Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2022)Volume 10, Issue 8
Lactiplantibacillus plantarum is widely used as a probiotic and had increase hair growth in vitro. However, it is still unclear whether L. plantarum can improve hair loss and gut microbiome in clinical trial. The purpose of this study is to examine the effect of L. plantarum on inhibiting hair loss, strengthening hair roots, improved gut microbiome. TCI999 (L. plantarum) was used to treat human Hair Follicle Dermal Papilla Cells (HFDPC), and examine mitochondrial activity and viability of hair follicle cells, and examined hair follicle growth inhibition-related genes (SRD5A1, AR and TGF-β). Additionally, 50 subjects were recruited, and divided into a placebo group and TCI999 group. It was taken once a day for 12 weeks, followed by hair testing, hair-related genes analysis, collection of hair loss and questionnaires. The results showed TCI999 significantly increased mitochondrial activity and hair cell growth, and significantly decreased SRD5A1, AR and TGF-β genes in vitro. Taking TCI999 for 12 weeks significantly increased hair root diameter, improving hair loss as well as scalp redness compared to the placebo group. In addition, TCI999 decreased the pro-inflammatory bacterial phase (Negativicutes, Gammaproteobacteria, Verrucomicrobia, Deltaproteobacteria, and Fusobacteria) and increased the anti-inflammatory bacterial phase (Actinobacteria, Bacteria, Clostridia). Thus, TCI999 can increase hair growth and improve gut microbiome.
Lactiplantibacillus plantarum; Gut microbiome; Hair loss; Follicle dermal papilla cells
In today's society, hair affects the appearance, impression and protection of the head. Hair loss, known as alopecia, is a common medical disorder that affects all genders [1]. Hair loss can occur as a result of several changes such as seasons, fatigue, stress, hormone imbalances, drugs and aging [2]. Hair growth can be divided into four stages: anagen (a rapid hair proliferation period), catagen (apoptosis-driven regression period), telogen (a relatively inactive period), and exogen [2]. Studies have shown that inactivated mitochondria will affect the normal growth cycle of hair follicle cells, shorten the hair growth period, and promote hair recession and rest period, resulting in hair loss [3]. Current treatments for hair loss include cosmetic products, surgical operation, and oral or topical medications such as finasteride (a 5 α reductase inhibitor) and minoxidil [4]. However, these treatments are only short-lived, their effectiveness diminishes once treatment is stopped, and side effects include itching, erythema, and dryness.
Mammalian skin and mucosal surfaces are composed of millions of microorganisms with diverse metabolic roles involved in immune regulation of the body [5]. These host-associated microbes play a well-established role in homeostasis in the Gastrointestinal (GI) tract [6]. Once the normal gut microbial balance is disrupted, it can trigger symptoms related to allergies, autoimmunity, and hair metabolism [5]. Intestinal bacteria are closely related to the secretion of many physiological hormones and hormones [7]. The imbalance of intestinal bacteria leads to the increase of inflammatory factors and the imbalance of hormones, which leads to the damage of hair follicle cells and promotes the symptoms of hair loss [8]. Therefore, supplements rich in certain probiotics can boost immune and metabolic pathways that restore tissue homeostasis and promote good health [9]. Study finds dietary supplementation with probiotic lactobacillus can be beneficial for hair. Lactiplantibacillus plantarum is widely used as a probiotic [10], and these lactic acid bacteria are not only found in nature, but are also easily found in fermented foods and the human gut. L. plantarum is commonly used for antimicrobial activity, Angiotensin Converting Enzyme inhibition (ACE), lipid metabolism and hair growth [11]. Studies have shown that the use of L. plantarum can increase hair growth and the Vascular Endothelial Growth Factor (VEGF), Keratinocyte Growth Factor (KGF) and Transforming Growth Factor-beta1 (TGF-β 1) associated with hair growth [12]. Although it has been reported that L. plantarum has hair growth effects, it is currently unknown whether L. plantarum can improve hair loss in human clinical.
In this study, we used TCI999 (L. plantarum) is isolated from fermented milk in TCI Co. Ltd. First, using TCI999 to treat Hair Follicle Dermal Papilla Cells (HFDPC), and observe the mitochondrial activity and growth of hair follicle cells. Second, we recruited 50 subjects, who were divided into placebo and TCI999 group. After 12 weeks of taking, hair test, blood collection, and questionnaire survey were performed. Finally, we collected fecal samples and then analyzed gut microbiome and questionnaires.
Isolation and identification of TCI999 strain
Sixteen samples of different objects including an old Chinese medicine scripture, goji berries, codonopsis root, solomon’s seal and astragalus root were collected from a local Chinese medicine pharmacy, located in Taipei, Taiwan. Eight strains of potential Lactobacillus cultures were isolated based on colony morphology. To isolate and proliferate the Lactobacillus cultures, MRS Broth (Merck, Germany) and MRS Agar (Merck, Germany) were used. Amplification was carried out in a thermal cycler ABI/2720 (Applied biosystems, USA). The reaction mixture contained Taq DNA Polymerase 2 × master mix red with 1.5 mM MgCl2 (AMPLIQON), 50-100 ng of bacteria, and primer used are 8F (5ꞌ-AGAGTTTGATCCTGGCTCAG-3ꞌ) and 1492R (5ꞌ-CGGTTACCTTGTTACGACTT-3ꞌ). DNA fragments were amplified as follows: initial denaturation at 95°C for 5 min, followed by 20 or 30 cycles consisting of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, extension at 72°C for 30 s, and a 7 min final extension step at 72°C. The products were stored at 4°C for subsequent Sanger sequencing. The sequenced 16S rRNA genes of each isolate are searched with GenBank DNA database by using the BLAST algorithm. The identities of the isolates were determined on the basis of highest score.
The manufacturing process of TCI999 strain
The yeast peptone, glucose, MgSO4-7H2O, NaCl, KH2PO4, K2HPO4, manganese gluconate, water for mixing and fermentation. After fermentation, L. plantarum TCI999 was added, and then the fermentation was carried out. After the fermentation was terminated, took a sample for confirmation. After centrifugation, the bacterium mud was collected, water was added to mix, the bacteria were heated and the supernatant was collected by centrifugation, and then filled and sterilized. Then add freeze-drying protective agent to mix, prior label, and stored in 4°C.
Cell culture
Hair Follicle Dermal Papilla Cells (HFDPC) (C12071, Promocell, Heidelberg, Germany) were purchased from the ATCC, and cultured in follicle dermal papilla cell growth medium (C26501, Promocell) with fetal calf serum, bovine pituitary extract, bFGF and insulin, supplemented with L-glutamine (X0550, Biowest, Nuaillé, France) and 1% (w/v) penicillin/streptomycin (P/S) (L0022, Biowest) in a humidified incubator of 5% CO2 at 37°C.
Cell viability assay
HFDPC (2 × 105 cells/ml) were seeded in 100 μl of 96-well plates for at least 24 h before use. The cells were treated with TCI999 for 24 h. The cell viability was measured by the MTT assay according to the manufacturer's instructions (Promega, Madison, WI). After the cells had been incubated for the indicated times, they were incubated with MTT solution (5 mg/ml) for four hours. The formazan precipitate was dissolved in 200 μl Dimethyl Sulfoxide (DMSO), and the absorbance at 570 nm was measured using an automated microplate reader (BioTek, Synergy™2, USA).
Quantification of gene expressions by real-time PCR
The treated HFDPC were harvested, and the total RNA was isolated from cells using an RNA purification kit (Geneaid, Taiwan). DNA-free total RNA was reversely transcribed to cDNA using a superscript™ Reverse Transcriptase (RT) kit (Invitrogen, Life Technologies Co., CA, USA). Quantitative real-time PCR was conducted using an ABI StepOnePlus™ Real-Time PCR System (Thermo Fisher Scientific, Inc., CA, USA) and the SYBR Green Master Mix (GMM) (KAPA Biosystems, MA, USA) for the transcript measurements. The gene-specific primers used in this study are listed in Table 1. The GAPDH gene was used as a normalization control (Table 1).
| Gene | Species | Direction | Sequence |
|---|---|---|---|
| SRD5A1 | Human | F | TCAGACGAACTCAGTGTACGG |
| Human | R | CGTAGTGGACGAGGAACATGG | |
| AR | Human | F | GGTGAGCAGAGTGCCCTATC |
| Human | R | GCAGTCTCCAAACGCATGTC | |
| TGF-β | Human | F | GCTCCTGTGACAGCAGGGATA |
| Human | R | CGTGGAGCTGAAGCAATAGTTG | |
| GAPDH | Human | F | ACAACTTTGGTATCGTGGAAGG |
| Human | R | GCCATCACGCCACAGTTTC |
Note: PCR: Polymerase Chain Reaction; AR: Androgen Receptor; TGF: Transforming growth factor
Mitochondrial activity assay
The mitochondrial membrane potential detection kit (JC-1) (Beyotime Institute of Biotechnology, China) was used to observe changes in the mitochondrial potential. Briefly, 5 mg/ml JC-1 working solution was added to the medium and incubated for 30 min at 37°C with CO2. The cells were subsequently washed with Phosphate Buffered Saline (PBS) to remove the JC-1 probe, and images were obtained via fluorescence microscopy (Olympus BX-61). The ratio of red to green fluorescence was analyzed using Image Pro Plus version 4.5 (Media Cybernetics, Inc., Rockville, MD, USA).
Clinical trial design
The clinical study was approved by the Antai-Tian-Sheng Memorial Hospital Institutional Review Board (TSMH-IRB 21-041-A), and the study was registered on ClinicalTrials.gov Identifier: NCT05077553. Fifty adult subjects (>20 years old, who suffered from hair loss) were recruited in this trial between July 2021 and July 2022. Informed consent was obtained from all subjects before the study at Chia Nan University of Pharmacy and Science. The subjects were divided into a placebo group (n=25) and a TCI999 group (n=25). Subjects were informed to take one sachet every day (100 mg/day, 1 × 1010 cfu) for 12 weeks. Subjects were instructed to eat normally and avoid mistakes caused by overeating. The hair condition of the subjects was measured at weeks 0, 4, 8 and 12 of the trial and they were not allowed to take any other supplements during the intervention period. The exclusion criteria includes: (i) Patients with diseases of the skin, liver, kidney; (ii) Subjects with known cosmetic, drug or food allergies, difficulty in digestive tract absorption or disorder; (iii) Pregnant and breastfeeding; (iv) Subjects with wounds on the scalp and subjects who had used drugs to treat the scalp, received hair transplants or other scalp treatments within six months.
Preparation of test sample
The TCI999 powder included L. plantarum (100 mg, 1 × 1010 cfu), citric acid, maltodextrin, sucralose. The placebo powder included citric acid, maltodextrin, and sucralose. The mass was the same between these groups. Each subject was required to examine the hair condition at weeks 0, 4, 8 and 12.
Hair detection
In hair root diameter, using a digital outer diameter measuring instrument (mitutoyo C/N293-100, Japan), the higher the detection value, the thicker the hair root. The detection position is to take three hairs for measurement, and then observe the thickness of the hair roots with a microscope (100 ×), measure the diameter of the hair from the root upwards, and evaluate the thickness of the hair. In hair robustness analysis, hair was pulled up in the frontal, temporal and occipital regions and assessment of hair loss volume and the lower hair amount indicated health and toughness. In hair loss volume determination, hair was collected from the drainage hole and after blow-drying. Before hair washing, subjects were asked to check and clean a drainage hole. After hair washing, subjects collected hair from the drainage hole and then photographed, recorded and weighed. In hair density determination, photographs recorded the sparse hair regions. In scalp health condition analysis, a soft plus analyzer was used (Soft Plus, Callegari, Italy), and then the scalp and hair root condition were examined through a microscope (40 ×), photographed, and recorded.
Fecal sample collection and gut microbiome analysis
Fecal samples were collected by the subjects at home using a validated protocol by BIOTOOLS Co., Ltd. The material was aliquoted into 1.5 ml Eppendorf tubes and stored in -80°C until further laboratory processing. Total genomic DNA from samples was extracted using the column-based method (e.g. QIAamp PowerFecal DNA Kit, Qiagen). DNA concentration was determined and adjusted to 5 ng/ μl for the following process. For the 16S rRNA gene sequencing, V3-V4 region was amplified by specific primer set (F: 5 ꞌ-CCTACGGGNGGCWGCAG-3ꞌ, R: 5ꞌ-GACTACHVGGGTAT CTAATCC-3ꞌ) according to the 16S Metagenomic Sequencing Library Preparation (MSLP) procedure (Illumina). In brief, 12.5 ng of gDNA was used for the PCR reaction carried out with KAPA HiFi HotStart ReadyMix (Roche) under the PCR condition: 95°C for 3 minutes; 25 cycles of: 95°C for 30 seconds, 55°C for 30 seconds, 72°C for 30 seconds; 72°C for 5 minutes and hold at 4°C. The PCR products were monitored on 1.5% agarose gel. Samples with bright main strip around 500 bp were chosen and purified by using the AMPure XP beads for the following library preparation. The Sequencing library was prepared according to the 16S Metagenomic Sequencing Library Preparation (MSLP) procedure (Illumina). In brief, a secondary PCR was performed by using the 16S rRNA V3-V4 region PCR amplicon and Nextera XT Index Kit with dual indices and Illumina sequencing adapters (Illumina). The indexed PCR product quality was assessed on the Qubit 4.0 Fluorometer (Thermo Scientific) and Qsep100TM system. Equal amount of the indexed PCR product was mixed to generate the sequencing library. At last, library was sequenced on an Illumina MiSeq platform and paired 300 bp reads were generated.
Statistical analysis
The comparison of measurement results for the skin parameters among groups and between groups was analyzed by one-way repeated measurement ANOVA and one-way ANOVA, respectively, followed by Tukey's post hoc test through GraphPad Prism 9, with p<0.05 considered statistically significant.
TCI999 increased the mitochondrial activity and growth of hair follicle cells
To investigate whether TCI999 can increase mitochondrial activity and growth in hair follicle cells, using TCI999 to treat HFDPC for 24 h, and then examined mitochondrial activity and cell viability. The results showed that TCI999 increased the mitochondrial activity by 5.2% compared to mock group as shown in Figure 1A. 20% Fetal Bovine Serum (FBS) treat Human Hair Follicle Dermal Papilla Cells (HFDPC) as positive control can increase viability by 2.1 times compared to mock group, and TCI999 increased the viability by 1.21 times compared to mock group as shown in Figure 1B. The results indicated that TCI999 enhance the mitochondrial activity of hair follicle cells and promote the growth of hair follicle cells (Figure 1A).
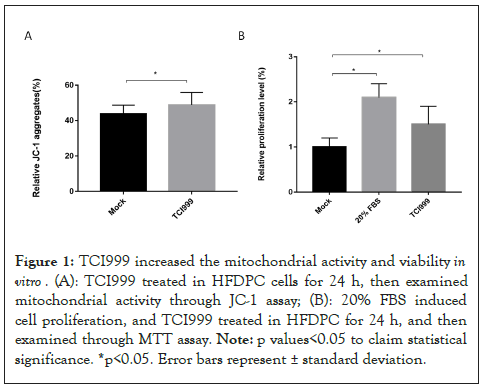
Figure 1: TCI999 increased the mitochondrial activity and viability in vitro. (A): TCI999 treated in HFDPC cells for 24 h, then examined mitochondrial activity through JC-1 assay; (B): 20% FBS induced cell proliferation and TCI999 treated in HFDPC for 24 h, and then examined through MTT assay. Note: p values<0.05 to claim statistical significance, *p<0.05. Error bars represent ± standard deviation.
TCI999 decreased hair follicle growth inhibition-related genes and strengthened hair roots
To evaluate the hair health efficacy of TCI999, 50 adult subjects suffering from hair loss were recruited in this trial. Blood collection, hair loss collection and a questionnaire survey were conducted after taking TCI999 for 12 weeks. First, we want to explore whether TCI999 regulated hair follicle growth inhibition related genes, steroid 5 α reductases (SRD5As), Androgen Receptor (AR) and TGF-β. The SRD5A1 is responsible for converting testosterone into Dihydrotestosterone (DHT), which inhibits hair follicles from producing hair. AR, a nuclear receptor, is mainly expressed in the Dermal Papilla Cells (DPCs) of the human hair follicle. When DHT enters the cell, the AR protein forms a dimer with it in the cytosol, translocate to the nucleus and acts as a transcription factor to promote the expression of the inhibitory proteins of hair growth such as TGF-β. Compared with the placebo group, taking TCI999 for 12 weeks decreased the hair follicle growth inhibition-related genes, SRD5A1, AR, TGF-β by 1.2, 2.7, 484.8 times respectively as shown in Figures 2A-2C. Then, we examined hair root diameter. Taking TCI999 for 12 weeks significantly increased hair root diameter by 4% compared to the baseline (week 0), and increased hair root diameter by 2.6% compared to the placebo as shown in Figure 2D. The results showed that TCI999 can help hair follicle growth and improve hair loss.
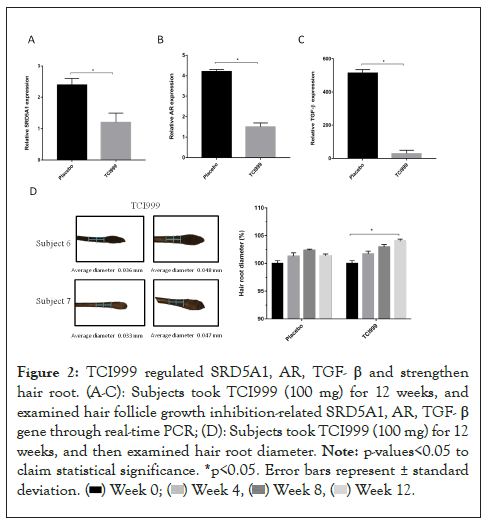
Figure 2: TCI999 regulated SRD5A1, AR, TGF- β and strengthen hair root. (A-C): Subjects took TCI999 (100 mg) for 12 weeks, and examined hair follicle growth inhibition-related SRD5A1, AR, TGF- β gene through real-time PCR; (D): Subjects took TCI999 (100 mg) for 12 weeks, and then examined hair root diameter. Note: p-values<0.05 to claim statistical significance, *p<0.05. Error bars represent ± standard deviation.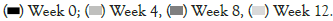
TCI999 increased hair density and improved scalp redness
Next, we examined hair follicle robustness, using the hands to pull up the hair in the frontal, temporal and occipital regions to observe the amount of hair loss. Taking TCI999 for 12 weeks significantly decreased pull hair loss by 36.4% compared to baseline (week 0), and decreased by 22.1% compared to the placebo group as shown in Figure 3A. Then, we examined the amount of hair loss after the subjects washed their hair, as well as hair density and scalp redness. Taking TCI999 for 12 weeks decreased hair loss by 9.7% compared to the baseline (week 0), and significantly decreased by 32.9% compared to the placebo group as shown in Figure 3B. In addition, TCI999 increased hair density and improved scalp redness as shown in Figures 3C and 3D.
Figure 3: TCI999 increased hair robustness and improved scalp redness. (A): Subjects took TCI999 (100 mg) for 12 weeks, and then examined hair follicle robustness; (B): Hair loss volume; (C): Hair density and (D): Scalp redness. Note: p-values <0.05 to claim statistical significance, *p<0.05. Error bars represent ± standard deviation.
TCI999 improved gut microbiome
Finally, we want to know if taking TCI999 can improve gut microbiome. Fecal samples were collected, and then analyzed by 16S metagenomic sequencing. After taking TCI999 for 12 weeks, it can effectively decrease the pro-inflammatory bacterial phase (Negativicutes, Gammaproteobacteria, Verrucomicrobia, Deltaproteobacteria, Fusobacteria) and increased the anti-inflammatory bacterial phase (Actinobacteria, Bacteria, Clostridia) (Figure 4). We also used questionnaires to investigate the somatosensory status of the subjects. After taking TCI999 for 12 weeks, subjects reported the oiliness, inflammation and overall hair loss had improved (Figures 5A-5D). These results suggested that TCI999 can regulate gut microbiome and improve hair and scalp condition.
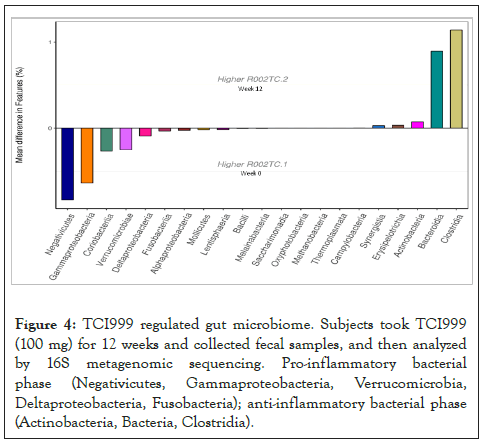
Figure 4: TCI999 regulated gut microbiome. Subjects took TCI999 (100 mg) for 12 weeks and collected fecal samples, and then analyzed by 16S metagenomic sequencing. Pro-inflammatory bacterial phase (Negativicutes, Gammaproteobacteria, Verrucomicrobia, Deltaproteobacteria, Fusobacteria); anti-inflammatory bacterial phase (Actinobacteria, Bacteria, Clostridia).
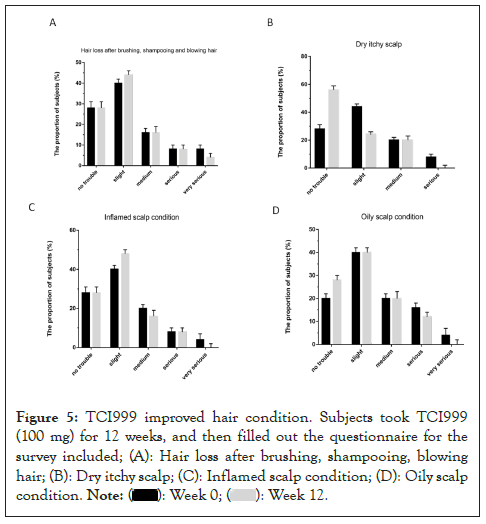
Figure 5: TCI999 improved hair condition. Subjects took TCI999 (100 mg) for 12 weeks, and then filled out the questionnaire for the survey included; (A): Hair loss after brushing, shampooing, blowing hair; (B): Dry itchy scalp; (C): Inflamed scalp condition; (D): Oily scalp condition.
The gut is an important conduit between the internal environment and the external environment of the human body. In addition to nutrient absorption and elimination, the gut is a complex and diverse ecology: the gut microbiome [13]. The complex relationship of the peripheral enteric nervous system and the gut immune system ultimately links the digestive system to many other parts of the body, including hair [14]. Therefore, the integrity of the gut microbiome is critical for optimal whole-body health. An imbalanced gut microbiome may fail to regulate important functions of the hair cycle, such as providing nutrients, synthesizing certain vitamins, and regulating the immune system, resulting in impairment of bodily functions, including hair loss [15]. In this study, TCI999 (L. plantarum) increased mitochondrial activity and growth of hair follicle cells. In clinical trial, taking TCI999 decreased hair follicle growth inhibition-related genes and strengthened hair roots and improved scalp redness. In addition, TCI999 increased the beneficial bacterial phase, suggesting TCI999 can affect hair growth by regulating gut microbiome.
The most commonly used probiotics belong to the Lactiplantibacillus and Bifidobacterium [16]. Dietary probiotic supplementation may improve scalp blood flow and reduce hair loss [17]. L. plantarum promoted vascular growth and hair growth in C57BL/6 mice. L. paracasei stimulated HFDPC proliferation and increased VEGF and Insulin-like Growth Factor-1(IGF-1) [18]. Oral administration of L. paracasei promoted hair growth and hair follicle maturation in the dorsal skin in mice. In addition, L. reuteri encouraged hair follicle development, promoted faster hair growth, prevented hair thinning, and even increased the hair number in the anagen stage of the hair cycle [19]. Taking yogurt containing L. rhamnosus with L. paracasei for 8 days, the effect of hair growth was better than the drug minoxidil in mice. Microscopic analysis showed that 70% of the hairs of yogurt-fed mice were in the anagen phase or the active growth phase of hair, whereas in the control group, which only had 30% of their hairs in the anagen phase [19,20]. L. paracasei along with other gut microbiome helped boost the production of biotin, a vitamin essential for hair growth [21]. Studies had shown that a lack of biotin can lead to thinning hair, which can lead to hair loss [22]. Consistent with our results, TCI999 (L. plantarum) increased mitochondrial activity and growth of hair follicle cells, and taking TCI999 decreased hair follicle growth inhibition-related genes and strengthened hair roots and improved scalp redness. Studies have showed that gut microbiome may be associated with hair loss. Intestinal microbiota can induce Th1 response, resulting in the production of Interferon gamma (IFN-γ), activating the Janus Tyrosine Kinase (JAK)/Signal Transducers and Activators of Transcription (STAT) signaling pathway, and causing hair loss [23,24]. Hair growth in two patients with alopecia areata treated with Fecal Microbiota Transplantation (FMT), suggesting gut microbiome regulates hair mechanism [25]. For Inflammatory Bowel Disease (IBD) patients, the abundance of the Negativicutes, Gammaproteobacteria, Verrucomicrobia, Deltaproteobacteria, Fusobacteria were increased [26,27]. Actinobacteria, Bacteria, Clostridia had anti-inflammatory effect [28]. Our results showed that TCI999 decreased the pro-inflammatory bacterial phase (Negativicutes, Gammaproteobacteria, Verrucomicrobia, Deltaproteobacteria, Fusobacteria) and increased the beneficial bacterial phase (Actinobacteria, Bacteria, Clostridia). However, more studies were needed to confirm how TCI999 regulated these bacterial phases.
The limitations of this study included that the majority of subjects were female (n=41), with a small proportion of men (n=9); It was best to set up a positive control group for comparison. Thus, TCI999 stimulated hair growth and inhibited hair loss. It was likely to be an alternative treatment for hair loss in the future. In conclusion, the advantage of TCI999 was that it can be eaten by eating fermented foods, such as wolfberry, codonopsis, bauhinia, and astragalus. On the other hand, supplementing the diet rich in TCI999 can strengthen the hair roots and improve the phenomenon of hair loss by regulating the gut microbiome.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Liang CH, Lin YH, Chan ST, Lin YK, Chiang CF (2022) Lactiplantibacillus plantarum TCI999 Probiotic Promoted Hair Growth and Regulated Gut Microbiome: Double-Blind, Placebo-Controlled Trial. J Prob Health. 10:285
Received: 01-Aug-2022, Manuscript No. JPH-22-18915; Editor assigned: 03-Aug-2022, Pre QC No. JPH-22-18915 (PQ); Reviewed: 17-Aug-2022, QC No. JPH-22-18915; Revised: 24-Aug-2022, Manuscript No. JPH-22-18915 (R); Published: 02-Sep-2022 , DOI: 10.35248/2329-8901.22.10.285
Copyright: © 2022 Liang CH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.