Research Article - (2023)Volume 7, Issue 2
Diabetes mellitus is a chronic metabolic disorder that is characterized by prolonged hyperglycemia. Some plant-based herbal formulations have been reported to lower blood sugar levels and control diabetic complications. Harnessing the inherent potentials in plants phytochemicals for specific health therapy remains a challenge. In this study, and using cold maceration and gravimetric methods for extraction, we isolated, identified and characterized some bioactive phytochemicals in Mangifera indica (mango leaf) and Annona muricata (soursop leaf). The phytochemicals were further screened and analysed using qualitative and quantitative techniques. The results of the both samples revealed the presence of alkaloids, flavonoids, tannins, phenols, terpenoids and saponins with 15.62% flavonoids and 20.50% saponin yield respectively for Mango Leaf (MGL) and Soursop Leaves (SSL); followed by phenol and tannin then alkaloids and terpenoids that gave the least yield. The antidiabetic potentials of six (6) selected phytochemicals were also investigated via alpha amylase inhibitory assay and glucose uptake capacity of yeast cells. Our data revealed impressive alpha amylase inhibitory activity and glucose uptake across the plasma membrane of the yeast cells, with Flavonoids, saponin and terpenoids fractions of the samples possessing the highest alpha amylase inhibitory activity and glucose uptake capacity. It is our conclusion, therefore, that Mangifera indica (mango leaf) and Annona muricata (soursop leaf) ethanolic extract posses anti diabetic potentials by inhibiting alpha amylase and increasing glucose uptake within the cell.
Mango leaf; Soursop leaf; Phytochemical; Antidiabetic; Extracts; Spectra
Diabetes has become a healthcare emergency; it is a chronic metabolic disorder that is characterized by prolonged hyperglycemia [1], due to the pancreas not producing sufficient insulin or the body cells not being able to respond adequately to the insulin secreted or both, resulting in defective carbohydrate, protein and fat metabolism [2]. It is associated with hyperglycemic emergencies [3], retinopathies, nephropathies, cardiopathies, stroke as well as amputations of the lower limb. Almost one in ten persons has been projected to be living with diabetes by 2050. Diabetes complications have been implicated as the leading cause of diabetic mortality and morbidity [1].
Herbal decoction, maceration, infusion and juice extracts of the stem, leaves, bark and seeds of plants have been recommended for the management of diseases [1,4]. Mango and soursop leaves extracts possess phytochemicals which include Alkaloids, Flavonoids, Glycosides, Polysaccharides, Sterols, Peptidoglycans, Amino acids and their derivatives, Saponins, Terpenoids, Carotenoids [5-7]. The use of most of these bioactive molecules to manage diseases including diabetes mellitus and as anti-microbial have been reported [8]. Some have been implicated in the reduction of blood sugar levels as they are thought to promote glycemic control, induce weight loss, and glucose uptake [9].
The use of herbal mixtures for the management of diabetes and its complication is on the increase [10-13]. This is thought to be as a result of the high cost of conventional diabetes medication, cheap, availability and fewer side effects of the herbal therapy [14]. Plants with antimicrobial and antidiabetic properties are known to thrive [15] and are well distributed across geographical locations, are not impeded by the various climatic conditions [16] and are usually consumed as food, spices or food supplements.
Plant specimen
The plant materials used are: Mangifera indica (Mango leaf) and Annona muricata (Soursop leaf),
Reagents
Reagent used include Alpha amylase (Molychem), Ethanol (Emsure), Methanol (Sigma-Alorich), Sodium Chloride (JHD), Petroleum Ether (JHD), Ammonium Hydroxide (JHD), n-butanol, n-hexane, Folin-Denis reagent, standard tannic acid, DPPH, Potassium ferrocyanide, H2SO4, Sodium phosphate, Ammonium molybdate, Starch azure, Sodium acetate buffer, Cacl2 and Trichloroacetic Acid (TCA), Acetone, Ammonium Sulphate (NH4)2SO4 all were sourced from ECCL England, Activated charcoal and Acetic acid.
Plant specimen preparation
We collected 200 g each leaf samples from the Botanical garden, Crop Science Department Federal University of Technology Owerri. They were identified by a Botanist, washed under tap water and air-dried at normal room temperature for 4 weeks, then pulverized into powder using an electric blender and stored in airtight ziplock samples bags to avoid moisture and contamination.
Extraction of phytochemicals
Using 95% ethanol in a sample to solvent ratio of 1:6 (w/v), we extracted the phytochemicals via crude ethanolic extraction and the extract stored in a well labeled air-tight specimen bottle. The powder was soaked at room temperature and allowed to stand for 3 days, with occasional agitation to ensure complete extraction, followed by filtration using muslin cloth folded into two. The extract was concentrated under vacuum in a rotary evaporator with the heating bath set at 45°C.
Phytochemical analysis
Phytochemical analysis and screening of the ethanolic extracts were carried out. Alkaloids and Saponin content were determined according to Roghini and Vijayalakshmi [17], Terpenoid content determined according to Malik [18], Flavonoid determined by the method reported by Ezeonu and Ejikeme [19], Total phenolic contents determined according to the method described by Phuyal, et al. [20] and Tannin content determined as described by Pearson [21].
Antioxidant assay
Antioxidant assay of the crude ethanolic extracts was carried out using 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) radical scavenging assay, Ferric Reducing Antioxidant Power (FRAP) Assay and Total Antioxidant Capacity Assay (TAC) as described by Rahman, et al. [22].
Antidiabetic assay (Alpha amylase inhibitory assay)
Antidiabetic assay of some selected phytochemicals was conducted using alpha amylase inhibitory assay according to the method of Hirasawa [23,24].
Glucose uptake assay
Glucose uptake capacity of yeast cells was carried out according to the method described by Cirillo [25].
The results of the phytochemical screening analysis of the crude ethanolic extracts of Annona muricata (Soursop leaf) labeled as SSL and Mangifera indica (Mango leaf) labeled as MGL, revealed abundance of alkaloids, flavonoids, tannins, phenols, terpenoids, and saponins.
Ethanolic extract of powdered leaf samples of MGL (Mango Leaf) and SSL (Soursop Leaf) by cold maceration yielded 15.62% and 20.50% respectively, which shows that soursop leaves produced a higher yield than Mango leaves.
In this study, the ethanolic extracts of MGL, SSL were screened to identify the phytochemicals present in the extract and based on the colour intensities of each phytochemical components screened, six phytochemicals; alkaloids, flavonoids, tannins, phenols, terpenoids, and saponins were selected for further study. The result collaborated the findings of Usunobun, et al. [26], Agu and Okolie [27], Uniyal and Rahal [28] but at slight variance with Nwaehujor, et al. [29] for Annona muricata (Soursop leaf) shown as Table 1.
| S/N | Extract | Tan | Cab | Alk | Sap | Fla | Qui | Gly | Phe | Ter | Cd | Nin | Cou | Anq | Ste |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SSL | +++ | +++ | ++ | ++ | ++ | - | + | +++ | +++ | - | - | +++ | - | ++ |
| 2 | MGL | +++ | +++ | +++ | +++ | +++ | - | + | +++ | ++ | - | - | ++ | - | ++ |
Note: Ethanolic extract of powdered leaf samples of MGL (Mango Leaf) and SSL (Soursop Leaf) by cold maceration yielded 15.62% and 20.50% respectively, which shows that soursop leaves produced a higher yield than Mango leaves.
Table 1: Qualitative analysis of ethanolic extract of Mango Leaf (MGL) and Soursop Leaf (SSL) Tannins, Carbohydrates, Alkaloids, Saponins, Flavonoids, Quinones, Glycosides, Phenols, Terpenoids, Cardiac glycosides, Ninhydrins, Coumarins, Anthraquinones and Steroids were screened.
Also, the quantitative analysis results of MGL and SSL samples collaborated the findings of Manimekalai and Chitra [30], Kazi, et al. [31] for Mangifera indica sample (Nwaehujor, et al. [29], Nguyen, et al. [32]) and for Annona muricata sample, showing high flavonoids, saponins, tannins and phenol contents. But the result of the crude ethanolic yield was similar to the observations of Aquisman, et al. [33] for mango leaves using 75% ethanol but slightly higher than that obtained by Suhendar [34] for soursop in Microwave Assisted Extraction (MAE) using 70% ethanol shown as Table 1.
However, antioxidant assay which was carried out to establish the free radicals scavenging ability and hydrogen atom donating ability of the samples provided an insight into their in-vitro response to oxidative stress. DPPH, FRAP and TAC assay results for MGL and SSL revealed high percentage antioxidant activities shown as Figure 1, collaborating Das, et al. [35], Orak, et al. [36], Qorina, et al. [37], Udem, et al. [38], though not comparable to the standard sample vitamin C. They were lower in comparison to Hasmila, et al. [39], Ibrahim, et al. [40] on Mangifera indica and higher for Annona muricata.
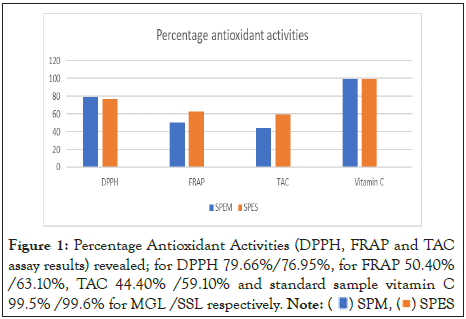
Figure 1: Percentage Antioxidant Activities (DPPH, FRAP and TAC
assay results) revealed; for DPPH 79.66%/76.95%, for FRAP 50.40%
/63.10%, TAC 44.40% /59.10% and standard sample vitamin C
99.5% /99.6% for MGL /SSL respectively.
Note: ( ) SPM, (
) SPM, ( ) SPES
) SPES
The antidiabetic activity guided search for potent phytochemicals were carried out on the six (6) selected phytochemicals through alpha amylase inhibitory assay and glucose uptake capacity of yeast cells. The crude extracts of the selected phytochemicals promoted glucose uptake across the plasma membrane of the yeast cell with more pronounced effect on the crude extracts of both MGL and SSL samples, followed by alkaloid and terpenoid of SSL (though, they both had very low yield) and phenol and flavonoid of MGL. Moreso, saponin of SSL gave the lowest glucose uptake followed by tannin of both MGL and SSL samples shown as Figure 2. However, the glucose uptake across the yeast cells were observed not to increase according to dose i.e. not dose dependent and this was consistent with Pitchaipillai and Ponniah [41] but in contrast with Pulivarthi, et al. [42].
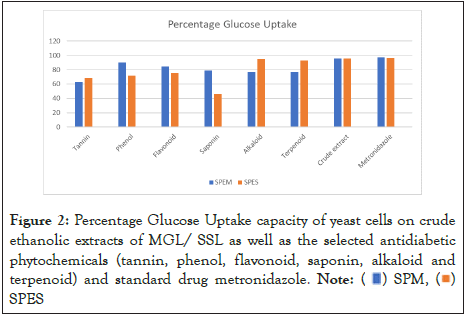
Figure 2: Percentage Glucose Uptake capacity of yeast cells on crude
ethanolic extracts of MGL/ SSL as well as the selected antidiabetic
phytochemicals (tannin, phenol, flavonoid, saponin, alkaloid and
terpenoid) and standard drug metronidazole.
Note: ( ) SPM, (
) SPM, ( )
SPES
)
SPES
Our data, however, showed that our samples have a potent alpha amylase inhibitory activity shown as Figure 3, which was high than that reported by Kulkarni, et al [43], Atanu and Francis [44]. The flavonoid and saponin fractions of MGL gave high alpha amylase inhibitory activities of 99.06% (for flavonoid) and 99.68% (for saponin). Moreso, the flavonoid and saponin fractions of SSL revealed an alpha amylase inhibitory activity of 98.75% and 99.16% respectively.
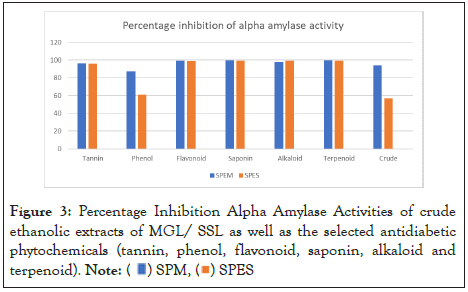
Figure 3: Percentage Inhibition Alpha Amylase Activities of crude
ethanolic extracts of MGL/ SSL as well as the selected antidiabetic
phytochemicals (tannin, phenol, flavonoid, saponin, alkaloid and
terpenoid).
Note: ( ) SPM, (
) SPM, ( ) SPES
) SPES
We conclude that, mango and soursop leave extract should be incorporated in herbal formulations for the management of diabetes mellitus and its complications. Therefore, to reduce the disease burden and its associated complications, achieve better glycemic control, reduce cardiovascular and oxidative stress, and enhance therapeutic efficacy: there is the need to standardize locally available phyto-compounds in herbal plants formulation for the management of diabetes and its complications.
[Crossref]
Citation: Amarachukwu OS, Chisom OC, Chidi IS, Nnanta GI, Oladimeji AT (2023) In-vitro Antidiabetic Assessment of Bioactive Phytochemicals in Locally used Mango and Soursop Leaves. J Pharma Reports. 07: 174
Received: 22-May-2023, Manuscript No. JPR-23- 21539; Editor assigned: 25-May-2023, Pre QC No. JPR-23- 21539 (PQ); Reviewed: 09-Jun-2023, QC No. JPR-23- 21539; Revised: 16-Jun-2023, Manuscript No. JPR-23- 21539 (R); Published: 23-Jun-2023 , DOI: 10.35248/JPR.23.7.174
Copyright: © 2023 Amarachukwu OS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.