Research Article - (2017) Volume 3, Issue 1
In Vitro Study of the Bacterial Anti-Bioresistance and the Use of Some Medicinal Plants in Avian therapy
*Corresponding Author: Hammadi K, Laboratory of Pharmacogonosy and Phytotherapy, University Abdelhamid Ibn Badis Mostaganem, Algeria, Tel: 213555114222 Email:
Abstract
This study addresses the problem of pathogenic bacteria resistant to antibiotics isolated from infected chickens and the use of medicinal plants and their bioactive substances in vitro.
On 75 strains isolated from a dead chickens belonging to the three families the Enterobactereaceae; the Staphylococeae; the Pseudomonaceae bacteria. The antibiogram tests were used to select ten resistant bacteria strains in this study.
Ethonobotanical study was done to select the most medicinal plants used for the therapy of animals in Algeria. The essential oils of six medicinal plants were extracted by Hydro distillation (Clevenger). The plants with their yields in essential oils are: Thymus vulgaris (2.75%), Salvia officinalis (2.50%), Rosmarinus officinalis (2.43%), Thymus capitatus (1.82%), Ruta chalepensis (0.93%), Artemisia herba alba (0.90%).
By measuring the activity of the oils on agar medium, this test has provided the following results: The essential oils of different plants gave the diameters of inhibition zones between 0 mm and 53.33 ± 1.53 mm, for the 5 μl discs, and between 0 mm and 52.33 ± 2.52 mm, for the 10 μl discs, while discs of 15 μl, those diameters vary between 0 mm and 56.67 ± 1.15 mm.
The results of MICS of oils studied are encouraging, oils of Thymus capitatus, Rosmarinus officinalis and Salvia officinalis share a minimum inhibitory concentration (MIC) between 1.25 and 20 (μL.mL-1) with an effect bactericidal/ bacteriostatic, except the oil Salvia officinalis presents a bactericidal effect. The oils of Thymus vulgaris, Artemisia herba alba and Ruta chalepensis have a MIC respectively of 1.25 to 10 (μL.mL-1), 5 to 40 (μL.mL-1), 1.25 and 40 (μL.mL-1). The effect is bactericidal effect for these oils.
Keywords: Essential oils; Antibacterial activity; Bacterial antibioresistance; Minimum Inhibitory Concentration (MIC)
Introduction
Algeria is a country of the North of Africa; recently indicated a worrying situation of antibiotic resistance, these last ten years have been trade mark by the emergence and dissemination of new resistance genes in particular in the north of the country [1]. Any use of antibiotics, either for the man, animal, plant or the technology of food processing, is likely to lead to a certain point in time, a bacterial resistance. Although many publications are beginning to appear, little is known on the different conditions of use in which the antibiotics preferably select, or select to a lesser extent, for bacteria resistant [2]. Bacterial resistance to antimicrobial agents is a problem of increasing importance in medical practice [3]. The history of aromatic and medicinal plants is associated with the evolution of civilizations. In all regions of the world, the history of peoples shows that these plants have always occupied an important place in medical therapy [4].
The 1990s of the last century have been marked by a general awareness in favor of the health of the Man and of the quality of the environment. Organic agriculture, the herbal medicine and the aromatherapy have sparked a renewed interest for the culture of aromatic and medicinal plants for use in a fresh, dried or in the form of an extract. The world demand for aromatic medicinal plant and their derivatives for the Agri-Food, phytotherapy, perfumes and natural cosmetic products have in fact increased. The aromatic medicinal plant, in the developing countries of Asia, Africa and Latin America, play an important role in the traditional pharmacopoeia and the power [5]. In phyto-therapy, essential oils are used for their antiseptic properties against infectious diseases of bacterial origin [6].
Our work is part of a contribution to the valorization of medicinal plants widely used by traditional Algerian breeders. To this end, our study encompasses two aspects, the first of which is based on microbiological character, isolation, identification and susceptibility. The second aspect of extraction, screening, antimicrobial activity.
Materials and Methods
The strains of germs causing respiratory diseases, digestive and nutritional status remain a major problem In the chicken farming sector, in addition to therapeutic failure in the face of the antibiotics. In this sense the pathogenic bacteria causing these diseases were isolated and identified. The levies are made according to the recommendations of the organization of International Epizootics (O.I.E). The chickens died recently were necropsied. Macroscopic observation aid has distinguished the infected body and that the return to the parameters of aspect, colors, the smell and sometimes presences of stains. The macroscopic examination of tissues and organs in order to detect possible changes lésionnelles. It is including the liver, heart, spleen, and intestine [7].
According to Pilet C, et al. [8], identification begins with the determination of the family, then of the genus (oxidase, Catalase) and finally of the different species by the classic gallery.
Bacteria are isolated from sick and dead animals. The media were selected according to the desired bacterial groups [9]. Representative colonies were selected in a random manner and subcultured by streaking on appropriate medium. The purification of the strains was performed by repeated cultures up to the obtaining of a pure culture [10]. Gram staining was a key trait for classifying families of bacteria.
Identification of strains of Enterobacteria: Colonies representative of Enterobacteria were selected randomly and transplanted by streaking on appropriate medium. The isolated bacterial strains were characterized using the API20 E [11]. The strains were identified by comparing their characteristics with those of known taxa as described in the Bergey'sManual for Determinative Bacteriology [11].
All strains were identified using standard bacteriological methods (production of catalase and coagulase and biochemical characteristics using the API20 staph [12]. The oxidase test is the differentiating character between Pseudomonas and other Enterobacteriaceae.
The bacterial strains isolated were characterized using the biochemical Gallery tests for Staphylococcus, Pseudomonas and Enterobacter bacteria with a high frequency of contamination and sensitivity.
The sensitivity of the isolated strains to antibiotics has been determined by the method of dissemination in agar as recommended by the Committee of the sensitivity of the French Society of Microbiology [13].
Aromatogramme test
The aromatogramme is a method of in vitro measurement of the antibacterial power of chemotyped essential oils. Different types of aromatogrammes, in solid, liquid, are exploitable [14].
The choice of our plants is based on a survey conducted earlier in the axis of the Ethno-Veterinary approach of the medicinal plants used in the region of Sidi Bel Abbes-Algeria [15]. The essential oils are obtained by Hydro distillation with a device of type Clevenger [16] for a period of three hours.
A bacterial suspension of equivalent density to the Standard 0.5 of Mac Farland (108 cfu.mL-1) is prepared and then diluted to the 1/100 fortified by 10% DMSO [17].
The method of dilutions of Kirby-Bauer
Minimum Inhibitory Concentration (MIC): one proceeds to a successive dilution by Progress. The following dilutions 1/2, 1/4 1/8, 1/16, 1/32, 1/64, 1/128, 1/256 [18]. The MIC (%, v/v) of the essential oil tested is deducted from the first tube of the range devoid of bacterial growth.
Minimum concentration bactericidal (MBC): The nutrient agar poured into petri dishes is seeded with ridges by 100 μL of the contents of the tubes with a concentration\MIC in the series of previous dilution [19]. The MBC is determined after an incubation period of 24 hours at 37°C. It is the lowest concentration which inhibits totally the growth.
The MBC (%, v/v) of the essential oil is deducted from the first box devoid of bacteria (Guinoiseau, 2010). The antibacterial effect was considered bactericidal or bacteriostatic in function of the report: MBC/MIC. Indeed, if MBC/MIC=1 to 2, the effect is bactericidal and if MBC/MIC=4 to 16.
Results
The isolated bacteria
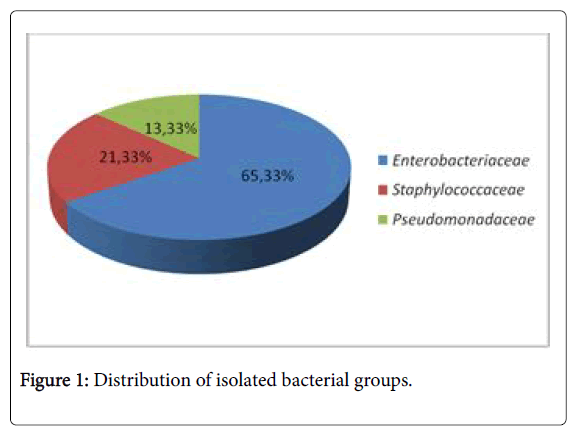
75 strains were isolated from the liver, heart, spleen, and intestine. Bacterial strains tested were the following: Enterobacteriaceae 65% (n=49), Staphylococcaceae 21% (n=16) Pseudomonaceae 13% (n=10) (Figure 1).
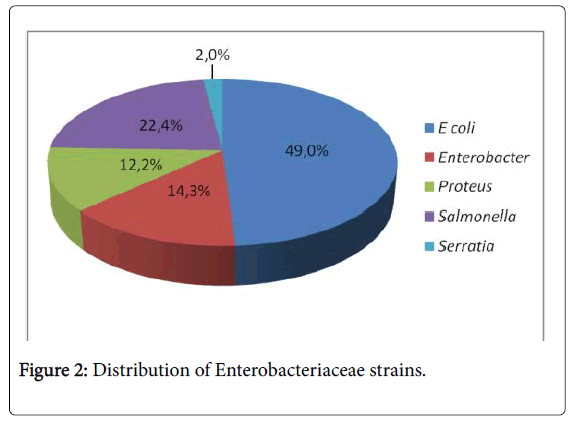
On a total of 49 strains of Enterobacteriaceae, we note E. coli 49% (n=24), Enterobacter 14% (n=7), Proteus 12% (n=6), Salmonella , 22% (n=11), Serratia 2% (n=1) (Figure 2).
Antibiogramme
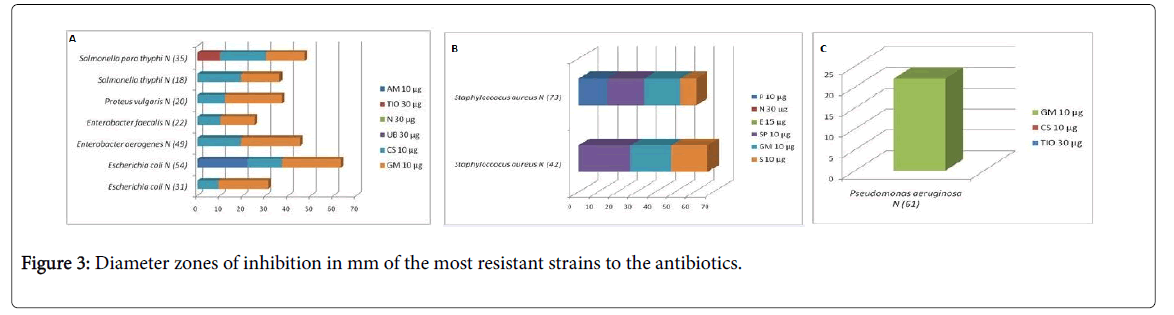
Selection of the most resistant strains: Isolates which follow this study of identification and aromatogramme are the one who presents a resistance to a many antibiotics tested (Figure 3).
Escherichia coli N (31) showed a resistance of 83.33% to all antibiotics tested except for gentamicin, whereas Escherichia coli N (54) had a 50% resistance to all the antibiotics tested except for ampicillin, Colistin and gentamicin (Figure 3).
The testicular antibiotics are ceftiofur, colistin and gentamicin. The antibiotic resistance of Pseudomonas aeruginosa N (61) is 66.66%, resistant to all antibiotics except gentamicin (Figure 3).
Enterobacter aerogenes N (49), Enterobacter faecalis N (22), Proteus vulgaris N (20), Salmonella thyphi N (18) and Salmonella para thyphi N (35) are sensitive to colistin and gentamicin at 66.66% with sensitivity to gentamicin (Figure 3).
AM: Ampiciline 10 μg; TIO: Ceftiofur 30 μg; N: Neomycine 30 μg; UB: Fluméquine 30 μg; CS: Colistine 10 μg; GM: Gentamicine 10 μg; P: Peneciline 10 μg; E: Erythromycine 15 μg; SP : Spiramycine 10 μg ; S: Streptomycine 10 μg.
The antibiotic testes are penicillin, neomycin, erythromycin, spiramycin, gentamicin and streptomycin. Staphyloccocus aureus N (24) and Staphylococcus aureus N (73) have a minimum resistance of 50%, susceptible to all antibiotics except spiramycin, gentamicin, streptomycin, While Staphylococcus aureus N (73) susceptible to Spiramycin and gentamicin.
The Results of Essential Oils
The results of the activity of the oils on agar medium
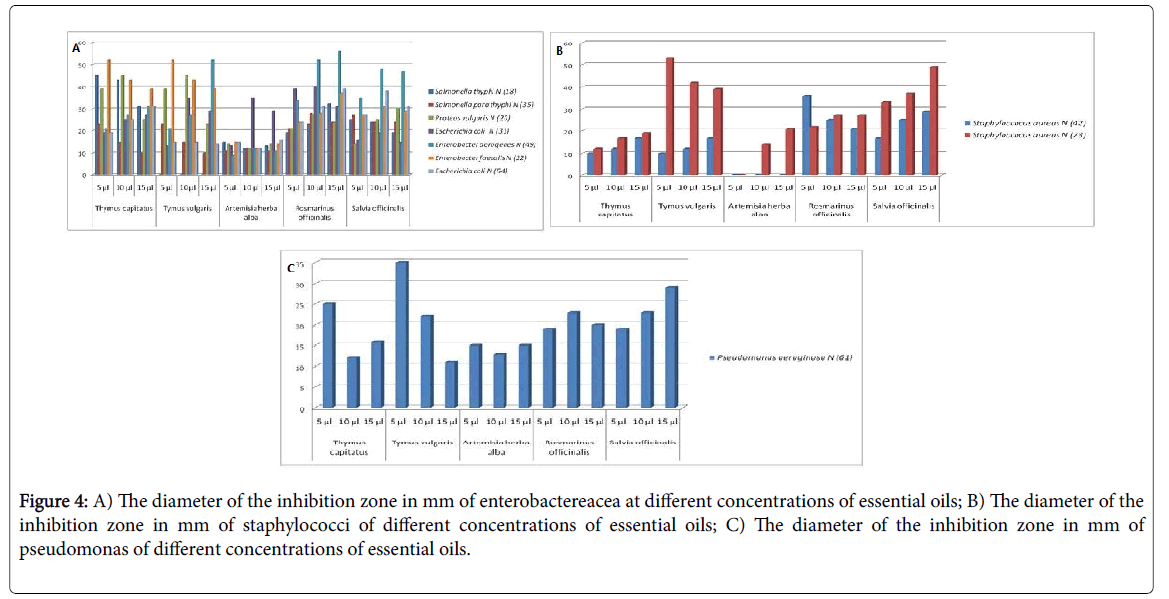
The results obtained are illustrated in the histogram by the (Figure 4). We note that the DMSO has no antibacterial activity, therefore a zone of inhibition around the disc soaked by DMSO, is totally absent.
Figure 4: A) The diameter of the inhibition zone in mm of enterobactereacea at different concentrations of essential oils; B) The diameter of the inhibition zone in mm of staphylococci of different concentrations of essential oils; C) The diameter of the inhibition zone in mm of pseudomonas of different concentrations of essential oils.
For the pure OE it generates zones of important inhibition what first appears is the sensitivity of the bacterial strains belonging to the three families Enterobacteriaceae, Staphylococaceae, Psedomonaceae with respect to the majority of the essential oils studied of concentration 5 μl, 10 μl and 15 μl.
The essential oils of Thymus capitatus and Thymus vulgaris show a powerful effect but not in relation to other plants, then that we note a clear resistance of Salmonella para thyphi with the oils of Thymus capitatus has 15 μl for a diameter of 10.33 ± 0.58 mm unlike S. aureus to same concentration. S. thyphi N (18) with the oils of Thymus vulgaris and this for the three concentrations.
The Results of the MIC and MBC
The determination of the minimum inhibitory concentration and the minimal bactericidal concentration are in (Table 1a and 1b).
| (μL.mL-1) | Thymus capitatus | Thymus vulgaris | Artemisia herba alba | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| Salmonella parathyphi N (35) | 20 | 40 | 2 | 10 | 10 | 1 | 40 | 40 | 1 |
| Salmonella thyphi N (18) | 5 | 5 | 1 | / | / | / | 20 | 40 | 2 |
| Proteus vulgaris N (20) | 2.5 | 5 | 2 | 2.5 | 2.5 | 1 | 10 | 20 | 2 |
| Escherichia coli N (31) | 2.5 | 5 | 2 | 5 | 5 | 1 | 20 | 40 | 2 |
| Enterobacter aerogenes N (49) | 1.25 | 2.25 | 1.8 | 5 | 10 | 2 | 5 | 10 | 2 |
| Staphyloccocus aureus N (42) | 10 | 40 | 4 | 1.25 | 1.25 | 1 | / | / | / |
| Enterobacter faecalisN (22) | 1.25 | 5 | 4 | 2.5 | 5 | 2 | 20 | 20 | 1 |
| Pseudomonas aeruginosa N (61) | 10 | 40 | 4 | 10 | 20 | 2 | 40 | 40 | 1 |
| Escherichia coli N (54) | 2.5 | 5 | 2 | 5 | 10 | 2 | 10 | 20 | 2 |
| Staphyloccocus aureus N (73) | 10 | 40 | 4 | 1.25 | 2.5 | 2 | 10 | 40 | 4 |
Table 1a: The MIC and the MBC of essential oils of different strains.
| (μL.mL-1) | Rosmarinus officinalis | Salvia officinalis | Ruta chalepensis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | MIC | MBC | MBC/MIC | |
| Salmonella parathyphi N (35) | 2.5 | 5 | 2 | 5 | 10 | 2 | 40 | 40 | 1 |
| Salmonella thyphi N (18) | 5 | 5 | 1 | 10 | 10 | 1 | 2.5 | 5 | 2 |
| Proteus vulgaris N (20) | 5 | 10 | 2 | 20 | 20 | 1 | 1.25 | 1.25 | 1 |
| Escherichia coli N (31) | 1.25 | 5 | 4 | 5 | 10 | 2 | 5 | 5 | 1 |
| Enterobacter aerogenes N (49) | 20 | 20 | 1 | 1.25 | 2.5 | 2 | 5 | 10 | 2 |
| Staphyloccocus aureus N (42) | 2.5 | 5 | 2 | 5 | 5 | 1 | 10 | 10 | 1 |
| Enterobacter faecalisN (22) | 10 | 10 | 1 | 2.5 | 5 | 2 | 1.25 | 2.5 | 2 |
| Pseudomonas aeruginosa N (61) | 5 | 10 | 2 | 5 | 10 | 2 | 5 | 5 | 1 |
| Escherichia coli N (54) | 2.5 | 5 | 2 | 5 | 5 | 1 | 5 | 10 | 2 |
| Staphyloccocus aureus N (73) | 1.25 | 1.25 | 1 | 2.5 | 2.5 | 1 | 2.5 | 5 | 2 |
Table 1b: The MIC and the MBC of essential oils of different strains (μL.mL-1).
Thymus capitatus
This This oil is very active by their strong inhibition on all bacteria Gram- tested and are belong to the family Enterobacteriaceae with a minimum inhibitory concentration between 1.25 and 5 (μL.mL-1) with a bactericidal effect/bacteriostatic, by against the pseudomonacea and Staphylococcus have an MIC of 10 (μL.mL-1) with a bactericidal effect/ bacteriostatic.
Thymus vulgaris
This oil is active by their strong inhibition on all bacteria tested except Salmonella typhi 6539 with a minimum inhibitory concentration (MIC) of 2.5 to 10 (μL.mL-1) for the Enterobacteriaceae and 1.25 (μL.mL-1) for Staphylococci and 10 (μL.mL-1) for the pseudomonacea. A bactericidal effect is given against these strains studied.
Artemisia herba alba
This oil carries active on all the three families studied with the exception Staphylococci, Enterobacteriaceae shows a sensitivity of (MIC) between 5 to 40 (μL.mL-1), Whereas the Pseudomonacea reached a MIC of 40 (μL.mL-1) with a bactericidal effect/ bacteriostatic.
Rosmarinus officinalis
This oil is very active by their strong inhibition on all bacteria tested. MIC for the Enterobacteriaceae between 1.25 to 10 (μL.mL-1) except the Enterobacters an MIC of 20 (μL.mL-1), a minimum inhibitory concentration (MIC) between 1.25 to 2.5 (μL.mL-1) for the Staphylococcus and Pseudomonaces MIC between 2.5 to 5 (μL.mL-1), with a bactericidal effect/bacteriostatic.
Salvia officinalis
This oil is very active by their strong inhibition on all strains tested with a minimum inhibitory concentration (MIC) between 1.25 and 20 (μL.mL-1) for the Enterobacteriaceae, 2.5 to 5 (μL.mL-1) for Staphylococci 5 (μL.mL-1) for the Pseudomonas, with a bactericidal effect on all of the strains.
Ruta chalepensis
This oil is very active by their strong inhibition on the Enterobacters, Proteus, Staphylococci and Salmonella with a minimum inhibitory concentration (MIC) between 1.25 and 5 (μL.mL-1), the Pseudomonas show an MIC of 5 (μL.mL-1), with a bactericidal effect on all of the strains.
Discussion
Flumequine resistance and sensitivity to gentamicin are the same findings [20], and disagree with the result of the lesser sensitivity of ceftiofur. Of the most commonly tested antibiotics, E. coli isolated from poultry have the least resistance to gentamicin [20]. The proportions of E. Coli are more than 90% stable and stable between 2003 and 2009 [21]. The high name Escherichia coli isolated from carcasses was probably related to the natural presence of this bacterial species in the digestive tract [22]. Ampicillin has become the less active antibiotic on E. coli [23].
E. aerogenes possesses a natural, inducible chromosomal cephalosporinase of low level of expression. E. aerogenes is naturally resistant to first-generation aminopenicillins and cephalosporins [24]. E. aerogenes can host, like all Enterobacteriaceae now, a plasmid encoding ESBL [24-26]. The natural resistance to cefalotine (i.e. to ampicillin and AMC) is constant. P vulgaris, by its sensitivity to CMA, are the exception [27] compared to other Enterobacteriaceae. Salmonella pose serious problems in both human and veterinary medicine and are all the more formidable as many of them are currently resistant to many antibiotics. Certain serotypes form the outset multiresistant [28]. Multi-resistant strains of S. typhi have also been reported in other countries [29]. According to the same author, Salmonella tested to the antibiogram, show a resistant and gentamycin.
Staphylococcus aureus has adaptive power and anti-staphylococcal resistance. More than 50% of strains of MRSA are resistant to gentamicin [12,30].
According to Abdallah et al. the highest rates were observed for gentamicin whereas the most constantly active antibiotics are resistance, this is the same result in our study. The presence of antibiotic-resistant Enterobacteria , Staphylococci and salmonella in the samples we analyzed; The proportion of resistant bacteria, pathogenic or saprophytic, is highest in animals intensively grown [31].
For the effect of the oil of Thymus capitatus our results are similar to those of Amarti et al. [32] who obtained complete inhibition of Escherichia coli and Staphylococcus aureus for an essential oil concentration of Thymus capitatus 1/2000 v/v. According to Amrani et al. this important bioactivity of Thymus capitatus oil is related to their high content of carvacrol and thymol.
The oil of Thymus vulgaris showed an antibacterial activity important against Escherichia coli and Staphylococcus aureus. Our results are aligned to Dorman et al. and El Ouali Lalami et al. [33,34].
The essential oils of Artemisia herba Alba are less interesting for the anti-bacterial plan and this for different concentrations, Staphylococci , Proteus , Salmonella and Enterobacter show a resistance of diameter between 0 and less than 15 mm. According to Ghanmi et al., in the study on the effect of harvest date on performance, the chemical composition and bioactivity of Artemisia herba-alba essential oils in the Guerçif region show that samples Collected in September are more active than those of the other months (April, June). We can also explain the difference observed between these three collection dates by the biosynthesis process of these main components [5].
The Salvia officinalis oil is very active at 5 μl, 10 μl and 15 μl against S. aureus and P. aerogenosa, these results do not converge with the work of Billerbeck was a result of 100 μl for P. aerogenosa.
Rosmarinus officinalis and Salvia officinalis showed efficacy against S. aureus and also approved by [35].
It appears that the essential oil of Ruta chalepensis has an effect on the strains studied. It has been reported that 2-undecanone was the major constituent of the essential oil of Ruta chalepensis and has a strong antibacterial activity [36,37].
We can confirm the importance and power of antibacterial Rosmarinus officinalis and Salvia officinalis in first position followed by Thymus capitatus and Thymus vulgaris, it remains Artemisia herba Alba of low activity in the face of the bacteria that have a multi resistance to antibiotics.
Conclusion
In conclusion this study which was intended to show the importance of essential oils against pathogenic strains, these strains have a resistance vis-a-vis the antibiotics used in animal husbandry avian, this resistance is the cause of a therapeutic failure is a major problem and disturbing to veterinarians and livestock producers. The sensitivity achieved has confirmed the presence of an undesirable phenomenon of antibioresistance among a variety of strains isolated. The oils of Thymus capitatus, Rosmarinus officinalis, Salvia officinalis, Thymus vulgaris, Artemisia herba alba and Ruta chalepensis share a series of minimum inhibitory concentration (MIC) between 1.25 to 40 (μL.mL-1) with a bactericidal effect/bacteriostatic. It is quite in agreement to test the activity of these oils in vivo, and thinking has oriented the breeders toward a breeding of less of antibiotics and based on organic products such as medicinal plants in the treatment of different avian pathologies.
Acknowledgment
We thank the engineer Fedoul Firdaous Faiza for the technical assistance.
References
- Tani ZBAK, Arlet G (2014) Actualité de la résistance aux antibiotiques chez les bacilles à Gram négatif en Algérie. Pathologie Biologie 62: 169-178.
- Acar J, Rostel B (2001) Antimicrobial resistance: an overview. Revue Scientifique et Technique-Office International des Epizooties 20: 797-807.
- Cohen R, Bingen E, Grimprel E, Raymond J, Gendrel D (2011) Résistance aux antibiotiques: un nouveau tournant à ne pas manquer. Archives de pédiatrie 18: 359-361.
- Bouzouita N, Kachouri F, Kamel MBH, Chaabouni MM (2008) Composition chimique et activités antioxydante, antimicrobienne et insecticide de l’huile essentielle de Juniperus phoenicea. J Soc Chim Tunis 10: 119-125.
- Ghanmi M, Satrani B, Aafi A, Isamili MR, Houti H, et al. (2010) Effet de la date de récolte sur le rendement, la composition chimique et la bioactivité des huiles essentielles de l’armoise blanche (Artemisia herba-alba) de la région de Guerçif (Maroc oriental). Phytothérapie 8: 295-301.
- Pellecuer J (1980) Essais d’utilisation d’huiles essentielles de plantes aromatiques méditerranéennes en odontologie conservatrice. Plantes Medicinales et Phytotherapie 14: 83-98.
- Ndiaye C (2010) Étude anatomo-clinique et bactériologique sur des cas suspects de colibacillose aviaire dans les régions de Dakar et thies (senegal) these. Université Cheikh anta diop de dakar.
- Pilet C (1979) Les entérobactéries Bactériologie médicale et vétérinaire : systématique bactérienne Doins. Paris 2e ed .
- Dho M, Mouline C (1983) Analyse qualitative et quantitative de la flore bactérienne dans la trachée du poulet sain. Ann Rech Vet 14: 189-194.
- Abeid AO, Zakaria M, Oudda H, Mohammed O (2015) Etude microbiologique et identification des souches isolés à partir du poisson (Mugil cephalus) séché-pilé «Lekhlia»(Microbiological Study and identification of strains isolated from the fish (Mugil cephalus) dried-pounded «Lekhlia») J Mater Environ Sci 6: 1142-1146.
- Koffi-Nevry R, Assi-Clair BJ, Emma FA, Affou SW, Koussemon M (2012) Origine des témoins de contamination fécale de l’eau d’arrosage de la laitue (lactuca sativa) cultivée dans la zone péri urbaine d’Abidjan. J Appl Biosci 52: 3669-3675.
- Elhamzaoui S, Benouda A, Allali F, Abouqual R, Elouennass M (2009) Sensibilité aux antibiotiques des souches de Staphylocoques aureus isolées dans deux hôpitaux universitaires à Rabat, Morocco. Médecine et maladies infectieuses 39: 891-895.
- Bonnet R, Caron F, Cavallo JD, Chardon H, Chidiac C, et al. (2013) Comité de l’antibiogramme de la société française de microbiologie. in Recommandations.
- Pibiri M, Seigniez C, Roulet, CA (2001) Assainissement microbiologique de l'air et des systèmes de ventilation au moyen d'huiles essentielles et leurs effets sur le bien-être des occupants. CISBAT 2001.
- Merazi Y, Hammadi K, Fedoul FF (2016) Approche Ethno-Vétérinaire Des Plantes Médicinales Utilisées Dans La Région De Sidi Bel Abbes-Algérie. European Scientific Journal 12: 1857- 7431.
- Clevenger J (1928) Apparatus for the determination of volatile oil. Journal of Pharmaceutical Sciences 17: 345-349.
- Guinoiseau E (2010) Molécules antibactériennes issues d'huiles essentielles: Séparation, identification et mode d'action. Université de Corse
- Oussou KR, Kanko C, Guessend N, Yolou S, Koukoua G, et al. (2004) Activités antibactériennes des huiles essentielles de trois plantes aromatiques de Côte-d'Ivoire. Comptes Rendus Chimie 7: 1081-1086.
- El Amri J, Elbadaoui K, Zair T, Bouharb H, Chakir S, et al. (2014) Étude de l’activité antibactérienne des huiles essentielles de Teucrium capitatium L et l’extrait de Siléne vulgaris sur différentes souches testées. Journal of Applied Biosciences 82: 7481-7492.
- Gay E, Chazel M, Danielle M, Haenni M, Calavas D, et al. (2010) Apport du Résapatha la problématique de l’antibiorésistance en santé animale: analyse des données recueillies en 2008 sur Escherichia coli dans les différentes filieres animales. Bulletin Epidémiologique AFSSA 36: 6-9.
- Eric J, Clairel C, Myriam C, Aureliel LR, Jean-Yves M, et al. (2011) Évolution de la résistance aux antibiotiques chez les E. Coli isoles d'infections chez la volaille (resapath). Neuviemes Journees de la Recherche Avicole, Tours, 29 et 30 mars, 2011.
- Maho A, Mbeurnodji L, Ndobale B (1997) Dominantes pathologiques aviaires à N’Djaména: étude de quinze fermes. Revue d’Elevage et de Médecine Vétérinaire des Pays Tropicaux 50: 277-280.
- Bouzenoune F, Boudersa F, Bensaad A, Harkat F, Siad N (2009) Les infections urinaires à Ain M’lila (Algérie). Résistance aux antibiotiques des 239 souches isolées entre 2006 et 2007. Médecine et maladies infectieuses 39: 142-143.
- Meunier O, Stoessel P, Saint-Laurent P, Lutun P, Jehl F, et al. (1997) Rôles des laboratoires d'hygiène et de bactériologie dans la prise en charge d'une épidémie à Enterobacter aerogenes multirésistant aux antibiotiques. in Annales de Biologie Clinique 55: 129-137.
- Bush K (1996) Is it important to identify extended-spectrum beta-lactamase-producing isolates? European Journal of Clinical Microbiology and Infectious Diseases 15: 361-364.
- Philippon A, Fournier G, Paul G, Vedel G, Nevot P (1988) Detection et distribution des beta-lactamases a spectre elargi chez les enterobacteries. Médecine et Maladies Infectieuses. 18: 869-876.
- Vu-Thien H (1998) Sensibilité aux antibiotiques des bactéries isolées dans les infections urinaires en pédiatrie. Arch Pediatr 5: 266S-268S.
- Chasseur-Libotte M, Ghysels G (1983) Evolution de la résistance aux antibiotiques des Salmonella en Belgique de 1975 à 1981. Médecine et Maladies Infectieuses 13: 332-337.
- Anderson E (1975) The problem and implications of chloramphenicol resistance in the typhoid bacillus. J Hyg (Lond) 74: 289-299.
- Bertrand X, Muller A, Thouverez M, Talon D, et al. (2004) Increased susceptibility to non-beta-lactam antimicrobial agents of MRSA isolates: relationship between genotype and antibiotype. Pathol Biol (Paris) 52: 480-485.
- Guillot JF (1989) Apparition et évolution de la résistance bactérienne aux antibiotiques. Ann Rech Vét 20: 3-16.
- Amarti F, Satrani B, Aafi A, Ghanmi M, Farah A, et al. (2009) Composition chimique et activité antimicrobienne des huiles essentielles de Thymus capitatus et de Thymus bleicherianus du Maroc. Phytothérapie 6: 342.
- Dorman H, Deans S (2000) Antimicrobial agents from plants: antibacterial activity of plant volatile oils. Journal of applied microbiology 88: 308-316.
- El Ouali Lalami A, El-akhal F, Ouedrhiri W, Ouazzani Chahdi F, Guemmouh R, et al. (2013) Composition chimique et activité antibactérienne des huiles essentielles de deux plantes aromatiques du centre nord marocain: Thymus vulagris et Thymus satureioïdis. Les Technologies De Laboratoire 8: 27-33.
- De Billerbeck, VG (2007) Huiles essentielles et bactéries résistantes aux antibiotiques. Phytothérapie 5: 249-253.
- Daoudi A, Hrouk H, Belaidi R, Slimani I, Ibijbijen J, et al. (2016) Valorisation de Ruta montana et Ruta chalepensis: Etude ethnobotanique, Screening phytochimique et pouvoir antibactérien Valorization of Ruta montana and Ruta chalepensis: Ethnobotanical study, phytochemical screening and Antibacterial activity. Journal of Materials and Environmental Science 7: 685-1063.
- Ferhat M, Kabouche A, Kabouche Z (2014) Comparative compositions of essential oils of three Ruta species growing in different soils. J Mater Environ Sci 5: 735-738.
Copyright: © 2016 2017 Yahya M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.