Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Letter - (2013) Volume 4, Issue 4
Chemical peeling results in a defect in the epidermal and dermal barrier. Therefore there is a possibility that chemical peeling may increase the risk for allergic contact dermatitis. In particular, from the viewpoint of allergic contact dermatitis, one should be cautious of peeling agents which destruct the epidermal and dermal barrier, penetrate deeply into the skin and increase the opportunity to interact directly with antigen presenting cells. However, to our knowledge, experimental trials on the skin sensitizing properties of peeling agents as a group have not been reported yet. In this study, we evaluated the skin sensitizing properties of five peeling agents, lactic acid, glycolic acid, trichloroacetic acid, salicylic acid and phenol, using the Human Cell Line Activation Test (h-CLAT), which is an in vitro test method. Lactic acid and glycolic acid were judged to be “non-sensitizers” by this test method. In addition, there are no reports of allergic contact dermatitis for them in clinical practice. Therefore, it can be concluded that they are quite safe as peeling agents from the aspect of allergic contact dermatitis. Trichloroacetic acid, salicylic acid and phenol were defined as “sensitizers” in this study. And there are a few case reports of allergic contact dermatitis for salicylic acid. It is very rare, but one should be aware that salicylic acid has a very weak sensitizing potential. Although it is unlikely that trichloroacetic acid and phenol are “sensitizers”, in fact, one should be aware that they have the ability to augment CD54 and CD86 expression, which are co-stimulatory molecules, on antigen-presenting cells. One should make a careful choice of nonsensitizing chemicals as a chemical peeling agent, since the skin is always exposed to the peeling agents under the unusual circumstance of losing its barrier functions during the chemical peeling procedure.
<Keywords: Chemical peeling; Allergic contact dermatitis; CD54; CD86; THP-1; h-CLAT
ACD: allergic contact dermatitis; h-CLAT: The Human Cell Line Activation Test; MHC: Major Histocompatibility Complex; LLNA: Local Lymph Node Assay; LA: Lactic Acid; GA: Glycolic Acid; TCA: Trichloroacetic Acid; SA; Salicylic Acid; DMSO: Dimethyl Sulfoxide; CV75: estimated concentration in μg/mL resulting in 75% cell viability; RFI: The Relative Fluorescence Intensity; MFI: Geometric Mean Fluorescence Intensity
Chemical peeling is a treatment method for some skin disorders such as acne and solar lentigines, and for aesthetic improvements to rejuvenate the skin [1,2]. The basic concept of chemical peeling is to destroy portions of the epidermis, and on occasion the dermis, with subsequent regeneration of the tissues based on wound healing. Chemical peeling results in a defect in the epidermal and dermal barrier, and gives potential contact sensitizers a greater opportunity to interact with the cutaneous immune system. Therefore, there is a possibility that chemical peeling may increase the risk for allergic contact dermatitis (ACD). In fact, Jung et al. previously investigated the relationship between chemical peeling and ACD [3]. In particular, from the viewpoint of ACD, one should be cautious of peeling agents which destruct the epidermal and dermal barrier, penetrate deeply into the skin and increase the opportunity to interact directly with antigen presenting cells. However, to our knowledge, experimental trials on the skin sensitizing properties of peeling agents as a group have not been reported yet.
In this study, we evaluated the skin sensitizing properties of peeling agents using the Human Cell Line Activation Test (h-CLAT), which is an in vitro skin sensitization method based on the augmentation of CD86 and CD54 expression in THP-1 cells (human monocytic leukemia cell line) [4,5]. It has been recognized that Langerhans cells play a pivotal role in the skin sensitization process because of their antigen processing and presentation abilities. Following antigen processing, Langerhans cells become mature dendritic cells with substantial phenotypic changes that include augmentation of class II major histocompatibility complex antigen (MHC class II), co-stimulatory molecules such as CD40, CD54, CD80 and CD86 [6,7]. The augmentation of these costimulatory molecules are considered to be necessary for dendritic cells to move through the dermis to the regional lymph nodes and interact with naïve T cells to induce a subset of sensitized T cells. Several studies have shown that skin sensitizers, not irritants enhance the expressions of these molecules on dendritic cells or dendritic-like cell lines [8-12]. Based on the above mechanism, h-CLAT, an in vitro test method for predicting skin sensitizing potential of chemicals was developed [4,5,13]. Yoshida et al. [12] and Sakaguchi et al. [5,13] indicated that measuring CD54 and CD86 expressions on THP-1 cells is the most suitable combination as prediction markers for the h-CLAT. The high predictive accuracy of the h-CLAT as compared with human data or Local Lymph Node Assay (LLNA) data has already been demonstrated [14,15]. The peeling agents destruct the epidermal and dermal barrier during chemical peeling, as the skin condition during the procedure make it easier for the peeling agents to directly contact with the antigen presenting cells than under normal skin condition. Due to this reason, we considered that the h-CLAT that measures the augmentation of co-stimulatory molecules on antigen presenting cells is an efficient test method to examine the skin sensitizing properties of chemical peeling agents and implemented the study.
THP-1 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in RPMI 1640 medium (Nissui Pharmaceutical Co., Tokyo, Japan) supplemented with 10% (v/v) fetal bovine serum (SIGMA-ALDRICH, St. Louis, MO, USA), 0.05 mM 2-mercaptoethanol and 1% penicillinstreptomycin solution (Invitrogen Corp., Carlsbad, CA, USA). Lactic acid (LA, SIGMA-ALDRICH), glycolic acid (GA, SIGMAALDRICH), trichloroacetic acid (TCA, Kanto Chemical Co., Inc., Tokyo, Japan), salicylic acid (SA, Kanto Chemical Co., Inc.) and phenol (SIGMA-ALDRICH) were all evaluated by the h-CLAT. LA, GA and TCA were first dissolved in normal saline, and SA and phenol were dissolved in dimethyl sulfoxide (DMSO, SIGMA-ALDRICH). The final concentration of DMSO in the culture media was less than 0.2%. In addition, 4 μg/mL dinitrochlorobenzene (DNCB, SIGMA-ALDRICH) as a positive control and 50 μg/mL sodium lauryl sulfate (SLS, SIGMAALDRICH) as a negative control were evaluated on the h-CLAT.
THP-1 cells were cultured in 24-well plates (1.0×106 cells/mL/well) with various concentrations of chemicals for 24 h. The cells were washed twice with phosphate buffer saline (PBS) containing 0.1% bovine serum albumin (BSA, Wako Pure Chemical Industries, Ltd, Osaka, Japan) (FACS buffer), stained with propidium iodide (PI, 0.625 mg/mL, SIGMA-ALDRICH), and then the cell viability was measured by flow cytometry (FACS Calibur Cell Quest, Becton Dickinson, San Jose, CA, USA). The CV75 (CV75: estimated concentration in μg/mL resulting in 75% cell viability) value for each test chemical was then calculated. THP-1 cells were plated at 1.0×106 cells/ml, and were treated for 24 h with each chemical. The dose for each chemical was set at the CV75 value determined previously. After treatment, a Fc receptor blocking procedure was performed: 0.01% globulins, and Cohn fractions II & III (Sigma-Aldrich) was added to the THP-1 cells for 10 min at 4°C. Next, cell immunostaining was performed using the following FITCconjugated monoclonal antibodies (mAbs): anti-human CD54 (clone; 6.5B5; DAKO Glostrup, Denmark), anti-human CD86 (clone; Fun- 1; BD-PharMingen San Diego, CA, USA) and FITC labeled-mouse IgG1 (clone; DAK-G01; DAKO) according to the manufacturer’s recommended conditions. FITC labeled-mouse IgG1 was used as an isotype control. The cells were incubated with these mAbs for 30 min at 4°C. After washing and re-suspending in FACS buffer, the fluorescence intensities of the THP-1 cell surface markers were analyzed by flow cytometry. A solution of 0.625 μg/mL PI was used to gate out any dead cells, and a total of 10,000 living cells were analyzed. The experiments for each chemical were conducted three times.
The Relative Fluorescence Intensity (RFI) was used as an indicator of CD86/CD54 expression, and was calculated by the following formula:
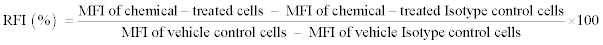
where MFI=Geometric Mean fluorescence intensity.
RFI values above 150 and 200 for CD86 and CD54 expression, respectively, were considered to represent a positive response following exposure to a chemical. When the cell viability was less than 50%, the data at that concentration were excluded from the analysis. The reason for this is because diffuse labeling of the cytoplasmic structures will occur due to cell membrane destruction, and this interferes with the fluorescent measurements [16].
We measured the RFI values for CD86 and CD54 expression following exposure to five kinds of peeling agents. The RFI values for CD86 and CD54 expression and the cell viabilities for each agent are shown in Figures 1A-1E. We defined the result as positive (a sensitizer) if either the RFI of CD86 or CD54 was more than 150% or 200%, respectively, and as negative (a non-sensitizer) if both RFIs for CD86 and CD54 were always below the cut-off. The results for the skin sensitization potential measured by h-CLAT and the presence or absence of ACD case reports for the five agents are summarized in Table 1.
Figure 1: Average RFI values and cell viability with each peeling agent. The RFI values for CD86 and CD54 expression on THP-1 cells were measured after exposure to the peeling agent. We defined the agent as a sensitizer if either the RFI of CD86 was equal to or more than 150% or the RFI of CD54 was equal to or more than 200%. It was defined as a non-sensitizer if both RFIs of CD86 and CD54 were below the criterion. The results are shown as means ± SD from three independent experiments with (A) LA, (B) GA, (C) TCA, (D) SA, and (E) phenol. ●=CD86; ■=CD54; ▲=Viability. Red dotted line=criterion for CD86 positivity; blue dotted line: criterion for CD54 positivity.
| Peeling agent | h-CLAT | Case reports about ACD | ||
|---|---|---|---|---|
| CD86 | CD54 | Judgement | ||
| Lactic acid | – | – | Negative | None |
| Glycolic acid | – | – | Negative | None |
| Trichloroacetic acid | + | + | Positive | None |
| Salicylic acid | – | + | Positive | [17,18] |
| Phenol | + | + | Positive | None |
Table 1: Results of the h-CLAT and the presence or absence of ACD case reports on the peeling agents.
LA and GA did not affect CD86 or CD54 expression at any dose, and were judged to be “non-sensitizers” by the h-CLAT. In addition, there are no reports of ACD for LA and GA in clinical practice to the extent of our research. Therefore, it can be concluded that LA and GA do not have any sensitization potential, and they are quite safe as peeling agents from the aspect of ACD.
SA-enhanced CD54 expression, and was defined as a “sensitizer”. There are a few case reports of ACD for SA [17,18]. It is very rare, but SA can provoke ACD and has a very weak sensitizing potential. It is thought that the h-CLAT detected this very weak sensitizing potential of SA.
TCA and phenol enhanced CD54 expression dramatically, and also enhanced CD86 expression. The h-CLAT defined these chemicals as “sensitizers”. However, there are no case reports of ACD to our knowledge. In addition, these chemicals have been used safely with respect to ACD as peeling agents for a long period of time. Although it is unlikely that TCA and phenol are “sensitizers”, in fact, one should be aware that they have the ability to augment CD54 and CD86 expression, which are co-stimulatory molecules, on antigen-presenting cells.
Chemical peeling agents are typically used at very high concentrations, and penetrate deeply into the epidermis and occasionally into the dermis during the peeling procedure. One should make a careful choice of non-sensitizing chemicals as a peeling agent, since the skin is always exposed to the peeling agents under the unusual circumstance of losing its barrier functions during the procedure.
We thank Dr. Takao Ashikaga and Mr. Hitoshi Sakaguchi for their technical advice.