It highlights the relationship between dosage and percentage of inhibition activity.
Figure 5: Photomicrographs showing antiangiogenic effect of A.crassna extracts against sprouting of microvessels in rat aortic explants. Images were taken on the 5th day of the experiment using an inverted phase-contrast microscope at 4 magnifications. (A) 0.5% DMSO showing extensive growth of microvessels as a negative control. (B) EOs 12.5 μg/ml. (C) EOs 25 μg/ml. (D) EOs 50 μg/ml. (E) EOs 100 μg/ml.(F) Suramin 100 μg/ml as a positive control.
Although there are many lines of ethnopharmacological evidence showing that Aquilaria species have been traditionally used in the Ayurvedic practice and Chinese medicine to treat various diseases specifically the inflammatory-related disorders. However, there is a paucity of information on traditional use of Aquilaria crassna towards cancer prevention or/and treatment. The reason may be due to the fact that cancer terminology is used in modern medicine to describe the diseases that related with uncontrolled cell growth in which inflammation and chronic pain is involved [19].
Solid tumors require several components for growth; angiogenesis the process which stimulate the development of new vasculature is an essential element in growth and metastasis of tumors [20]. Cancer colonies remain undetected and dormant for many years, and under certain circumstances the tumor cells switch into angiogenic phenotype, induce formation of new blood vessels, then start to grow and migrate to other organs [21]. In the past two decades tremendous efforts have been made for discovery of antiangiogenic agents from natural sources to treat angiogenesis related disease specifically cancer. The direct step of angiogenesis is proliferation, differentiation, migration and tube formation of endothelial cells, therefore endothelial cell could be good target for cancer therapy [22].
A. crassna is a plant with diverse traditional medicinal properties. A number of studies have confirmed that the essential oil is an active component of A. crassna stem bark [23,24]. However, very little is known about the pharmacological properties of this plant. The present study clearly demonstrates that the essential oil extracted from A. crassna using the hydrodistillation method had a remarkable antiproliferative and antiangiogenic properties. In the cytotoxicity studies, the essential oil mixture from hydrodistillation shows significant anti-proliferation activity against HCT 116 (IC50 28.0 ± 1.5 μg/mL), PANC-1 (IC50 32.0 ± 3.2 μg/mL), PC3 (IC50 79.0 ± 4.2 μg/mL) and MCF-7 (IC50 110.2 ± 3.5 μg/mL) human cancer cell lines. On the other hand, ethanol extract exhibits moderate or poor cytotoxic effect. Thus, essential oils from hydrodistillation approach was selected and used as the active extract of A. crassna for further phytochemical analysis and antiangiogenic study.
Compelling data implicate angiogenesis and tumor-associated neovascularization as a central pathogenic step in the process of tumor growth, invasion, and metastasis. A significant correlation was shown to exist between the degree of tumor angiogenesis (microvessel density) and survival in patients presenting with solid tumors [25]. Therefore, identification of anti-angiogenic phytochemicals with multiple biological functions that are non-toxic and effectively act at various steps of angiogenic process could be a good therapeutic approach against tumor growth and metastasis. Recently, essential oils and their constituents are found to act by multiple pathways and mechanisms involving apoptosis, antimetastatic and antiangiogenic. For instance, essential oils from orang Citrus sinensis has been reported to inhibit angiogenesis and metastasis in colon cancer cells via modulation of apoptotic factors Bax/Bcl2 and inhibition of vascular endothelial growth factor (VEGF) [26]. Other study showed that the essential oil of Origanum onites has successfully blocked tube formation and cell migration of endothelial cells [27]. In this study, two angiogenesis platform were employed to assess the antiangiogenic effect of A. crassna essential oils. In the In vitro platform assay HUVECs were used as a model cell line of angiogenesis because it forms the internal tube-like structure of the blood vessels. The result shows that A. crassna EOs not cytotoxic towards endothelial cells with IC50=53.0 ± 2.7 μg/ml. Subsequently, EOs markedly inhibited tube-like structure networks in a matrigel matrix in a dose dependent manner (Figure 4). Noteworthy, during the last steps of angiogenesis, endothelial cells formed tubuleslike structure by self-organization, where they adheres into the basement membrane, then they migrate and differentiate to form tubes-network [28]. Thus, Inhibition of three-dimensional branching formation and tube-like structures of endothelial cells elucidates a valuable approach of antiangiogenic potency of the agent on vascularization. Moreover, rat aortic ring assay was used in this study as ex vivo platform to evaluate the effect of EOs on the growth of new blood vessels (Angiogenesis) from explanted tissue. The results showed that EOs perturbed the new blood vessels formation from rat aortic ring explants with IC50 43.13 μg /ml. Taken together, the results of the MTT assay on HUVECs and rat aortic ring assay suggest that the antiangiogenic effect demonstrated by EOs is not attributed to the cytotoxic nature of the extracts, but may be more related to inhibition of one or more of other angiogenesis cascade such as endothelial cell differentiation. Since angiogenesis is a process that is generally down regulated in the normal condition, the antiangiogenic approach of cancer therapy should be selective and nontoxic also may not lead to side effects even after prolonged exposure [3]. In this study, EOs exhibited strong dose dependent antiproliferative activity against colorectal and pancreatic cancer cells. Moreover, the EOs demonstrated considerable antiangiogenic effect on tube formation and rat aortic ring assays. These results may be attributable to the presence of bioactive components. Particularly, sesquiterpenes compounds such as β-Caryophyllene, α-Caryophyllene and Caryophyllene oxide. Several studies indicate that these compounds have promising capability to suppress certain type of cancer cells by inducing apoptosis pathways [29-31]. Furthermore, β-Caryophyllene was identified as a major constituent (35%) in the essential oil of Cannabis sativa L, which makes the compound an important dietary cannabinoid with high selectivity binding to cannabinoid receptor 2(CB2) [32]. Recent study has suggested that CB2 receptor play central roles in the modulation of inflammatory and neuropathic pain responses [33]. Interestingly, angiogenesis and inflammation are closely integrated processes [14]; reducing inflammation may significantly correlate with angiogenesis suppression. Overall, we suggest that the EOs of A. crassna and its phytochemical constituent’s exhibits significant activity against colorectal cancer cells through a mechanism that appears to involve apoptosis induction and angiogenesis suppression.
In conclusion, the present study revealed that of the two extraction methods tested, hydrodistillation produced an extract comprising greater amounts of A. crassna biologically active essential oils. This extract was found to exert significant antineoplastic and antiangiogenic effects. Overall, the biological activities observed in the study could be attributed to the versatility of the phytochemical constituents of the essential oils of A. crassna. Based on the findings of the present study, it was suggested that these EOs may be promising candidates for a novel chemopreventive or chemotherapeutic formulation of, perhaps, minimal side effects. Further studies are in progress in our laboratory to investigate the toxicity profile of EOs and their in vivo anticancer activity.
We have no conflict of interest to declare.
Saad S Dahham would like to acknowledge Universiti Sains Malaysia for the USM Fellowship.