Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2013) Volume 4, Issue 1
Keywords: Genotoxicity; Diabecon and mahalaxmivilas ras; Ayurvedic preparations; Comet assay; DNA damage; lymphocyte
Genotoxicity describes a deleterious action on a cell’s genetic material regardless of the mechanism by which the change is induced. Genotoxins like aromatic amines are responsible for causing mutations because of their nucleophillic nature which form strong covalent bond during formation of aromatic amine, DNA adducts and preventing the replication. Such type of changes at molecular level (in the genetic material) of organism can be detected by using various genotoxicity assay system like chromosomal aberration, sister chromatid exchange, micronucleus assay and comet assay having different end points [1]. The comet assay which is also known as single-cell gel electrophoresis test, is an useful and reliable method of detecting DNA strand breakage (double, single, and alkali-labile sites expressed as single strand breaks) in virtually any nucleated cell. Other significant advantages of the comet assay over other known genotoxicity tests (mentioned above) are its fairly straight forward technique, sensitivity, requirement for small numbers of cells (making the assay conducive to non-lethal testing) and rapid production of data [2].
Ayurvedic preparations (Drugs) for the treatment of chronic ailments have been used since time immemorial. Although no systematic pre-clinical and clinical studies on the efficacy and toxicity of these preparations are published, however such type of preparations are considered to be safe in view of clinical experience as recorded in the ancient Indian documents.
Diabecon, which is a glucose lowering agent and stimulates β-cells of the pancreas, and Mahalaxmivilas Ras were used in the present study to check the genotoxic effect on human lymphocytes. We selected these drugs for our studies due to their extensive clinical use in India.
Samples
The drugs- Diabecon and Mahalaxmivilas Ras were obtained commercially.
Chemicals
Low melting agarose (Hi Media), Normal melting agarose (Hi Media), Ethidium Bromide (Hi Media), Tris base (Hi Media), Triton X 100 (Hi Media), DMSO (Hi Media), RPMI-1640 (Hi Media).
Preparation of drug solution
The two commonly used Ayurvedic drugs i.e Diabecon, Mahalaxmivilas Ras, were selected to study their genotoxicity. The percentage solutions of drugs were prepared by dissolving 0.5 gm, 0.7 gm, 0.8 gm, 1.0 gm and 1.5 gm of the particular drug in double distilled water in order to make a final volume of 100 ml.
Experimental procedure
Fresh blood from a healthy non–smoking donor was collected with the help of a physician. The syringe was rinsed with heparin solution (75 μl /ml). The 0.2 ml blood was poured in eppendorf tube (capacity 1.5 ml) pre- filled with 0.5 ml PBS (Ca++ ion and Mg++ ion free) to final volume of 0.7 ml and gently inverted to mix. Blood was stored at 22°C up to 4 hour prior to lymphocyte isolation. Viability of lymphocytes was determined by Trypan blue exclusion analysis. Before performing cell viability test lymphocytes were incubated for 2 hours at 37°C with each drugs at 0.5%, 0.7 gm, 0.8%, 1% and 1.5 in RPMI 1640. The control group includes lymphocytes in RPMI 1640 medium as well as RPMI 1640+H2O2 (1%). The assay is based on principle that the viable cells are non permeable for the dye while the dead cells lost this membrane property and turned blue. The peripheral blood lymphocytes obtained form each blood sample was mixed with 0.7% low melting point agarose dissolved in Phosphate-Buffered Saline and casted to frosted microscope slides pre-coated with 1% normal melting agarose. The cells were then lysed for 1 h at 4°C in a buffer consisting of 2.5 M NaCl, 100 mM EDTA, 1% Triton X-100, 10 mM Tris, and pH 10. After lysis, the slides were placed in an electrophoresis unit, allowing DNA to unwind for 20 min, in the electrophoretic buffer consisting of 300 mM NaOH, 1 mM EDTA, pH>13. Electrophoresis was conducted at ambient temperature of 4°C for 25 min at electric field strength 18 V (0.7–1.0 V/cm) 300 mA [3]. The slides were then neutralized with 0.4 M Tris, pH 7.5, stained with 75 μl Ethidium bromides and covered with cover slips. To prevent an additional damage all the steps described above were conducted under dimmed light. The objects were observed at 400x magnification in a fluorescence microscope. For DNA damage analysis, 100 cells were scored per slide. Scored at least two slides per sample and 25 comets per slides using image analysis software attached CCD camera with fluorescent microscope having specific filters. Visual scoring of comet cells (at least 100) can be done by oculometer and categorizing them in to 4 classes and giving them the score 1-4. (Class IV indicates more than 80% damage; [4] (Figure 1).
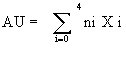 (1)
(1)
Where ni is the number of cells with damage degree i (0, 1, 2, 3 or 4).
The Ayurvedic drugs Diabecon and Mahalaxmivilas Ras induced genotoxicity assessment in human lymphocytes in vitro results are discussed as under.
Assessment of viability test
The results of in vitro treatment of human lymphocytes with different concentration of Diabecon and Mahalaxmivilas Ras are presented in chart 1. The sub-lethal concentrations for of Diabecon and Mahalaxmivilas Ras were determined as 0.8%, 1%, 0.5%, 0.7% and 1.5% (w/v) aqueous solutions. All the tested drugs caused a concentration dependent decrease of cell viability.
Assessment of genotoxicity
Single Cell Gel Electrophoresis or Comet assay: By treating the lymphocytes culture in RPMI 1640 medium with various sub-lethal concentrations of each Ayurvedic drug Diabecon and Mahalaxmivilas Ras the extent of DNA damage was determined. The results obtained by comet assay (single cell gel electrophoresis) are presented in chart 2. Chart 2 indicates that comet tail length is greater in Diabecon as compared to other drugs. The longest comet tail length was observed with positive control treated with H2O2. The comet tail length observed was 3.5 ± 1.6 μm which was at 0% concentration of H2O2 (negative control), and the comet tail length was 94.3 ± 4.3 μm in positive control. The longer tail length observed was 54.6 ± 9.1 μm which was at 0.8% concentration of Diabecon and the tail lengths of Mahalaxmivilas Ras were 37.3 ± 13.4 μm.
Coefficient of variation and standard deviations are presented in chart 2. It shows the possible variations in observation of these 2 Ayurvedic drugs. The coefficient of variation shows the comparative accuracy of the data with respect to these two drugs tested. It was found that Diabecon was more consistent as compared to other drug. Diabecon is a glucose lowering agent that generally stimulates the beta cells of the pancreas in the human body. Mahalaxmivilas Ras is very efficacious in all kinds of diseases of head and nose and ranked 2nd position in order of decreasing genotoxicity. The comet assay is presently used world wide in pharmaceutical industries for preliminary screening of drugs and genotoxicity assessment of heavy metal contaminations in various test species. This assay requires fewer samples and is cost-effective. It detects DNA damages in the form of Single and double-strand breaks and alkali-labile sites (ALS).
We wish to thank to Department of Biological Sciences, R.D.V.V., Jabalpur (M.P.) for providing the necessary infrastructure to relate with my work.