Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2020)Volume 12, Issue 4
Human Cytomegalovirus is a ubiquitous opportunist pathogen capable of infecting newborns, immune compromised and immunosuppressed patients. Till date only a limited number of clinically approved drugs are available for treatment. All these drugs prove to be very effective against active HCMV infection but with their respective share of drawbacks. Anti-viral agents derived from natural compounds are the need of the hour. They have potent anti-viral property but lack the cytotoxic effect associated with their synthetic counterparts. In this regard the medicinal mushrooms have proven to be quite an interesting candidate of choice. We collected and isolated extracts from four different edible mushroom species and used them on in vitro cultured MRC5 and 1B4 cell lines. Cytotoxicity and anti-viral response for each mushroom was tested in a dose dependent and time dependent fashion. Among the four mushroom types, only Pleurotus sp. and Lentinus sp. were found to have potent anti-viral response with minimum cytotoxicity against human cytomegalovirus. Their crude extracts showed 100% inhibition of HCMV replication at 180 μg/ml and 160 μg/ml respectively. Our study highlights that extracts from both of these mushrooms can be used as alternative HCMV therapeutic strategy.
Human cytomegalovirus; Natural compounds; Medicinal mushrooms; Cytotoxicity; Anti-viral response
Human cytomegalovirus (HCMV) is a double strand DNA virus, member of the beta- herpesvirideae capable of causing life threatening infections in immunosuppressed patients and in immunocompromised individuals such as individuals suffering from AIDS. HCMV can also cause severe congenital birth defects in neonates [1,2]. Furthermore, HCMV infection is asymptomatic or mildly symptomatic in immunocompetent hosts, even if in these subjects it represents a risk factor for the development of immunosenescence and vascular diseases [2,3]. To date, no vaccine is still available, and to treat HCMV infections there is a limited number of commercial drugs like ganciclovir, valganciclovir, cidofovir, foscarnet and recently approved letermovir [4]. In neonates primary toxicity of intravenously administrated ganciclovir shows moderate to severe neutropenia after six weeks of treatment [5,6]. AIDS patients associated with symptomatic HCMV retinitis, can develop severe thrombocytopenia, drug fever, neuropathy after prolonged treatment with ganciclovir [7]. Not only long term uses of these drugs yield several drug resistant HCMV strains but also create a huge problem in disease management. Current study reveals that mutations in HCMV DNA polymerase protein UL54 and in the kinase UL97 are strongly responsible for the development of drug resistant HCMV strains. Several nucleotide polymorphism in UL54 gene produces ganciclovir and cidofovir resistant HCMV strains [8,9]. Due to such limitations natural compounds extracted from medicinal herbs are the best solution for this new-aged antiviral therapy. For this reason, several mushrooms and their constituents have been tested for their antiviral potentials. They could inhibit the activity of several viral enzymes, responsible for synthesis of viral nucleic acids or adsorption and uptake of viruses into mammalian cells [10]. Several bioactive compounds isolated from Ganoderma lucidum, Coriolus varsicolor, Schizophyllum commune, Lentinus edodes mushrooms showed antiviral activity against HIV, herpesvirideae [11]. Ganoderiol F and godermanontriol are the two major bioactive terpenoid extracted from Ganoderma lucidum inhibit HIV-1 induced cytopathic effect in MT4 cell line [12]. Ganodermadiol isolated from Ganoderma lucidum shows antiviral response against Herpes simplex virus type-I [13]. Protein bound polysaccharides from Trametes versicolor were also found to be showing promising antiviral response against HIV and cytomegalovirus in vitro [14]. Recent findings suggest a bioactive plant-derived alkaloid, berberine (BBR), and plant-derived flavonoid deguelin (DGN) can inhibit HCMV viral lytic replication in fibroblast cell lines [15-17]. The purpose of this study was to explore and identify naturally occurring medicinal mushrooms with significant anti-cytomegaloviral properties.
Isolation and preparation of mushroom extract
Fruit bodies of two non-edible wild mushrooms (Polyporus alveolari, Phellinus sp.) and two edible wild mushrooms (Pleurotus ostreatus and Lentinus squarrosulus) were collected during the rainy season from different parts of West Bengal, India and transported to the laboratory by aseptic biodegradable polythene bags. Morphological identification of the collected mushrooms was followed by standard taxonomic keys of basidiomycota [18,19]. The surfaces of the collected basidiocarps were cleaned by running tap water and air dried for 24-48 h. The cleaned and dried fruit bodies then crashed and powdered in Wiley mill (Wiley India Pvt.Ltd, Kol.01, India) and sieved by 60 mash screen. The extraction from test fungi was carried out employing the modified methods of Mizuno et al. and Wang et al. [20,21]. The powdered basidiocarp (10 g) were treated with 200 ml of 80% methanol (1:20 ratio) repeatedly refluxed and heated at 60°C ± 2°C temperature for 6 h. The extracted materials were collected and again treated in rotary evaporator at 56°C ± 2° for 3 h to remove the remaining traces of organic solvent. The semi dried extracted materials were collected in fresh autoclaved centrifuge tube and preserved at 4°C.
Human cell culture and virus stock preparation
Human Lung Fibroblast cell line MRC5 (ATCC/CCL/344- USA) was maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with L-Glutamine, FBS (10%) streptomycin (100 μg/ml) and Penicillin (100 IU/ml). For cell based assay, U373-pUL112-113 (clone 1B4) cells, which stably express EGFP under the control of the IE2-dependent early UL112-113 promoter of HCMV, had been cultured in DMEM (10% FBS) supplemented with G418(750 μg/ml) was a gift from Dr. Anna Luganini (University of Turin) [22]. Laboratory strain of Human Cytomegalovirus AD169 was also purchased from ATCC (VR-538) and cultured in MRC5 cell line in 5% CO2 incubator until a marked cytopathic effect was observed. Further purification of the virus had been done by ultracentrifugation of the cell, and culture filtrate and the stock were preserved in -80°C for future use.
Cytotoxicity determination of the mushroom extract
Prior to testing the cytotoxicity, the semi dried each extract was dissolved in 0.1% DMSO for better solubility. For cytotoxicity assay MRC5 cells (5 X 104 cells/well) and 1B4 cells were seeded in 96 wellplate and incubated at 37°C in 5% CO2 for 24 h. After the incubation, cells were washed with 1X PBS, different concentrations of mushroom extract were applied in triplicate at a final DMEM concentration 100 μl/well and again incubated at 37°C in 5% CO2 for 5 days. Triton (0.1%) reagent was used as a negative control. After 5 days 10 μl of MTT reagent (Hi-Media EZcount) was added in each well. After 5 h incubation in 37°C purple colour formazan crystals formed were dissolved with 1% DMSO solution. The absorbance was measured in ELISA reader (SpectraMax M2) at 570 nm. The cell viability was measured by [(Am - Ab ) / Cm ] × 100% formula, where Am is the mean absorbance of each dilution and Ab is cell free blank, Cm is mean absorbance of untreated control respectively [23].
Antiviral assay by MTT reagent and EC50 determination by dose dependent assay
Antiviral activity of the selected mushrooms were carried out using standard Laboratory Human Cytomegalovirus strain AD169 on MRC5 cell line.MRC5 cells were seeded in (4 X 104 cells/well) 96 well plate and incubated at 37°C for 24 hrs. The day after, the wells were washed with 1X PBS and infected with AD169 strain (MOI of 0.5 PFU/cell) in serum free medium for 6 hrs at 37°C. Then, the viral inocula were removed and the cells treated with different concentration of mushroom extracts (1 mg/ml-0.1 μg/ml) in triplicate. Plates were incubated in 5% CO2 at 37°C for 5 days. After incubation the viability of the cells i.e effective concentration of the mushroom extracts were measured by MTT reagents according to manufacturer ’ s protocol. Effective concentration had been calculated as: [(Ai+t- Ai)/(Ac-Ai)] x 100%. Where Ai+t is the absorbance of compound treated infected cell and Ai and Ac are absorbances of untreated infected cells, absorbance of untreated uninfected cells, respectively [24]. 50% effective concentration of the extracts had been determined and it also represents the 50% reduction of virus induced cell mortality.
Qualitative estimation of EGFP expression in cell based assay
For qualitative cell based EGFP expression study, 1B4 cell lines were seeded in 12 well plates and incubated in 5% CO2 incubator for 24 hrs. Next day the cells were infected with HCMV AD 169 strain (MOI of 0.5 PFU/cell) for 6 h and after that freshly prepared DMEM (10% FBS) supplemented with G418 (750 μg/ml) had been added and treated with the previously determined EC50 of Pleurotus sp (PE) and Lentinus sp (LE) extracts. EGFP expression had been examined after 72 hours post infection (hpi) under inverted fluroscence microscope (Zeiss 4.1) to measure the number of infected cells.
Viral load determination by real time PCR
To analyze the effect of mushroom extracts on viral replication, MRC5 and 1B4 cell lines were infected with HCMV AD169 at MOI of 0.5 PFU/cell and, following virus adsorption (6 h at 37°C), were incubated with effective concentration of the PE and LE. HCMVinfected and treated cells were then collected at h.p.i., and total DNA was isolated by using Nucleopore DNA Sure Blood Mini kit(#NP-61105) and viral load was quantified by quantitative real time PCR using following forward primers of HCMV glycoprotein gH (5’CGTGGAAGATGACCGAAGAT3’) and reverse (5’ATCGGCCACACTTTAACCAG 3’) using SYBR green real time master mix (HiMedia). A 25 μl of total volume of reaction mixture was made with 2 μl of DNA, 12.5 μl of 2x master mix containing SYBR green gH forward/reverse primers and water. The cycling conditions were as follows, denaturation at 94°C for 5 mins, followed by 35 cycles of 94°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds and final extension at 72°C for 7 mins.
Time dependent antiviral response of mushroom extracts
MRC5 cells were seeded in 6 well plates (1.2 X 106 cells/well) and incubated in 5% CO` incubator for 24 hrs. After the incubation cells were infected with HCMV AD169 strain (MOI of 0.5 PFU/ml) and kept 6 h for complete viral absorptions in serum free DMEM. Then infection media was replaced by freshly prepared DMEM supplemented with 10% FBS. After that EC50 concentration of PE, LE and ganciclovir as positive control were added. Plates were incubated at 37°C in 5% CO2. At different time interval virus infected cells were harvested (12,24,36,48,60,72 hpi) and total viral DNA has been extracted using Nucleopore DNA Sure Blood Mini kit and total viral load has been estimated using previously described primers of HCMV glycoproteins gH. Percentage of viral load inhibition was calculated as follows: 100 -[(Vt / Vc ) x 100], where Vt and Vc represents viral copy number in treated cells and untreated control cells in particular hpi.
Cytotoxic activity of the mushroom extract
The cytotoxic activity of Pleurotous sp. (PE) and Lentinus sp. (LE) extract showed less cytotoxicity than Phellinus sp (PhE) and Polyporus sp. (PoE). PE extract showed 50% cytotoxicity (CC50) at 502.12 μg/ml on MRC5 cell line and 822.28 μg/ml on 1B4 cell line. Moderate cytotoxicity CC50 has been observed in LE. CC50 value of LE in MRC5 cell line was 429.61 μg/ml and 468.07 μg/ml in 1B4 cell line. Whereas PhE and PoE show high cytotoxicity on both MRC5 and 1B4 cell lines. CC50 value of PhE was 39.08 μg/ml on MRC5 cells and 40.43 μg/ml on 1B4 cell line respectively. For PoE the CC50 values on both MRC5 and 1B4 cell lines were 53.43 μg/ml and 50.54 μg/ml. For further study due to less cytotoxicity of Pleurotous sp. and Lentinus sp mushrooms were chosen to check if there is any antiviral activity against HCMV over high toxicity of Polyporus sp and Phellinus sp.(Figure 1).
Figure 1: Image of (a) Pleurotus ostreatus and (b) Lentinus squarrosulus on their natural habitat.
Antiviral response
Antiviral effect of PE and LE were evaluated by using MTT assay on MRC5 cell line. EC50 value of PE was 80.39 μg/ml and 69.48 μg/ml in LE respectively. The selectivity index [(S.I)=CC50/EC50] of these two extracts were 6.24 for PE. and 6.18 for LE. Due to very High CC50 values no significant antiviral response had been observed in case of Phellinus sp and Polyporus sp extracts. The detailed analysis has been shown in Table 1.
| CC50 value* | Antiviral activity (EC50)* |
Selectivity index (S.I)=CC50/EC50 | ||
|---|---|---|---|---|
| Name of the Mushroom | MRC5** | 1B4** | MRC5 | MRC5 |
| Pleurotus sp.(PE) | 502.12 ± 3.34 | 822.28 ± 1.27 | 80.39 ± 1.266 | 6.24 |
| Lentinus sp.(LE) | 429.61 ± 1.59 | 468.07 ± 0.80 | 69.48 ± 1.68 | 6.18 |
| Phellinus sp.(PhE) | 39.08 ± 1.29 | 40.43 ± 1.06 | - | - |
| Polyporus sp.(PoE) | 53.43 ± 2.36 | 50.54 ± 1.56 | - | - |
*All the experiments were done in triplicate
**Mean value of mushroom extract (µg/ml) represented with S.D.M
Table 1: Cytotoxicity and antiviral activity of selected mushrooms.
Dose dependent antiviral response and EGFP expression of mushroom extract
To determine the dose dependent activity of the Pleurotus sp and Lentinus sp various concentration of extracts were used on MRC5 cells after infecting with AD169 strain. The results represent in Figure 2(a), Crude extract of Pleurotus sp and Lentinus sp both shows 100% inhibition of HCMV replication at 180 μg/ml and 160 μg/ml respectively. The EC50 Value of PE and LE was 80.39 μg/ml and 69.48 μg/ml respectively whereas EC50 value of the ganciclovir was 1.301 μg/ml represent in Figure 2(b). Qualitative estimation of HCMV induced cytopathic effect on MRC5 cell line and number of 1B4 cells expressing EGFP had been significantly reduced after treatment with EC50 concentration corresponding to 80.39 μg/ml and 69.48 μg/ml of PE and LE extracts respectively (Figure 3).
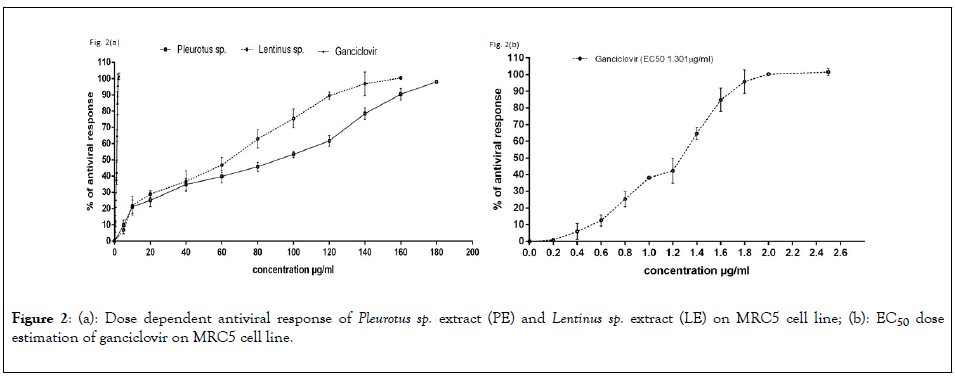
Figure 2: (a): Dose dependent antiviral response of Pleurotus sp. extract (PE) and Lentinus sp. extract (LE) on MRC5 cell line; (b): EC50 dose estimation of ganciclovir on MRC5 cell line.
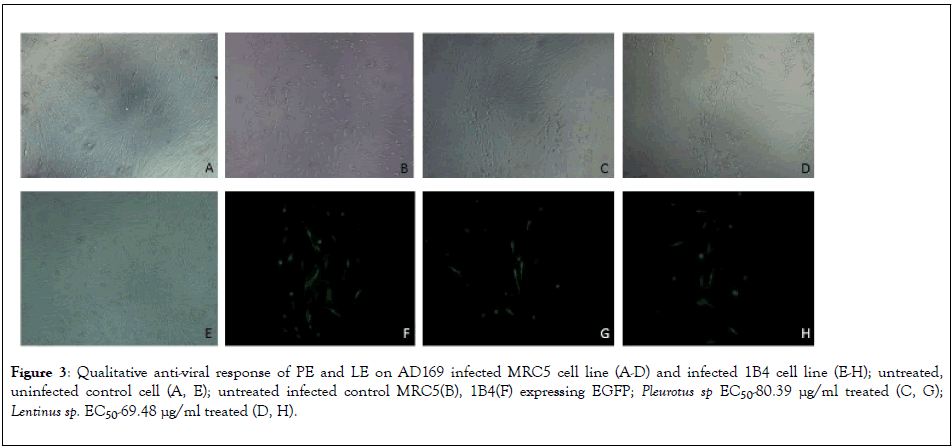
Figure 3: Qualitative anti-viral response of PE and LE on AD169 infected MRC5 cell line (A-D) and infected 1B4 cell line (E-H); untreated, uninfected control cell (A, E); untreated infected control MRC5(B), 1B4(F) expressing EGFP; Pleurotus sp EC50-80.39 μg/ml treated (C, G); Lentinus sp. EC50-69.48 μg/ml treated (D, H).
Quantitative real time analysis for viral load determination
To quantify the efficiency of antiviral activity of PE and LE on both MRC5 and 1B4 cell lines were infected with HCMV AD169 strains and the viral load was quantified after 72 hpi. Viral log copy numbers were calculated by using a standard curve for HCMV glycoprotein H gene. Viral log copy number of untreated but infected MRC5 cells and infected 1B4 cells were 5.79 and 5.48 respectively. In case of infected cells treated with PE the log viral load decreased significantly, 2.97 and 3.27 respectively for MRC5 cells and 1B4 cells respectively. LE extracts showed slightly poor response than PE extract on infected MRC5 cells (3.55) and nearly similar responses on infected 1B4 cell line (3.38) represented in Figure 4(a).
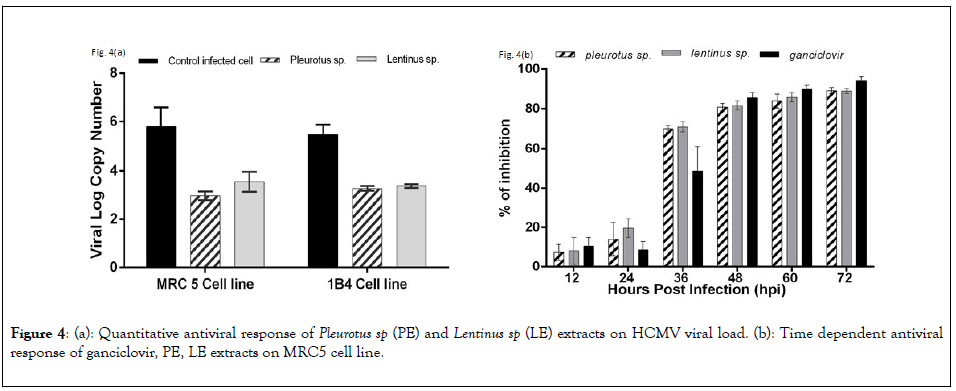
Figure 4: (a): Quantitative antiviral response of Pleurotus sp (PE) and Lentinus sp (LE) extracts on HCMV viral load. (b): Time dependent antiviral response of ganciclovir, PE, LE extracts on MRC5 cell line.
Time dependent antiviral response of PE and LE
Time dependent response of PE and LE showed significant positive response along with the standard drug ganciclovir. The percentage of viral load inhibition was calculated in each drug treated cells with the comparison to untreated infected control cells by using the following formula, [100 - ( V log CT / V log CC x 100)], where VlogCT and VlogCC was the viral log copy no of extract treated cell and untreated cells respectively. The percentage of inhibition of PE treated cases were 7.30 and 14.04 and LE treated cases 8.13 and 19.65 had been observed in 12 and 24 hpi respectively. At 36 hpi percentage of viral load inhibition of both PE and LE were 69.65 and 70.57. So this data clearly indicates that the mushroom extracts start to effectively reduce the viral propagation at an early stage of viral replication expressed in Figure 4(b).
In the present study we have demonstrated that among the four wild mushrooms (Polyporus alveolari, Phellinus sp, Pleurotus ostreatus, Lentinus squarrosulus) analyzed for their antiviral activity, only Pleurotus sp. and Lentinus sp mushrooms could be a potential source of antiviral agents against Human cytomegalovirus. Their cytotoxic activity shows distinctive response based on the cell type used. In 1B4 cell line Pleurotus sp. and Lentinus sp. mushroom extracts show lower CC50 value (822.28 ± 1.27 and 468.07 ± 0.80 µg/ml respectively) in comparison to MRC5 cell line (502.12 ± 3.34 and 429.61 ± 1.59 µg/ml respectively). This data clearly indicates that astrocytoma/glioblastoma cell lines are able to withstand the cytotoxicity of Pleurotus sp mushroom more than the human fibroblast cell line but no significant cytotoxic difference was found on both the cell lines when treated with Lentinus sp extract. Comparatively high cytotoxicity have been observed on both cell lines when treated with Phelinus sp. (39.08 ± 1.29 µg/ml in MRC5 and 40.43+1.06 µg/ml in 1B4) and Polyporus sp. (53.43 ±2.36 µg/ml in MRC5 and 50.54+1.56 µg/ml in 1B4 cell line) extract as both these mushroom contains high amount of glycosylated proteins and proteoglycans which exhibit high cytotoxicity and potential antitumor substances [25]. Phellinus linteus contains β-(1 → 3) linked glycan which exhibit anti metastasis and immunomodulation in cancer cells, Hispolon another phenolic compound extracted from Phellinus sp. also induced apoptosis in breast cancer cell line [26,27]. As well as Lectins isolated from Polyporus adusta exhibit anti-proliferative effect on cancer cells [28].
As described above, due to high cytotoxicity of Phellinus sp. and Polyporus sp. the antiviral response was studied only using the extract of Pleurotus sp. and Lentinus sp. It has been reported that mycelial extract of Pleurotus eryngii shows antiviral activities against influenza virus [29]. JLS-S001, a flavonoid fraction of Lentinus edodes blocks HSV-1 replication at the late stage of the replication cycle [30]. The EC50 value of Pleurotus sp. and Lentinus sp. were 80.39+1.26 µg/ml and 69.48 ± 1.68 µg/ml respectively clearly indicates that both these mushrooms have some distinctive antiviral chemical constituent. In order to achieve 100% efficiency of the antiviral responses both these extracts were applied in dose dependent manner where Pleurotus sp exhibit complete antiviral response at 180 µg/ml and 160 µg/ml in case of Lentinus sp extract. The selectivity index of both Pleurotus sp and Lentinus sp were 6.24 and 6.18 respectively.
HCMV UL-112/113 encodes four phosphoproteins (p34, p43, p50, p84) which enhance the Immediate Early-2 gene mediated transactivation of early gene promoters [31,32]. So, UL-112/113 transfected stable cell line 1B4 had been used to qualitative screening of anitiviral compounds from Pleurotus sp. and Lentinus sp extract by EGFP expression. Number of fluorescent cells observed in case of AD169 infected 1B4 cell line treated with 50% effective concentration of both the mushroom extracts were much less (less than equal to 13 for PE and less than equal to 12 for LE) compared to the untreated control cells (less than equal to 20). In case of MRC5 cells the number of foci is also reduced in treated cells compared to infected cells without treatment. We can hypothesize that as the mushroom extracts were added on the cells after 6 h post infection, the bioactive compounds were probably entering the cells and interfering with the effective transcription of the viral genes.
To find out whether the bioactive compounds are responsible for interacting with the viral transcriptomes, quantitative real time analysis of glycoprotein H (gH-UL-75) gene has been done. HCMV UL-75 is a late response gene which is required for complete infectious virus production and can only be expressed by the activation of IE2 genes associated with UL-79/87/95 proteins [33]. Quantification of viral load using HCMV glycoprotein H gene as reference gave log viral copy number of 5.79, 2.97 and 3.55 for untreated, treated with PE and treated with LE respectively in MRC5 cell line. Similarly, the log viral copy numbers for untreated, treated with PE and treated with LE in 1B4 cell line presented 5.48, 3.27 and 3.38 values respectively. Time dependent antiviral response showed that other than ganciclovir both these mushroom extracts show similar response to HCMV replication as a result viral load decreases in course of time. 80%-90% inhibition was achieved at 48 to 72 hpi but at 36 hpi both PE and LE strongly reduced viral load (70%-72%) in comparison to ganciclovir (50%-55%). These data clearly represent that the crude extracts of both the mushroom were able to effectively inhibit the propagation of human cytomegalovirus in vitro [34-39].
Our results shows that both PE and LE inhibit propagation of Human Cytomegalovirus, and thus, might be a potential candidate for the development of alternative natural anti-HCMV compounds. These results could improve the current knowledge on alternative antiviral therapies, although the exact anti-viral mechanism of action of PE and LE needs further investigation.
This work has been funded by West Bengal Department of Science and Technology, Memo No: 934(Sanc.)/ST/P/SandT/ 1G-10/2016 and we are very much thankful to ICMR-NICED Virus Laboratory for giving us the opportunity to do the total in vitro work and Ramakrishna Mission Vivekananda Centenary college for providing infrastructure to perform some biochemical analysis.
None declared
Citation: Roy D, Ansari S, Chatterjee A, Luganini A, Ghosh SK, Chakraborty N (2020) In vitro Search for Antiviral Activity against Human Cytomegalovirus from Medicinal Mushrooms Pleurotus sp. and Lentinus sp. J Antivir Antiretrovir.12:201. DOI: 10.35248/1948-5964.20.12.201
Received: 05-Jun-2020 Accepted: 19-Jun-2020 Published: 26-Jun-2020 , DOI: 10.35248/1948-5964.20.12.201
Copyright: © 2020 Roy D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.