
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2015) Volume 6, Issue 6
Natural products represent a rich and promising source of novel, biologically active chemical entities for treating Leishmaniasis. This study investigates the anti-leishmanial activity of n-butanol, aqueous, and chloroform fractions obtained from crude methanolic extract of Albizia gummifera seed in in-vitro (peritoneal mice macrophage) model after one time treatment. The anti-lesihmanial activity was determined from the minimum inhibitory concentration (MIC), infection rate (IR) and multiplication index (MI) against L. donovani. Cyto-toxicity of the fractions was also assessed against Vero cells. In addition to this secondary metabolites were determined by phytochemical screening. The MIC value of n-butanol, aqueous, chloroform and amphotericin B were 11.2 μg/ml, 33.5 μg/ml, >89 μg/ml and 1.32 μg/ml, respectively. N-butanol and aqueous fractions significantly inhibit the growth of intra-cellular L. donovani amastigote (P>0.05) compared to amphotericin B whereas the chloroform fraction did not revealed any significant leishmanicidal effect (P<0.05) with their respective parasitic infection rate, multiplication index and MIC levels. Based on these data, the n-butanol and aqueous fractionates of A. gummifera seeds exhibit considerable in-vitro anti L. donovani activity and may be promising anti-leishmanial herbal remedy candidate that support the traditional use of the plant.
Keywords: Albizia gummifera ; In-vitro , Leishmania donovani amastigote stage; Anti-amastigote assay
Leishmaniasis is a disease caused by protozoan parasite of the genus Leishmania and is an obligatory intracellular parasite of recticulendothelial cells [1]. Transmission of the parasite is usually through the bites of the various species of infected female sand flies. Approximately 350 million people live in areas of active leishmania transmission, with 12 million people being directly affected by Leishmaniasis in Africa, Asia, Europe and America [2]. Although it is one of the neglected tropical diseases, it is currently among the top ten diseases causing high burden among people living in developing nations [3]. Leishmaniasis is traditionally classified in three major types on the basis of clinical symptoms. The first one is visceral Leishmaniasis (VL) which leads to full-blown disease and ultimately to death if left untreated; and the others are cutaneous Leishmaniasis (CL) and mucocutaneous Leishmaniasis (MCL) which are responsible for considerable morbidity of a vast number of people in endemic foci. The parasites have a complex life cycle that involves phlebotomine sandy fly vectors and mammalian hosts, with amastigotes being within the phagolysosome of macrophages and promastigotes in the vector’s mid gut [4].
In Ethiopia, visceral Leishmaniasis (VL) is reported to be widespread over the arid and semi-arid areas and majorly caused by L. donovani [5]. Cutaneous Leishmaniasis occurs in high lands of Ethiopia [6]. L. aethiopica found to be the major causes of CL in high lands of Ethiopia [7], whereas L. major and L. tropica are the causes of CL in low lands of Ethiopia [8]. The preferences for first-line and second-line treatment vary on the type of disease and are often guided by regional practice. Pentavalent antimonials are presently first-line available treatment which given for both visceral and cutaneous Leishmansis while amphotericin B and Pentamidine solely applied as second line drugs for visceral form [9]. In Ethiopia, the treatment regimens follow the national guidelines in all treatment centers. The first-line drugs for the management of primary visceral Leishmaniasis in Ethiopia are a combination of antimonials with paromomycin while Liposomal Amphotericin B for special situations like in pregnant women and in Leishmania/HIV co-infected patients [10]. The second line treatment for visceral Leishmaniasis in Ethiopia is liposomial amphotericin [11]. Natural products, primarily plant-derived substances of diverse structural classes have pharmacological activities against Leishmaniasis as described in literatures [12]. The seeds of Albizia gummifera were considered for investigation on the basis of its traditional use for local and systemic condition of Leishmaniasis and based on similarity in the chemical composition with plants belonging to the same genera that have anti-leishmanial activity. Previous study of the crude hydro alcoholic extract of A. gummifera using Alamar blue assay showed the highest potency against the promastigotes of L. donovani with IC50 value of 8.65 μg/ml [13]. The current study was undertaken to examine the anti-amastigote stage L. donovani activity of A. gummifera . In addition to this, cytotoxicity assay was undertaken to demonstrate the safety of the fraction of A. gummifera for mammalian cells. The ethanol and water fraction of Albizia gummifera showed to inhibit the growth of L. donovani in peritoneal mice macrophages.
Culture medium
Minimum essential medium (from Sigma-Aldrich, Life science, USA); penicillin-streptomycin, and sodium bicarbonate (Sigma- Aldrich Laborchemikalien GmbH, USA) and fetal calf serum (Grand island, NY, 14072. USA) were used for culture medium.
Reference drug
Amphotericin B (Fungizon®, Bristol-Myers Squibb, and Rueil- Malmaison, France) was used as a reference drug (from Ethiopian Public Health Institute).
Test strains, cell line and animals
Reference L. donovani isolate (MHOM/50/68/15) from Ethiopian public Health institute, Leishmania Diagnostic and Research Laboratory; African green monkey kidney (Vero) cells (donated from National veterinary Institute, Debrezeit); White Swiss albino mice (from Ethiopian public Health institute).
Plant specimen preparation, extraction and fractionation
Seed of Albizia gummifera was collected from Southern part of Ethiopia, about 550 km away from Addis Ababa, near Dilla town in October, 2014. The plant was identified by a taxonomist and a voucher specimen (herbarium number, AG-2006) was stored at the herbarium of Modern and Traditional Medicine Directorate, Ethiopian Public Health Institute. The air-dried and powdered seeds (500 g and 100 g) were then extracted using one liter of 70% ethanol and 100 ml of distilled water by maceration, respectively. The resulting ethanol extract was concentrated using rotary evaporator (Buchi type R-210/215, Switzerland) at reduced pressure and at a temperature of 35°C to give gummy residue of 23 g. The aqueous extract was dried using lyophilizer and yielded 10.5 g of amorphous powder. The concentrated extract residues were then kept in wide mouth tightly stopper bottles in a refrigerator until used for MIC determination and anti-amastigote activity tests. The extract that showed pronounced anti-amastigote activity was further fractionated by partitioning, using immiscible solvents to enrich possible active constituents.
Portion of the70% ethanol A. gummifera (5 g) extract was then suspended in distilled water and fractionated with dichlromethane and n -butanol until the partitioning solvents became colorless. The concentrated dichloromethane fraction (0.22 g) labeled as fraction one. The dried n -butanol partition (0.98 g) labeled as fraction two. The aqueous residue that was left following the two solvents partitioning dried using freeze dryer (lyophilizer) to give a dried amorphous solid of 1.22 g and labelled as fraction three. The dichlromethane and nbutanol partitions of A. gummifera , and the aqueous residue were then kept in wide mouth tightly stopper bottles in a refrigerator until used for subsequent investigation for their efficacy against anti-amastigote activity.
Preparation of stock solutions
A stock of amphotericin B solution was prepared and kept at -200C in refrigerator until used. N -butanol and dichloromethane A. gummifera plant fractionates were dissolved in DMSO and aqueous partion residue was dissolved in phosphate buffer solution (PBS) to make 40 mg/ml stock solution and kept in +4°C until used.
Phytochemical analysis
Phytochemical screening of the n -butanol, dichloromethane and aqueous fractions of A. gummifera seeds of ethanol extract for the presence of alkaloids, saponins, terpenoids, anthraquinones, phytosterols and withanoids, coumarins, tannins, flavonoids and phenolics compounds were performed following standard methods [14- 16].
Cultivation of Leishmania donovani parasite
Leishmania donovani parasites were cultivated in minimum essential medium (MEM) (Sigma Che. Co., St. Louis, USA) supplemented with 10% Fetal bovine serum (FBS), (100U/100 μg/ml) penicillin/streptomycin, and (2 mM/ml) and L-glutamine at pH 7.2 in 25 cm2 Costar culture flasks were incubated at 26°C following methods described in manual of parasitological laboratory, Ethiopian Public Health Institute [17].
Biological assay
All biological assays were performed at the Ethiopian Public Health Institute, Leishmania laboratory. In each assay, the concentration of DMSO was adjusted to less than 1.0% of the complete culture medium, concentration that showed no growth inhibitory effect on L. donovani and non-toxic to mice macrophages. Assays were done based on methods described by Ogaro et al. and Ngore et al. [18-21] with slight modifications such as medium type, volume of medium used in the assay and initial extract concentrations.
Determination of minimum inhibitory concentration (MIC)
In 96-well micro-titer plate fractions were serially diluted in 1:3 ratios in several concentrations ranging from 1 μg/ml to 1 mg/ml in 100 μl culture medium with each test concentration in duplicate. Then 100 μl suspensions containing 1.2 ×106 promastigotes per ml in a logarithmic phase were added to each well. Contents of the plates were then maintained at 26°C under a 5% CO2 incubator. The cell density, motility and morphology for each treatment were determined daily with an inverted microscope and the anti-leishmanial activity was expressed as the MIC values after 72 h of incubation. The lowest concentration of the samples that prevented the growth of Leishmania parasites in vitro was considered to be the MIC. Biological tests were performed two times, with each test concentration in duplicate. Amphotericin B was used as positive control whereas medium alone and 1% DMSO was employed as negative controls.
Anti-amastigote assay
Mice peritoneal macrophages collection, culture and infection was done based on methods described previously with slight modification [18-22]. Mice were injected with 2 ml of 2% starch (cold) before two days. After two days, mice were anesthetized by dislocating the spinal cord and up on disinfection of mice body surface with 70% ethanol the abdominal skin was sheared dorso-ventrally to expose the peritoneum. Using a sterile syringe and needle, 10 ml of sterile cold phosphatebuffered saline (PBS) was injected into the peritoneum. After gentle massaging, the mouse peritoneal macrophages were harvested by drawing 6-8 ml exudates of the PBS. The contents were transferred into a sterile 50 ml centrifuge tube. The suspension was centrifugally washed at 1400 rpm for 10 minutes at room temperature and the pellet was re-suspended in complete minimum essential medium (MEM). Macrophages were seeded in 8-well chamber slides and allowed to adhere for 1.5 h at 37°C in a 5% CO2. Non-adherent cells were washed with pre-warmed 5% complete MEM in PBS and then fresh complete media was added to adhered macrophages and then incubated overnight at 37°C in a 5% CO2. Adherent macrophages were infected with parasite at macrophage ratio of 10:1 and again incubated at 37°C in the 5% CO2 for 4hours. Unattached promastigotes were removed by extensive washing with pre-warmed PBS and the cultures were incubated in complete medium for 24 h. A one-time treatment of infected macrophages with fractionates was done. Amphotericin B was used as a reference drug for comparison of parasite inhibition. The medium and reference drug were replenished daily for 3 days. After 5 days, the monolayers were washed with PBS (pre-warmed at 37°C), fixed (in methanol) and stained with 10% Giemsa. The numbers of amastigotes were determined by counting at least 100 macrophages in duplicate cultures. The results were expressed in terms of infection rate (IR) and the multiplication index (MI) was calculated as follow:
IR = Number of infected macrophages in 100 macrophages

Cyto-toxicity assay
African green monkey kidney (Vero) cells were cultured and maintained in minimum essential medium (MEM) supplemented with 10% FBS at 37°C in 5% CO2 for 24 h. Then, they were harvested by trypsinization, pooled in a 50 ml centrifuge tube and 100 μl cell suspensions (1 × 105 cells) was added to each well in a 96-well microtitre plate. Each sample was replicated 2 times. A volume of 200 μl of the highest concentration of the extract of the test samples (a serial dilution, prepared in MEM) was added and the experimental plates further incubated at 37°C for 48 h. The cells in media without the extract were used as controls. In each well, 10 μl 3 -(4 , 5 - dimethylthiazol-2-yl )-2 5 -diphenyltetrazolium bromide) (MTT) was added, and the cells were incubated for 3 h, until a purple precipitate was clearly visible under a microscope. The medium together with MTT was aspirated off the wells. MTT solubilising solution (100 μl) was added, and the plates were shaken for 5 mins. The absorbance for each well was measured at 595 nm (reference, 655 nm) in a micro-titre plate reader and the percentage cell viability was calculated manually using the following formula:
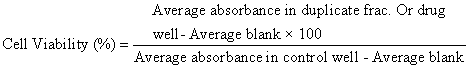
Ethical clearance
Ethical approval for the study was obtained from Ethiopian Public Health Institute Institutional Review Board (IRB) with project number SERO-014-4-2015.
Statistical analysis
Data represent the mean number (± SD) of replicated samples from two independent assays. Comparison between the means of n -butanol, dichloromethane and aqueous fractions of A. gummifera seed ethanol extract, untreated infected negative controls and amphotericin B (standard drug controls) was done using the student’s t-test. The 50% inhibitory concentration (IC50) and 50% cytotoxcity (CC50) values were calculated using dose–response curves from regression equations. Selectivity index (SI) was also obtained from the ratio between CC50 and IC50. All the analyses were carried out at 5% level of significance.
Phytochemical constituents and biological activity
The qualitative phytochemical screening indicated the presence of alkaloids, saponins, terpenes, flavonoids and phenolic compounds in the n -butanol, dichloromethane and aqueous fractions of A. gummifera . MICs of these fractions were determined by investigating morphological, density and motility changes of parasites treated with different concentration of fractions under light microscope after 72 h of incubation. Results of this experiment are illustrated in Table 1.
| AG fractions/Amphotericin B | MIC (µg/ml) |
|---|---|
| n-butanol | 11.20 |
| Aqueous | 33.50 |
| Dichloromethane | 89.00 |
| Amphotericin B | 1.32 |
| MEM | - |
| 1% DMSO | - |
Table 1: Minimum inhibitory concentration (MIC) values for fractions of A. gummifera against L. donovani promastigotes.
Anti-amastigote activities
The ethanol and aqueous extracts of A. gummiefra were first screened for their anti L. donovani amastigote activity and the infection rate, multiplication index after treatment with the extracts were compared between each other (Table 2). Based on the result presented on Table 2, the solvent fraction of the ethanol extract of A. gummifera (n -butanol, dichloromethane and aqueous) were used to determine the infection rate, multiplication index and IC50 using amphotericin B as positive control (Table 3), and the infection rate and multiplication indices were shown to decrease with increasing the concentration of fractions.
| Variable | Ethanol | Aqueous |
|---|---|---|
| IC50 (µg/ml) | 2.29 | 2.16 |
| Infection rate (IR) | 11.2** | 26.2** |
| (13.4) | ||
| P-value | 0.29 | |
| Multiplication Index (MI) | 2.43** | 6.87** |
| (3.42) | ||
| P-value | 0.22 |
Table 2: Anti L. donovani amastigote activity of water and ethanol extracts of A. gummifera
| Variables | n-butanol | Aqueous | DCM | Amphotericin B |
|---|---|---|---|---|
| Infection Rate | 30.1** | 38.1** | 76.6*** | 26.1 |
| (14.6) | (15.7) | (12.2) | ||
| P-Value | 0.79 | 0.46 | 0.001 | |
| Multiplication Index | 20.6** | 16.7** | 72.3*** | 21.9 |
| (15.8) | (15.5) | (15.1) | ||
| P-Value | 0.94 | 0.74 | 0.006 | |
| IC50 (µg/ml) | 2.16 | 2.27 | 4.85 | 1.10 |
Table 3:Anti L. donovani amastigote activity of water and ethanol extracts of A. gummifera
Cytotoxicity
The n -butanol, water and dichloromethane fractions were screened for cytotoxcicty activity against Vero cells using the MTT assay in comparison with amphotericin B, (Table 4) and selectivity indices (SI) were also calculated from IC50 and CC50 values (Table 5). The present study shows as the concentrations of the fractions and reference drug increases viability of the Vero cells decreases.
| n-Butanol | Aqueous | Dichloromethane | Amphotericin B | |
|---|---|---|---|---|
| Mean viability of Vero cell (%) | 48.42** | 33.62*** | 72.7 | 64.8 |
| (17.2) | (16.7) | (13.6) | ||
| P-value | 0.363 | 0.091 | 0.573 | |
| CC50 (µg/ml) | 6.64 | 5.68 | 9.67 | 8.65 |
Table 4: Viability of Vero cells for A. gummifera fractions and amphotericin B.
| Cytotoxcity CC50/ Anti-leishmaniasis IC50 = SI | |||
|---|---|---|---|
| Fractionates/drug (µg/ml) | Cytotoxcity, CC50 | Anti-L. donovani, IC50 | Selectivity Index, SI |
| n-butanol | 6.64 | 2.16 | 3.0 |
| Aqueous | 5.68 | 2.27 | 2.5 |
| Dichloromethane | 9.67 | 4.85 | 2.0 |
| Amphotericin B | 8.65 | 1.1 | 7.9 |
| MEM | 0 | 0 | - |
| 1% DMSO | 0 | 0 | - |
Table 5: Cytotoxicity activity of amphotericin B and fractions of A. gummifera against Vero cell.
Phytochemical constituents
Chemical screening of the crude extract of Albizia gummifera seed in previous studies was reported to contain alkaloids, glycosides, phenols, saponins and terpens [23,24]. The aqueous fraction was also reported to have very polar compounds such as saponins, phenolic glycosides and terpens. Terpens, saponins, phenols, alkaloids and glycosides were reported to be present from the n -butanol fraction [23]. Saponins, alkaloids, terpinoids and flavinoids were also reported to be present in leaves and barks of Albizia species previously [25].
Biological activity
Minimum inhibitory concentration (MIC): The polar fractions were found to be more active than the non-polar fractions, and n -butanol fraction has better activity than aqueous fraction. The dichloromethane fraction was found to have less activity compared to amphotericin B. The negative controls (1% DMSO and MEM alone) were devoid of any antileishmanial activity.
The highest activity of n -butanol and aqueous fractions may be due to the presence of alkaloids, falvonoids, tannins, coumarins, saponins and phenolic compounds. The anti-leishmanial activities of these secondary metabolites were reported from other plant species [26]. For instance, the quinoline alkaloids, from the Bolivian plant Galipea longiflora have also shown oral activity in experimental VL and CL mouse models and saponins purified from the Vietnamese plant Maesa balansae showed excellent activity after parenteral administration against VL and CL in rodent models [27]. The mechanism of action of the reported alkaloids and flavonoids has been suggested due to their ability to form complexes with the parasite cell wall and inhibiting the action of DNA polymerase, respectively [18]. In another study done on leishmania amazonensis promastigotes alkaloids and terpens were found to have antileishmanial activity [4]. Similarly, tannins which are naturally found in C. nucifera , were also shown to have antileishmanial activity [28]. The less activity of dichloromethane fraction might be due to absence and/or less amount of secondary metabolites present in the fraction.
Anti-amastigote activities: The discovery of new and original leads suitable for optimization and drug development is dependent on the ability to screen many compounds; assays should be rapid, inexpensive and reproducible and for pathogens displaying several life stages like leishmania, there is a need to determine the best parasite stage to target [29]. Promastigotes can be transformed into amastigotes in a wide range of mammalian cells [30]. Promastigotes and amastigotes clearly differ in their morphology, bioenergetics, gene expression and protein phosphorylation and expression of membrane proteins, including gp63, LPG, and a metalloproteinase [31,32]. So, correlation of data from promastigotes and amastigotes is unreliable [33].
Axenic amastigotes can also be screened efficiently but by removing the parasite from its intracellular niche, the assay does not test for penetration of the compound into the host cell nor for activity in the peculiar environment of the macrophage phagolysosome [29]. In addition, axenic amastigotes may have different metabolic processes with intracellular amastigotes [34-36]. The preferable method for in vitro testing of compounds against Leishmania amastigotes involves growing amastigotes in mammalian macrophages. However, quantifying the growth of intracellular amastigotes is usually done by direct visual examination of stained cells on glass microscope slides [33].
Although, it is labor intensive and cannot be automated, direct counting assay enables determination of the percent of infected cells and the number of amastigotes per cell, which has been used for screening compounds for efficacy against intracellular amastigote forms of Leishmania [37]. Infected cell cultures, on a chamber slide system, are stained with Giemsa, and then drug activity is evaluated microscopically by determining the percentage of infected cells as well as the number of amastigotes per cell through examination of 50-300 macrophages [38]. However, this model lacks information about the behavior of macrophages during the treatment, their possible influence on drug activity, or possible damage received due to toxicity [39].
The ethanol extract showed a better activity than the aqueous one in reducing the number of inter-celluar amastigotes of L. donovani but there was no statistically significant difference among the mean number of amastigote in the two experiments. The IC50 for 70% ethanol and aqueous fractions were 2.16 μg/ml and 2.27 μg/ml, respectively. The mean number and infectivity of L. donovani amastigotes in macrophages treated with ethanol and water fractions of Albizia gummifera were not significantly different compared to amphotericin B at all concentrations. But the mean number of L. donovani amastigotes and infectivity in macrophages treated with dichloromethane fraction was significantly lower compared to amphotericin B.
N -butanol and aqueous fractions showed lowest IC50, and dichloromethane fraction had highest IC50 compared to the reference drug, amphotericin B. Effects of the fractions of n- butanol, aqueous, and dichloromethane were dose dependent, where higher concentrations resulted in low infection rate and multiplication indexes. N -butanol fraction of A. gummifera was more effective in inhibiting L. donovani in mice peritoneal macrophage, but dichloromethane fraction was less effective.
Cytotoxicity
in vitro cytotoxicity test is mainly performed to screen for potential toxicity of compounds that affect basic cellular functions. Ethanol extract of A. gummifera did not cause lysis at concentrations less than 400 μg/ml as reported from previous studies [13]. In the current study, cytotoxicity using in vitro on African green monkey kidney (Vero) cells to assess safety of the fractions included in this study with increasing concentrations of fractions.
N -butanol and dichloromethane show less toxicity (P>0.05) to healthy Vero cells with CC50 of 6.64 and 9.67, respectively compared to amphotericin B. There was no significance difference (P>0.05) in the mean viability of Vero cell treated with n -butanol, dichloromethane and amphotericin B.
The mean toxicity for Vero cell and the activity against L. donovani were compared using the selectivity index (SI) ratio expressed as, Cytotoxicity CC50/ Anti-Leishmaniasis IC50=SI. The selectivity index of n -butanol and aqueous fractions were 3 and 2.5 respectively, indicating their relative selectivity against intracellular L. donovani , which means n -butanol and aqueous fractions are selective to the intracellular amastigotes by 3 and 2.5 times than the Vero cells respectively. Amphotericin B (Fungizon®) is 8 times more selective to the parasite than Vero cells but dichloromethane fraction is less selective. The fractions are less selective to L. donovani amastigote than the reference drug amphotericin B which is attributable due to the presence of toxic secondary metabolites such as tannins, alkaloids, and saponins.
As per the present study, the n -butanol and dichloromethane fractions are safer than aqueous fraction at serially diluted different concentrations. Amphotericin B is safer because of its higher selectivity index. As the concentration of any of the fractions or amphotericin B increases, the percent viability of Vero cells decreases.
In conclusion, our In-vitro study indicated that Albizia gummifera seed fractions have anti-leishmaniatic effect with more selective cytotoxicity to parasites than the host cells at concentrations used to inhibit growth of the parasites, n -buthanol and aqueous fractions having more effect. The effect may be attributed to the presence of one or more of the secondary metabolites. The present study may provide a proof for the potential use of A. gummifera hydro-ethanolic extract solvent fractions as anti-leishmania effect.
We would like to express our sincere gratitude to Ato Sendew Mekasha, Ato Abebe Mengesha, Ato Ashenif Tadele, Dr. Getachew Addis and W/r Frehiwot Teka for their great technical, material support and encouragement during the study period. Our special thanks should also go to all staffs of vaccines and diagnostic production directorates, Parasitological, Bacteriology and zoonosis directorate and National Veterinary Institute. Last but not least, we also appreciate the financial support of Ethiopian Public Health Institute (EPHI), Addis Ababa University, and WHO-Ethiopia.