Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2020)Volume 9, Issue 2
Objective: The current study investigated the mechanism(s) of action of the antisickling property of Cnidiscolus aconitifolius ethanol extract and determined its histopathological effects on the liver, kidney and spleen of Wistar rats.
Methods: The antisickling properties of C. aconitifolius (CA) ethanol extract was tested at different concentrations, using the inhibitory and reversal models. Its effect on the density and mean cell volume of Hb S red blood cells was evaluated using the discontinuous density gradient assay. Ciklavit®, an antisickling nutritional supplement was employed as positive while phosphate buffered saline (pH 7.0) was the negative control. Changes in percentage Red Cell Count (RBC) and Mean Cell Volume (MCV) in each density fraction were determined. Quantification of extracellular potassium leakage of RBC before and after incubation with CA extract was also conducted. CA was evaluated for its acute toxicity (LD50) while sub-acute toxicity was evaluated by oral administration to Wistar rats for 28 days at 125, 250 and 500 mg/kg body weight. Rats were anaesthetized on the 29th day and blood collected through cardiac puncture for hematological analysis. The liver, kidney and spleen of the rats were harvested for histopathological examinations.
Results: The results showed that the percentage of dense cells in blood treated with CA was reduced significantly (p<0.05) compared to positive control. CA increased MCV of cells and inhibited basal potassium efflux with a rightward shift in the dose-response curve. The median lethal dose (LD50) of CA was 3808 mg/kg; neither mortality nor structural or histological alterations were observed in the liver, kidney and spleen at the administered dose. The extract of CA enhanced erythropoiesis.
Conclusion: C. aconitifolius extract prevented potassium efflux and reduced red cell density in vitro. The CA crude extract was safe for consumption and increased the production of red blood cells in vivo.
Sickle cell disease; Potassium efflux; Dense cells; Cnidoscolus aconitifolius; Man cell volume; Toxicity
Presence of dense (dehydrated) sickle cells contributes excessively to the obstruction of blood flow in the microcirculation [1]. Variability in the amount of these cells has made the steady state unstable rheologically, thus contributing to the development of subclinical and clinical events [2]. The increase in cell density progressively decreases the deformability of Red Blood Cell (RBC) and this reduction in deformability plays a role in the senescence of normal RBCs [3,4]. RBCs of Sickle Cell Disease (SCD) have characteristic high density (ρ>1.120 g/cm3) and this reduces erythrocyte lifespan in patients with the highest percentage dense RBCs [5,6]. In a study by Bartolucci et al. [7] they reported that patients who experienced renal dysfunction, skin ulcers or priapism have significantly higher percentage dense RBCs. Dense RBCs are more susceptible to being trapped in vivo causing persistent blockage of small post-capillary venules, with acute effects resulting in perfusion deficits [8]. Akinola et al. [2] detected a sub-population of poorly deformable dense cells at the onset of vaso-occlusive crisis, while Ballas and Smith [9] established a significant association between the percentage dense RBC and Mean Cell Hemoglobin (MCH), percentage Hb F and Red cell Distribution Width (RDW).
Cell dehydration is one of the distinguishing characteristics of sickle RBCs. The loss of potassium, Cl– and water from erythrocytes leads to dehydration and increases Hb S concentration which causes rapid sickling in hypoxia. This effect is caused by the activation of a Ca2+activated K+ channel of human RBCs called The Gardos channel [10]. Obstruction of K+ loss from the erythrocyte should maintain the mean cell volume, prevent increase in Hemoglobin S concentration and reduce sickling. Many studies have reported the antisickling properties of various agents, the most notable of which is hydroxycarbamide (hydroxyurea, HU). This is an antineoplastic agent that has been shown to increase the concentration of Hb F variably in patients with SCD. It was the only FDA-approved drug for SCD treatment in the US for about 20 years until L-glutamine (Endari) was approved in 2017. HU improves the bioavailability of nitric oxide amongst other effects, but has some unwanted effects which may or may not be reversible [11]. Bone marrow transplant is the only cure available for SCD and the procedure is expensive with high risk. It requires a compatible family member as donors which are hard to come by. There is at best only an 85% diseasefree survival rate, with a 7% transplant-related mortality rate and a 9% graft failure rate [12]. Significant progress has been made in gene therapy approaches. However, the procedures are still at investigational stages, and will likely be cost-prohibitive for the majority of SCD patients in the near term. Uses of medicinal plants singly or in combinations have been researched into over the years. Several plants have been reported to possess antisickling properties amongst which are: Carica papaya, Sorghum bicolor, Zanthoxylum zanthozyloides, Cajanus cajan, Moringa oleifera, Telfairia occidentalis, Cyperus esculentus, Alchornea cordifolia, Parquetina nigrescens, Khaya senegalensis [13-21]. The search for herbal antisickling agents which are non-toxic, accessible and easily available continues.
Cnidoscolus aconitifolius (Mill.) I.M. Johnst. (Family: Euphorbiaceae) originated as a domesticated leafy green vegetable in the Maya region of Guatemala, Belize, and Southeast Mexico during pre- Cambrian period [22]. It was introduced into West Africa from an agricultural research station in Puerto Rico in 1977 [23]. C. aconitifolius (CA) was first cultivated in Ghana from where its cultivation spread into neighboring Nigeria in the ensuing years [23]. C. aconitifolius is also referred to as Chaya and took on new names in the communities where it was introduced [24]. It is called, “efo Iyana Ipaja”, “Efo Jerusalem”, in Southwestern parts of Nigeria [25], while in the Niger Delta of Nigeria; it has been nicknamed “Hospital Too Far”, because of its numerous traditional claims [26]. The phytochemicals contained in the leaf extract are alkaloids, amino acids, tannins, saponins, flavonoids, terpenes, glycosides and steroids [27]. Gonz´Alez-Laredo [28] reported that methanol extract of CA leaves showed a hydrogen cyanide concentration of 2.37 mg/100 g of dry sample by the acidic titration method and 4.25 mg/100 g by the alkaline titration method. These values are very low compared to normal level (200-300 mg/100 g) reported in plant foods such as lima beans, cassava and other green leafy species [29] and are below the maximum (20 mg/100g) allowed by the FDA in foods. This might confer some trust for consuming the plant in salads or as a raw vegetable. The leaves of the plant are collected fresh, cut, cooked as soup and eaten to treat individuals with low PCV especially, “sicklers” in Southwestern Nigeria. This claim of its use in the treatment of anemia prompted the investigation of its effect on SCD in vitro using the inhibitory and reversal models [30]. This current, subsequent study is aimed at determining the mode of action of the alcoholic extract of C. aconitifolius as an antisickling agent and ascertain its safety. This was assessed by determining its effects: on the density, mean cell volume of Hb SS RBCs using a discontinuous density gradient [2]; on the inhibition of potassium leakage in vitro, and on vital organs of Wistar rats in vivo. It is expected that any drug that reduces RBC density and prevents dehydration would increase deformability of sickle cells and may be useful in the management of vaso-occlusive crisis.
Preparation of the extracts
Cnidoscolus aconitifolius (Mill.) I.M. Johnst (Family Euphobiaceae) was identified at IFE Herbarium, Obafemi Awolowo University, (OAU) Ile-Ife, Nigeria by G. Ibhanesebor. Fresh leaves were collected at OAU campus and voucher number IFE 17256 was allotted. Leaves were dried in the oven at 40°C, powdered and extracted by macerating in absolute ethanol for 72 h. The ethanol extract was filtered and the filtrate evaporated to dryness in vacuo at 40°C. Extract was dissolved using 0.1 mL Tween 80 and appropriate volume of distilled water added. A stock of 16 mg/mL of CA extract was prepared and serially diluted to give concentrations 1, 2, 4, and 8 mg/mL.
Antisickling assay
Blood samples from confirmed SCD patients attending the Hematology Clinic were collected for the assays after appropriate informed consent was obtained. Ethical approval was obtained from the Ethics and Research Committee of Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Osun State, Nigeria (Certificate number: IRB/IEC/0004553) for this study.
RBC Sickling Inhibitory Test: Hb SS red blood cell suspension, obtained after centrifugation of whole blood, was washed three times in phosphate buffered saline. Red blood cell suspension (5 mL) was incubated with 2 mL CA extract at different concentrations of 1, 2, 4, 8, and 16 mg/mL. The bottles were covered with parafilm and incubated for 1 h at 37°C. After 1 h, ultra-pure nitrogen gas was bubbled into the cell suspension for another 1 h to induce deoxygenation [31]. 200 μL of treated RBCs was thereafter fixed in 5% buffered formalin solution for counting [30].
RBC Sickling Reversal Test: Ultra-pure N2 gas was bubbled into boujol bottles containing 5 mL washed Hb SS red blood cells, covered with parafilm, for 1 h at 37°C to induce sickling. Without fixing, 2 mL of CA extract at different concentrations (1, 2, 4, 8, and 16 mg/mL) was added, mixed and incubated for another 1 h. 200 μL of the cell suspension was thereafter fixed in 5% buffered formalin solution ready for counting [30]. One percent Tween 80 in phosphate buffered saline was employed as the negative control while 2 mL Ciklavit® (a nutraceutical made from the extracts of Cajanus cajan and used in the management of sickle cell anaemia) was used as positive control.
Slides were prepared from the fixed cells, and viewed under the microscope at high power objective (400X). Total of 400 red cells (both sickled and unsickled) were counted and the % sickled cells was calculated. All experiments were set up in triplicates.
Red cell fractionation assay
A 10% RBC suspension of Hb SS red cells (5 mL) was incubated with 2 mL CA extract at 37°C for 1 h covered with parafilm. After 1 h of incubation, the suspension was centrifuged and 40% RBC prepared by adding 3 mL PBS (pH 7.0) to 2 mL packed red blood cells. A discontinuous density gradient method reported by Mackie et al. [32] was modified to provide sufficient cells in each cell fraction of SS RBCs [2]. The assays were carried out with Hb SS and Hb AA blood samples. Phosphate buffered saline was used as negative control while Ciklavit®, an antisickling preparation that is readily available, was used as the positive control.
Quantification of potassium efflux
C. aconitifolius extract was first dissolved completely with DMSO4 before final dissolution in H2O to make a stock concentration of 100 mg/mL. Desired concentrations of 3 mg, 10 mg and 30 mg were made from the stock concentration. Blood samples were centrifuged at full speed for 5 minutes and the plasma discarded. Red blood cells were washed three times with phosphate buffered saline at same speed. Aliquot (2 μL) of RBC was introduced into the earlier prepared concentration of extracts (3 mg, 10 mg, 30 mg and 100 mg). Each RBC-Extract mixture was incubated at room temperature for 1 h. After incubation, the absorbance of respective concentration was taken at 405 nm [33]. Phosphate buffered saline was used as blank and folic acid as the standard drug.
For effective quantitation of extracellular K+ leakage, TECO® Diagnostic (Lakeview, CA) Potassium Reagent was used. 2 μL of RBC-Extract mix was added to 10 μL of the potassium reagent provided by TECO® in Eppendorf tubes. The absorbance of the reaction mix was immediately taken by mass spectrophotometer at 540 nm [33]. The absorbance of standard potassium reagent with concentration 4 mEq/mL was taken. The equation
C2=(C1 × n2)/n1 ………………………………………. Equation 1
was used to calculate the potassium concentration. Where C1 is concentration of the standard potassium reagent; C2: concentration of the test extract; n1: absorbance of standard potassium reagent and n2: absorbance of test extract.
Statistical analysis was performed on Graph pad Prism thus: The calculated concentration values of CA extract at different concentrations were linearly transformed using the equation for range normalization:
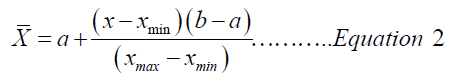
Where 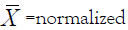 value; Xmin= minimum arbitrary value;
Xmax= maximum arbitrary value; a= minimum range value;
b = maximum range value
value; Xmin= minimum arbitrary value;
Xmax= maximum arbitrary value; a= minimum range value;
b = maximum range value
The exponential concentrations were transformed into their logarithm. The dose response curve was determined according to the log (agonist) vs. normalized response - (variable slope) available on Prism 7.
Toxicity assay
Animals: Wistar rats male (100-140 g) and mice of either sex (18- 25 g) were purchased and kept for one week in the Animal House, Department of Pharmacology, Faculty of Pharmacy, OAU, Ile- Ife to acclimatize. Beddings were changed three times per week. The animals were fed with standard diet and water ad libitum. All experiments were carried out according to the guidelines for care and use of experimental animals and approved by Institutional Animal Ethical Committee (IAEC).
Acute toxicity: The median lethal dose (LD50) of the crude extract was determined using Lorke’s method [34]. Phase 1 required the use of nine mice divided into three groups of three animals per group. Each group of animals was administered orally with CA crude ethanol extract at different doses of 10, 100 and 1000 mg/ kg body weight. The animals were placed under observation for 24 h for behavioral and mortality studies. The experiment proceeded to the second phase with doses of 1600, 2900 and 5000 mg/kg administered to four different groups of mice. The animals were monitored for 24 h and mortality recorded. The LD50 value was determined using the formula:
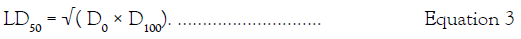
D0=Highest dose that cause no mortality.
D100=Lowest dose that cause mortality.
Sub-chronic oral toxicity: Wistar rats [24] were grouped into six rats each in four cages. Group 1, the negative control, was treated with 5% Tween 80 (0.1 mL/100 g), while groups 2-4 were orally administered with 125, 250 and 500 mg/kg body weight respectively for 28 days. The animals were fed with standard rat feed and water ad libitum throughout the period. On the 29th day, the rats were anaesthetized, and blood collected through cardiac puncture into EDTA bottles for hematological analysis using Auto analyzer (H18 Light). Thereafter, kidney, liver, and spleen were harvested and stored in 10% formalin-normal saline solution for histopathological analysis.
Histopathological analyses: Following dehydration and embedding of the fixed kidney, spleen and liver, sections were cut at 2-3 microns with microtome, stained with Hematoxylin and Eosin (H&E) Stains. Light microscopic examination of multiple tissue sections from each organ in all groups was performed and images representative of the histological profile were examined and photomicrograph taken.
Statistical analysis
Each assay was performed in triplicates and the one way ANOVA was used to detect significant differences and standard errors of the mean values. Level of significance was set at p<0.05.
Antisickling effect
The antisickling properties (both inhibitory and reversal) of C. aconitifolius extract increased as concentration increased from 1 mg/mL to 4 mg/mL however, reduction in activities was observed as concentration increased from 4 mg/mL to 16 mg/mL (Figure 1).
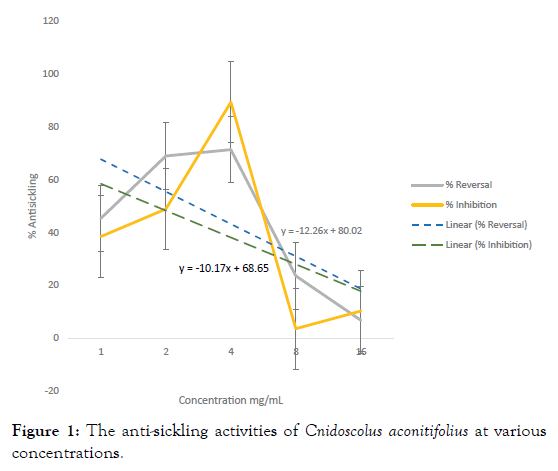
Figure 1: The anti-sickling activities of Cnidoscolus aconitifolius at various concentrations.
Changes in the percentage of red blood cells in each density fraction
The red blood cell count of the density fractions F2 and F3 of Hb AA blood before and after incubation with CA extract and Ciklavit® were not significantly different. There was no dense cell layer (F4) in Hb AA blood cell fraction (Figure 2). However, Hb SS blood before incubation gave a high red blood cell count in the F4 fraction which significantly reduced after incubation with CA and Ciklavit® (Figure 3). The reduction of the dense cells in the F4 fraction caused an increase in the count of cells in F3 fraction.
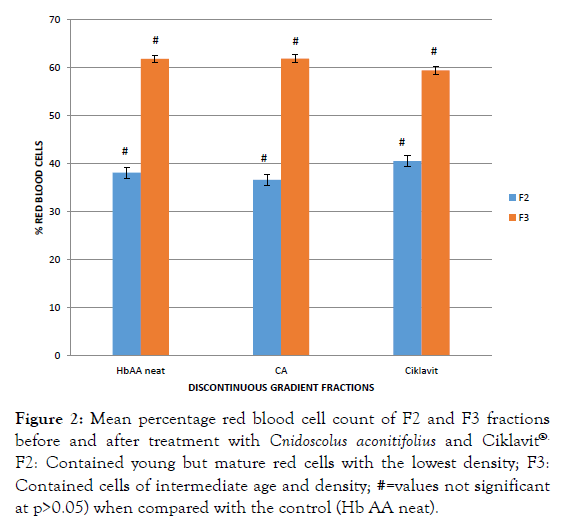
Figure 2: Mean percentage red blood cell count of F2 and F3 fractions before and after treatment with Cnidoscolus aconitifolius and Ciklavit®. F2: Contained young but mature red cells with the lowest density; F3: Contained cells of intermediate age and density; #=values not significant at p>0.05) when compared with the control (Hb AA neat).
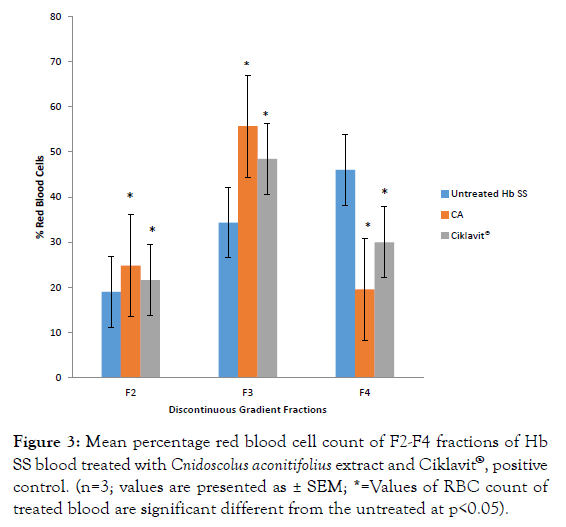
Figure 3: Mean percentage red blood cell count of F2-F4 fractions of Hb SS blood treated with Cnidoscolus aconitifolius extract and Ciklavit®, positive control. (n=3; values are presented as ± SEM; * =Values of RBC count of treated blood are significant different from the untreated at p<0.05).
Changes in the mean cell volume (fL)
There was an increase in the mean cell volume of Hb SS blood cells incubated with CA extract only. Ciklavit® on the other hand had no effect on the mean cell volume of Hb SS red blood cells (Table 1).
| Fractions from the Discontinuous Density Gradient |
Mean MCV of Hb SS neat (negative control) (fL) | Mean MCV of Hb SS + CA extract (fL) | Mean MCV of Hb SS + Ciklavit® (positive control) (fL) |
|---|---|---|---|
| F2 | 84.30 ± 4.00 | 87.40 ± 9.10 | 84.40 ± 5.95 |
| F3 | 82.47 ± 5.20 | 95.70 ± 7.43* | 82.97 ± 5.86 |
| F4 | 80.10 ± 4.53 | 96.53 ± 7.40* | 80.33 ± 4.31 |
Table 1: The mean cell volume of HbSS blood cells after treatment with Cnidoscolus aconitifolius extract and Ciklavit® (positive control).
Inhibition of potassium leakage
Basal potassium efflux from normal erythrocytes studied in the presence of folic acid and CA extracts dissolved in Phosphate buffered saline vehicle, showed no shift in the dose-response curve when compared with the vehicle alone (Figure 4a). Figure 4b showed the result of basal potassium efflux from HbS erythrocytes studied in the presence of folic acid and CA extracts dissolved in Phosphate buffered saline. While Folic acid slightly shifted the dose-response curve with about 3% loss of efficacy, CA significantly (p<0.05) inhibited basal potassium efflux with a rightward shift in the dose-response curve and reduced the efficacy of the process by 18%.
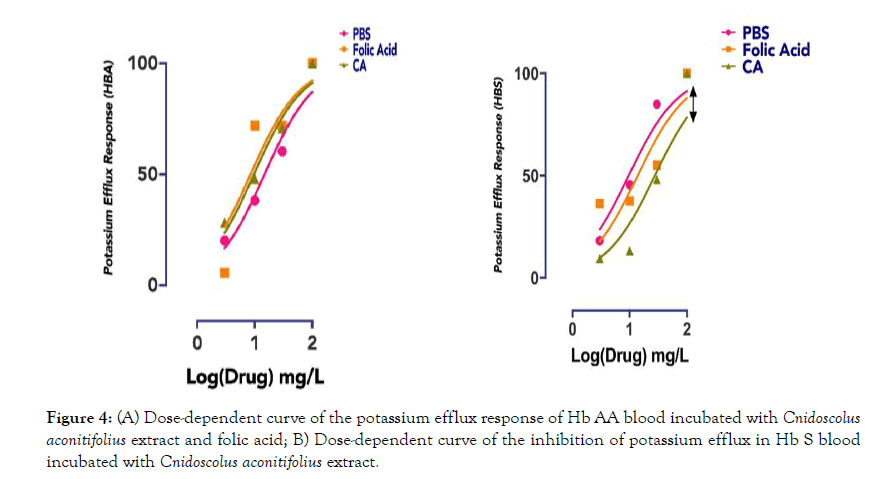
Figure 4: (A) Dose-dependent curve of the potassium efflux response of Hb AA blood incubated with Cnidoscolus aconitifolius extract and folic acid; B) Dose-dependent curve of the inhibition of potassium efflux in Hb S blood incubated with Cnidoscolus aconitifolius extract.
Acute toxicity
In the acute toxicity studies, only one mortality was observed at the maximum dose of 5000 mg/kg body weight using Lorke’s method guidelines. The LD50 value was 3807 mg/kg in mice. There were no changes in normal behavioral pattern of the animals and no signs or symptoms of toxicity.
Sub-chronic oral toxicity: There were no changes in the normal behavioral pattern of the animals and no signs or symptoms of toxicity.
Hematological analysis: The effect of CA extract on the hematological parameters of rats is presented in Table 2. There was a significant increase in RBC values compared with the control.
| Parameters | Control (mg/kg) | 125 mg/kg | 250 mg/kg | 500 mg/kg |
|---|---|---|---|---|
| LYM % | 79.93 ± 0.94 | 72.6 ± 2.90 | 77.58 ± 3.19 | 80.42 ± 2.94 |
| MON % | 7.35 ± 0.56 | 11.62 ± 2.11 | 9.64 ± 1.43 | 10.25 ± 1.08 |
| GRAN % | 12.73 ± 1.05 | 15.78 ± 2.15 | 12.78 ± 2.16 | 9.33 ± 1.93 |
| RBC | 7.01 ± 0.35 | 7.32 ± 0.17 | 7.53 ± 0.19 | 7.52 ± 0.06 |
| HGB | 13.93 ± 0.51 | 13.92 ± 0.36 | 15.0 ± 0.42 | 14.97 ± 0.20 |
| HCT | 39.75 ± 1.94 | 39.3 ± 0.91 | 41.58 ± 0.93 | 41.22 ± 0.66 |
| MCV | 56.85 ± 1.44 | 53.82 ± 1.02 | 55.3 ± 0.47 | 54.90 ± 0.77 |
| MCH | 19.88 ± 0.51 | 18.97 ± 0.31 | 19.86 ± 0.20 | 19.85 ± 0.18 |
| MCHC | 35.03 ± 0.46 | 35.35 ± 0.25 | 36.02 ± 0.26 | 36.3 ± 0.32 |
| RDW-SD | 40.88 ± 1.29 | 37.03 ± 2.39 | 36.44 ± 0.47 | 35.62 ± 0.78 |
| RDW-CV | 18 ± 0.39 | 16.92 ± 0.41 | 16.36 ± 0.27 | 16.13 ± 0.26 |
| PLT | 557.5 ± 204.63 | 612.67 ± 24.36 | 586.2 ±53.38 | 534.83 ± 55.11 |
| MPV | 7.98 ± 0.38 | 7.92 ± 0.24 | 7.66 ± 0.08 | 7.5 ± 0.09 |
| PDW | 10.15 ± 0.95 | 10.23 ± 0.38 | 9.76 ± 0.41 | 9.85 ± 0.25 |
| PCT | 0.46 ± 0.18 | 0.48 ± 0.03 | 0.44 ± 0.04 | 0.39 ± 0.04 |
| P-LCR | 8.5 ± 3.36 | 9.43 ± 1.51 | 7.74 ± 1.2 | 7.08 ± 1.33 |
Table 2: Hematological parameters for rats after 28 days treatment with crude extract of Cnidoscolus aconitifolius at different doses.
Histopathology: Histological evaluation of the effects of C. aconitifolius leaf extracts on the liver, kidney and spleen of Wistar rats harvested after 28 days of treatment. The kidney, liver and spleen showed normal histology as shown in the Photomicrograph of sections of harvested organs presented in Figures 5-7.
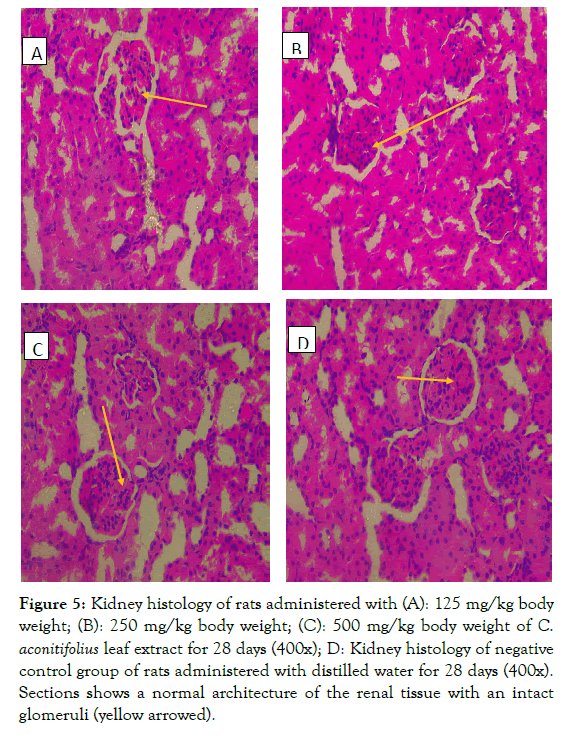
Figure 5: Kidney histology of rats administered with (A): 125 mg/kg body weight; (B): 250 mg/kg body weight; (C): 500 mg/kg body weight of C. aconitifolius leaf extract for 28 days (400x); D: Kidney histology of negative control group of rats administered with distilled water for 28 days (400x). Sections shows a normal architecture of the renal tissue with an intact glom eruli (yellow arrowed).
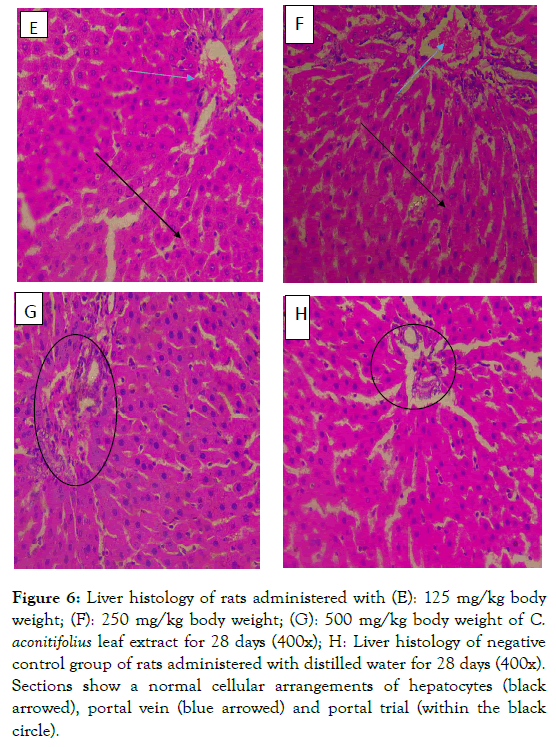
Figure 6: Liver histology of rats administered with (E): 125 mg/kg body weight; (F): 250 mg/kg body weight; (G): 500 mg/kg body weight of C. aconitifolius leaf extract for 28 days (400x); H: Liver histology of negative control group of rats administered with distilled water for 28 days (400x). Sections show a normal cellular arrangements of hepatocytes (black arrowed), portal vein (blue arrowed) and portal trial (within the black circle).
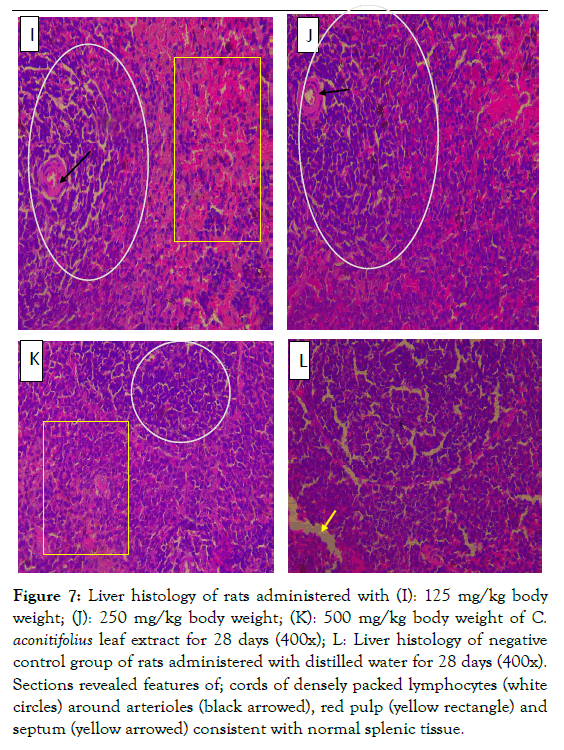
Figure 7: Liver histology of rats administered with (I): 125 mg/kg body weight; (J): 250 mg/kg body weight; (K): 500 mg/kg body weight of C. aconitifolius leaf extract for 28 days (400x); L: Liver histology of negative control group of rats administered with distilled water for 28 days (400x). Sections revealed features of; cords of densely packed lymphocytes (white circles) around arterioles (black arrowed), red pulp (yellow rectangle) and septum (yellow arrowed) consistent with normal splenic tissue.
The highest antisickling activity of the ethanol extract of CA was observed at 4 mg/mL. Both inhibitory and reversal activities of CA showed a steady increase in activity as concentration increased up to 4 mg/mL however a decrease in activity was observed as concentration increased from 4 mg/mL to 16 mg/mL. The optimum concentration with the optimal activity was therefore 4 mg/mL of the extract. The dose-response relationship describes that different levels of doses of a drug or biological agent, can cause the change in effect on an organism after a certain exposure time. Doses lower than the threshold produced no response while those in excess of the threshold exert no additional response [35]. Increasing the dose of a drug with a small therapeutic index increases the probability of toxicity or ineffectiveness of the drug [36]. Hence 4 mg/mL of CA which was observed to give the highest inhibitory and reversal activities, could be said to be the threshold of the antisickling activity since a reduction in activity was produced at higher concentrations (Figure 2). There has been a search to generate new antisickling agents that require relatively low doses, rapidly enter the blood stream, transverse the red blood cell membrane, bind specifically with Hb S and inhibit cell sickling with minimal side effects, [37] Due to the chronic nature of SCD, and the hemoglobin concentration in the body which requires large and frequent doses of drugs to effectively treat the disease, a low therapeutic dose is essential [37]. The effective concentration (EC50) for inhibitory activity was calculated to be 1.83 mg/mL while that of reversal was 2.50 mg/mL. The antisickling activities of CA at 4 mg/mL had been reported to be significantly higher (p<0.05) than that of Cikalvit® (64.19 ± 1.30% inhibition and 76.9 ± 0. 8% reversal activities) at the same concentration [30].
On a discontinuous density gradient, Hb AA neat blood produced only three bands: F1, F2 and F3 (the least dense fraction, F1, contained reticulocytes and cell fragments; the second band (F2) contained young but mature red cells with the lowest density; the third band (F3) contained cells of intermediate age and density). The absence of F4 band is an indication of the absence of dense cells in Hb AA blood. There is no significant difference (p>0.05) in the percentage red blood cell count of the F2 and F3 layers of treated and untreated Hb AA blood (Figure 3). On the other hand, Hb SS neat blood (untreated) showed four fractions F1, F2, F3 and F4. The fourth band (F4) at the bottom (F4) contained the densest cells. Treatment with CA extract and Ciklavit® (positive control) gave a reduction in the percentage red blood cells in the F4 layer and an increase in that of the F3 layer (Figures 4 and 5). This implies that there was a reduction in the density of the red blood cells during incubation with the extracts. The mean percentage change in red cell count of Hb SS blood treated with CA compared to Hb SS control was 26.46 ± 0.55% while that of Hb SS blood treated with Ciklavit® was 16.04 ± 0.16%. Suggesting that the administration of CA reduced the dense cells in Hb SS more effectively than the positive control Ciklavit® at p<0.05. This sub-population of poorly deformable dense cells accumulates at the onset of vaso-occlusive crisis and fluctuates in the steady state thus making it rheologically unstable [2].
The MCV of the red cells in each fraction, particularly in F3 and F4, significantly (p<0.05) increased after treatment with CA extract, but not with Ciklavit® when compared to the untreated values (Table 1). This increase in MCV infers that the extract of CA rehydrates the sickle red cells, which then become more deformable, but the clinical importance of these larger cells in the densest fraction is uncertain, albeit they are fewer.
Loss of cell K+ through the Gardos channel plays a role in preventing colloido-osmotic lysis during transient complement activation on the red cell surface in normal red cells. In sickle cells however, the Gardos channel, either alone or in conjunction with K-Cl cotransport plays a major role in cell dehydration. In vitro dehydration of sickle erythrocytes depends on external Ca2+ and can be prevented by inhibitors of the Gardos channel such as charybdotoxin, nifedipine or clotrimazole [10]. There is no information on any herbal recipe used as an inhibitor of Gardos channel. Whilst CA has no observable effect on extracellular potassium leakage of normal erythrocyte in vitro (Figure 6a), it is a representative blocker of potassium efflux in Hb S erythrocytes because increasing concentration of CA makes it less likely for K+ efflux (rightward shift in dose-dependent curve) (Figure 6b).
Blood parameters are key indicators in diagnosing the actual physiological status of an organism [38]. The assessment of hematological parameters can be diagnostic of adverse effects of foreign compounds in the blood constituents of an animal [39]. Administration of the chemical compounds at toxic doses often results in changes in blood parameters that are indicative of hematological disorders such as anaemia which is characterized by low hemoglobin content [40]. From this study, the Red Blood Cell count (RBC), Hemoglobin (HGB) and MCHC count indicated a significant increase at p<0.05 as concentration increased when compared with the control group (Table 2). This is an indication of the erythropoiesis effect of CA extract in vivo. The extract promotes the production of red blood cells and this might be the rationale behind the ethnomedicinal use as blood booster.
The results of the acute toxicity test showed that mortality was recorded at 5000 mg/kg and the LD50 was over 3800 mg/kg in mice signifying that the extract is non-toxic at doses below 2000 mg/kg per oral [41]. The doses used in the 28-day repeated dosing viz. 125-500 mg/kg were far below the estimated LD50, hence, it can be suggested that the doses used in this study were safe and non-toxic. Furthermore, the animals showed no physical signs of toxicity throughout the period of treatment compared to the negative control group.
The histopathological study of ethanol of extract CA was necessary due to the reported content of cyanide in the leaf, which could be harmful when consumed. Due to the fact that liver serves as detoxifier in the body it is imperative to investigate it for possible damage. In this study the leaves were oven dried at 40°C before extraction hence the ethanol extract of CA was not acutely toxic with LD50 of 3808 mg/kg. Akachukwu et al. [27] reported 0.003 ± 0.01% of cyanide in the aqueous leaf extract of CA and this is too low to cause toxicity. At different doses of 125, 250 and 500 mg/kg of CA administration for 28 days, there was no physical signs of toxicity nor mortality. Kidneys are saddled with the responsibility of filtration, metabolism as well as excretion of compounds. Probable damages include test-article-induced lesions, spontaneous renal lesions and an age-related disease of rodents namely Chronic Progressive Nephropathy (CPN) [42]. The kidneys of rats administered with CA extract were seen to be intact with no lesions at all doses administered. Section of the kidney showed normal histo-architecture of a renal tissue revealing glomeruli, tubules, interstitium and corpuscles (Figure 5A-D). This thus means that the ethanol extract of C. aconitifolious is safe for long term use and does not pose any danger to the kidney.
The liver filters and detoxifies harmful substances such as alcohol, drugs, solvents, pesticides, and heavy metals from the blood [43]. Liver is an organ of the body which is involved in many metabolic functions thus it is prone to injuries induced from these activities [44]. In this study there were no structural alterations in the liver. Histological sections of the liver of wistar rats administered with 125, 250 and 500 mg/kg body weight doses of CA extract showed normal liver tissue composed of hepatocytes, portal vein, hepatic artery and interlobular bile duct portal (triad) as it is in the negative control group (Figure 6E-H). The results of both histopathology and hematology suggested that the extract had no adverse effects on the biosynthetic abilities of the liver.
The spleen initiates immune reaction to blood-borne antigens used for filtering the blood of foreign material as well as old damaged red blood cells. The functions are achieved by the two main compartment of the spleen, the white pulp and the red pulp. Enlarged spleen causes great pain as in the case with SCD. In this study the structures of the white pulp as well as the red pulp were not altered by the CA extract administered at 125, 250 and 500 mg/ml body weight dose levels in Wistar rats. Normal histological features of the splenic tissue with areas of white pulp, red pulp and septum were clearly distinguishable (Figure 7I-L).
The ethanol extract of C. aconitifolius, a nutritious vegetable, has been shown for the first time to increase MCV of sickle cells in vitro thus making them more deformable and to reduce the formation of the poorly deformable dense cells that accumulate at the onset of vaso-occlusive crisis. CA also inhibits potassium efflux thereby preventing dehydration of red cells and can serve as an inhibitor of the Gardos channel. CA extract did not show any adverse effect on Wistar rat organs hence safe for consumption.
Authors have no conflict of interest.
M.C.C: Conceptualization, data curation, formal analysis, investigation, and writing-original draft; NOA: Experimental design, Supervision, writing-review and editing; T.A: Investigation, Data collection, analysis and manuscript writing; I.A.O: Methodology, supervision, writing-review and editing; RAB: experimentation, visualization, and Data analysis; JMA: Supervision, writing- review and editing.
Citation: Cyril-Olutayo MC, Akinola NO, Temitope AA, Idris AO, Bejide RA, Agbedahunsi JM (2020) In vitro Antisickling and Sub Chronic Toxicity Studies of the Ethanol Extract of Cnidoscolus aconitifolius in Wistar Rats. Med Aromat Plants (Los Angeles) 9: 344. doi: 10.35248/2167-0412.20.9.344
Received: 14-Feb-2020 Accepted: 03-Mar-2020 Published: 10-Mar-2020 , DOI: 10.35248/2167-0412.20.9.344
Copyright: © 2020 Cyril-Olutayo MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.