Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2019)Volume 8, Issue 1
Brazil is the country with the greatest biodiversity; however, most of the plants are used empirically, without scientific evidence. Luehea candicans, popularly called as-açoita-cavalo, is a specie characteristic of the Brazilian Cerrado Biome, used for fight hemorrhages, dysenteries, diarrhea, rheumatism and tumors. On the other hands, there are practically no studies about chemical composition and assess of their biological effects. Thus, the aim of this investigation was characterize and evaluate the antitumor potential of crude hydroethanolic extract from the bare stem parts of this plant, as well as its ethyl acetate and methanolic fractions. For the characterization, the crude extract was submitted to qualitative (phytochemical screening and analysis by direct flow injection by mass spectrometry-FIA-ESI-IT-MS) and quantitative tests (determination of phenolic compounds and determination of total flavonoids and anthocyanins). In order to study the antitumor potential, MTT technique was used to evaluate the cellular metabolism of canine breast cancer tumor cells (TCM) and osteosarcoma (OST). The results demonstrated the presence of polyphenols, mainly flavonoids and tannins. In addition, were observed an increase in metabolism when TCM and OST cells were incubated with all extracts of L. candicans. Based on this, L. candicans in these tumor types should be careful use, and that in vivo studies should be performed to prove the true effects of this plant.
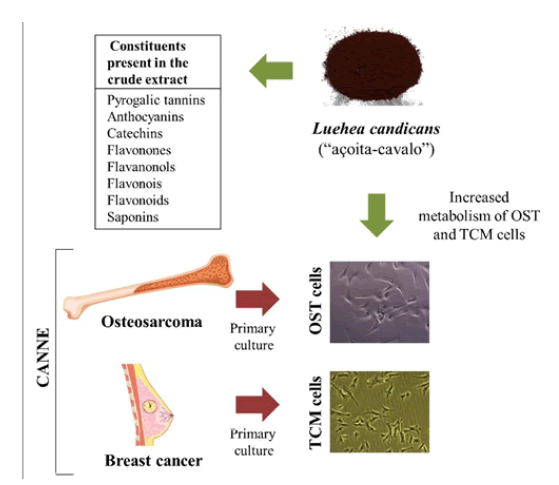
Antitumor effect; Breast cancer; Characterization; Mass spectrometry; Osteosarcoma tumor
Medicinal plants are defined as those capable of alleviating or curing diseases, based on traditional use as a remedy in a population or community. Phytotherapy is based on ancient traditions, widely disseminated through salespeople, healers, faith healers, part of the culture of indigenous people, and rural areas [1]. According to the World Health Organization (WHO), approximately 85% of the population of developing countries depended on medicinal plants and herbal medicines for primary health care [2], even could be considered them as a complementary therapies [3]. Medicinal plants plays an important role in pharmacological research, not only when their constituents are used directly as therapeutic agents, but also as raw materials for the synthesis or models for pharmacologically active compounds [4]. Approximately 7000 substances showing pharmaceutical importance have been isolated from known medicinal plants properties, and several constituents processed into pharmaceuticals. However, thinking about a higher level, of all the 365.000 species of flowering plants, it is estimated that only 8% of them supported systematically studied in terms of constituents and proven biological effects [1]. Brazil has around 15 to 20% of all described plants, and most of them are characteristic of the Cerrado Bioma [5]. Based on this, the analysis of the bioactivity of Cerrado Bioma medicinal plants could provide the basis for the production of new drugs [1]. Luehea candicans, popularly known as açoita-cavalo is a tree plant that can reach 8-12 m in height. Although not endemic in Brazil, it is distributed from the South to the North of the country, and its predominance occurs on Cerrado Bioma, Amazon Rainforest, Caatinga, Atlantic Florest and Pantanal [6]. It has popular use for dysentery, hemorrhage, arthritis, diarrhea, clear blood, leucorrhea, rheumatism and tumors [7]. However, only two studies were performed in order to prove its effects, being demonstrated inhibitory effect on the proliferation of human kidney cancer cells [8] and antimicrobial activity [7]. Cancer is a leading cause of non-communicable morbidity and mortality wide world [9]. In human and veterinary medicine, mammary tumors (TCM) represent the most frequently diagnosed neoplasia [10], with shared features between human and dogs [11]. In addition, osteosarcoma (OST) is the most common primary bone cancer in human and dogs [9,12], also sharing several aspects [12]. Then, for both TCM and OST, dog could be an elective model to prognosis and treatment [9,11]. As L. candicans is used empirically for the treatment of cancer, and dogs could be a good model, we aimed to characterize the L. candicans crude extract and organic fractions, as well as, evaluate the in vitro antiproliferative potential in canine breast cancer (TCM) and osteosarcoma (OST) cells.
Collection and identification of the species Luehea candicans
The vegetal species Luehea candicans was collected in Morros, Maranhão, Brazil. The location was recorded by GPS, Garmin, model, Edge 1000 and is under coordinates 2° 55 '23.4 "S/43° 55' 31.9" W. After collection, the specimen was identified and cataloged at Herbarium Seabra Rosa Mochel, of the Department of Biological Sciences, State University of Maranhão with their taxonomic identifications under number 4586. The stem skeleton was collected and transported to the Laboratory of Natural Products of Maranhão Federal Institute (IFMA), São Luís-Monte Castelo Campus, to selection and cleaning. The vegetable was dried at room temperature and shaded in an aerated area for five days. The coarser, after drying, was ground in a Tecnal 651/2 electric mill (Laboratory 6-Natural Products-IFMA) with different meshes (10 mm, 5 mm and 1 mm diameter). The material obtained from the grinding process was stored in an amber container and stored in a refrigerator.
Preparation of crude extract
The botanical material, after crushing, was subjected to the percolation process in 80% hydro alcoholic solution (EtOH/H2O) in the proportion 1:3 (m/v) [54]. The mixture was placed in a suitable vessel and mechanically stirred. After 24 hours the mixture was filtered and the solid was put back into the vessel and a new hydro alcoholic solution was added, this procedure being repeated twice more, totaling three extractions, thereby obtaining the crude hydroethanolic extract which was then stored in amber bottle.
Obtaining the methanolic fractions (MeOH) and ethyl acetate (AcEt)
From the crude hydroethanolic extract, the liquid-liquid fractionation process was started to obtain the organic phases. Partitioning of the crude extract followed in increasing order of polarity where in a settling funnel the extract and hexane were placed. The mixture was homogenized and after 24 hours there was separation of the hexane phase. Shortly after completion with hexane, ethyl acetate was added under the same conditions as described for the hexane phase. Remaining aqueous phase, n-butanol was added, homogenized and after 24 hours separation was carried out. This procedure was repeated two more times to give the n-butanol phase (F-BuOH). This procedure is used to obtain the methanolic phase (F-MeOH), as shown in Figure 1.
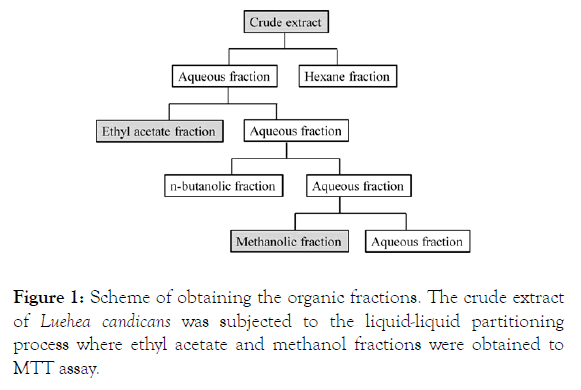
Figure 1: Scheme of obtaining the organic fractions. The crude extract of Luehea candicans was subjected to the liquid-liquid partitioning process where ethyl acetate and methanol fractions were obtained to MTT assay.
Calculation of dry weight and extraction yield
The yield calculation is based on the arithmetic mean of the extract weight after the total evaporation of the solvent. To do this, three test tubes were weighed on analytical balance and the weight with four decimal places was noted. Then, 1 mL of the extract was added to each tube, which was submitted to the evaporation of the solvent in an oven at 110ºC for 1 h. To obtain the weight of the dried sample of each tube the difference between the filled and empty tube was made. The yield calculation was done through the relationship between the amount of powder and the amount of mass extracted in the extraction process.
Phytochemical screening
The preliminary prospecting test was carried out from the crude hydroethanolic extract, submitted to the chemical analyzes through a series of reactions with acidic and basic solutions, which in turn presented color variations and intensity, in addition to the precipitation of some of the investigated metabolites, such as phenols, tannins, anthocyanins, anthocyanidins, flavonoids, leucoantocyanidins, catechins and flavones, flavonols, flavanones, flavanones and xanthones, steroids, triterpenoids, saponins and alkaloids [13].
Determination of phenolic compounds
Total phenolic compounds were quantified by spectrophotometric method using the Folin-Ciocalteu reagent. The samples were analyzed in triplicate. The solution was then homogenized and allowed to stand for 3 min, after which time, 1 mL of saturated anhydrous sodium carbonate solution was added and allowed to stand for 1 h. Then the absorbance readings were taken in Digital spectrophotometer (model SP-22, brand Biospectro) at 720 nm. In order to calculate the amount of total phenolic compounds, the equation of the straight line of the standard curve constructed with gallic acid in the concentrations of 0; 10; 15; 20; 25 and 35 mg/L [14].
Determination of total flavonoids and anthocyanins
For the determination of flavonoid and anthocyanin content a spectrophotometer was used at different wavelengths, in the reading range from 325 nm to 1000 nm. The analysis was performed with 10.0 mL of 95% ethanol solution+1.5 N hydrochloric acid (HCl) which was previously prepared (85:15). The determination of anthocyanin content was done by absorbance at 535 nm. As for flavonoid analysis, the same procedure was followed with absorbance of 374 nm [14]. Calculation of the anthocyanin content was determined by multiplying the absorbance with dilution factor and dividing by 98.2. The result was correlated to 1 mL and the amount of mL in 100 g was determined.
Analysis using direct flow injection by mass spectrometry (FIA-ESI-IT-MS)
The sample direct flow infusion was performed in Thermo Scientific LTQ XL linear trap type analyzer equipped with electrospray ionization source (ESI) in negative mode (Thermo, San Jose, CA, USA). A stainless-steel capillary tube at 280°C, a spray voltage of 5.00 kV, capillary voltage of -90 V, -100 V tube lenses and a flow of 5 μL/min were used. The complete scan analysis was recorded in the m/z range of 100-1000. Multiple-stage fragmentation (ESI-MSn) was performed using the collision-induced dissociation (DIC) method against helium for ion activation. The first scanning event is given by a complete mass spectrum data acquisition on ions m/z range. The second scanning event was an MS/MS using data scan dependent on the [M-H]¯ compounds molecules of interest with collision energy of 30% and activation time of 30 ms. The ions produced were then subjected to greater fragmentation under the same conditions, until no further fragments were observed [14].
In vitro assays
Cell culture: All assays were approved by the Ethics Committee on the Use of Animals, from Faculty of Veterinary Medicine and Animal Science (FMVZ)/USP (CEUA nº 1100210217). The canine mammary solid carcinoma (TCM) and osteosarcoma (OST) were acquired during the elective surgeries under tutor’s authorization. Canine tissues were mechanically and enzymatically (type I collagenase) dissociated and cells were placed in sterile 75 cm2 growth bottles containing medium High glucose DMEM (#41965062, Gibco) supplemented with antibiotic (10 mL/L, penicillin- streptomycin (#15140163, Gibco) and 10% (v/v) fetal bovine fetal serum (FBS, #16000044, Gibco). The bottles were placed in the humidified incubated at 37°C with 5% carbon dioxide (CO2). The medium was replaced every two or three days, according to the confluence of the cell monolayer, and the subcultures were performed when the cells had 80% confluence.
Cell count with trypan blue: This method is based on the microscopic observation that viable cells are impermeable to the dye, whereas the non-viable cells have permeability due to the formation of pores in the membrane; this allows the penetration of the blue dye [15]. When the cells reached 80% confluence they were washed with PBS (136.9 mM of NaCl, 26.8 mM of KCl, 14.7 mM of KH2PO4 and 8.1 mM of Na2HPO4.7H2O; pH 7.2) and harvested with 0.25% trypsin solution (#R001100, Gibco, and centrifuged at 1500 rpm for 5 minutes. After centrifugation, the supernatant was withdrawn and the pellet resuspended in 1 mL of medium. Then, 10 μL of the cell suspension and 10 μL of trypan blue were mixed and 10 μL being added to the Neubauer chamber and counted under a microscope.
Cell metabolism assay with 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazole bromide: The MTT technique is a quantitative, colorimetric and easy to perform assay. The method is based on the reduction of tetrazolium MTT to formazan crystals through the action of mitochondrial enzymes, such as succinate dehydrogenase, thus providing a measure of mitochondrial function. The amount of formazan crystals formed is directly proportional to the number of viable cells [16]. Cells were plated (1 × 104 cells) in 96-well microplates, and cultured as described before. After 24 hours, the culture medium was removed and medium and the crude extract and fractions (ethyl acetate and methanol) were added at different concentrations (10-1000 μg/mL), the control group receiving only medium, and cultured for 24, 48 or 96 hours. After the incubation period, the supernatant was removed and 200 μL per well of a solution containing 5 mg/mL MTT in DMEM was added and incubated for 1 h at 37°C. The MTT solution was then removed and 100 μL of dimethylsulfoxide (DMSO) were added to each well. The absorbance was read on spectrophotmeter reader at 570 nm (MQuant-Bio Tek Instruments, VT, USA). To evaluate the cellular metabolism (%), the following formula was used, in which 100% was assigned to the control: cell metabolism (%)=(absorbance of treated cells/control absorbance) × 100.
Statistical analysis
For the MTT test, the statistical analyses were performed by least square means analysis of variance with GLM procedure of SAS (version 5.1). The model included main effects (concentration and time) and standard errors. Graphs were plotted on GraphPad Prism (version 5.0).
Yield of the crude extract and fractions
The mass and yield of the Luehea candicans stem bunch and organic phases crude extract are shown in Table 1. It can be observed that the methanolic fraction showed the highest percentage of yield (40%), followed by the ethyl acetate fraction (19.70%). The crude extract showed the lowest yield (15.13%).
| Compounds | Mass used (g) | Obtained mass (g) | Yield (%) |
|---|---|---|---|
| Crude extract | 600 | 90.77 | 15.13 |
| Ethyl acetate fraction | 9.83 | 19.7 | |
| Methanolic fraction | 19.96 | 40 |
Table 1: Results of extractions of crude extract and organic phases.
Phytochemical prospecting
The results of the phytochemical prospection of the crude extract showed a phytochemical Profile composed of hydrolysable tannins, flavonoids, anthocyanins, catechins, flavones, flavanones, xanthones, catechins, steroids, saponins and alkaloids with their proper classifications (Table 2). The identification of chemical constituents was related by the presence of color intensities, precipitation and foaming, and the results are consistent with the classification in abundant (+++), moderate (++), low (+) and absent (-).
| Constituents | Results |
|---|---|
| Phenols | - |
| Pyrogalic tannins (hydrolysable tannins) | + + + |
| Flobabenic tannins (condensed or cathected tannins) | - |
| Xanthones | + + |
| Anthocyanidins | + + |
| Anthocyanins | + + + |
| Leocoantocyanidins | - |
| Catechins | + + |
| Catechins (confirmation) | + + + |
| Flavonones | + + + |
| Flavanonols | + + + |
| Flavonois | + + + |
| Flavonoids | + + + |
| Free Steroids | + |
| Alkaloids | + + |
| Saponins (saponin heterosides) | + + + |
| Confirmation of saponins | + + + |
Table 2: Result of preliminary phytochemical prospecting of the hydroethanolic extract. Abundant (+++), moderate (++), little (+), absent (-).
Content of phenolic compounds
The total phenol content was expressed as gallic acid equivalents (EAG)/g extract, which was detected through the analytical curve (y=0.0069x+0.0062) in 1076.8 ± 3.86 mg. EAG.g-1, with a standard deviation of 0.0066 between measures.
Flavonoids and anthocyanins content
The total anthocyanin and flavonoid contents in the crude extract were determined by visible spectrophotometry at wavelengths 535 and 374 nm. The results are shown in Table 3, where it can be observed that the constitution of flavonoids is much higher than that of anthocyanins (29.30 and 8.33, respectively).
| Compounds/λ (nm) | Mean (mg/100g) | Concentratio (mg/100g) |
|---|---|---|
| Anthocyanins/(535) | 0,409 ± 0,003 | 8,330 |
| Flavonoids/(374) | 1,438 ± 0,005 | 29,300 |
Table 3: Total anthocyanin and flavonoid contents found in the crude hydroethanolic extract.
Analysis using direct flow injection by mass spectrometry (FIA-ESI-IT-MS)
From the analysis by FIA-ESI-IT-MS it was possible to observe 16 molecular ions considered as base peaks, fingerprint spectra (Figure 2).
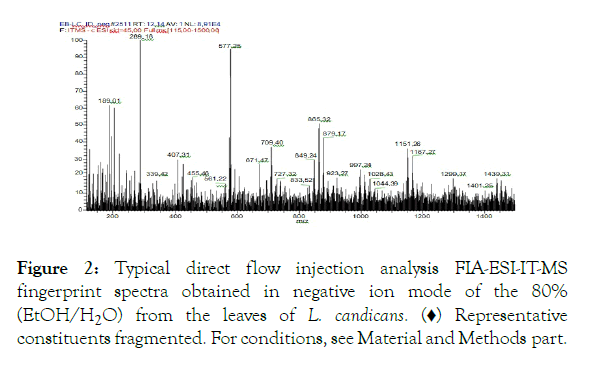
Figure 2: Typical direct flow injection analysis FIA-ESI-IT-MS fingerprint spectra obtained in negative ion mode of the 80% (EtOH/H2O) from the leaves of L. candicans . (♦) Representative constituents fragmented. For conditions, see Material and Methods part.
The structural identification of the corresponding compounds was done by analyzing the peaks generated by the fragmentation in the mass spectrometry. The chemical constituents identified from the Luehea candicans bare stem crude hydroethanolic extract are found in Table 4. In general, the secondary metabolites were composed by catechins, procyanidins and their derivatives and anthraquinone class, all of which are within a larger group, the polyphenols. In Figure 3 it is possible to observe the compounds identified in the Luehea candicans crude extract, according to Table 4.
| Numbers | [M- H]- (m/z) | Fragments (msn) | Compounds |
|---|---|---|---|
| 1 | 289 | 271; 245; 203 | Catechin |
| 2 | 341 | 297; 179; 161 | Cafeoil-o-hexoside |
| 3 | 407 | 289; 257; 243 | Dimeric procyanidin derivative |
| 4 | 423 | 405; 379; 299; 261; 243 | Dimer prodelfinidine derivative |
| 5 | 577 | 425; 407; 273 | Dimeric procyanidin B |
| 6 | 591 | 573; 289; 259 | Methylated dimer |
| 7 | 605 | 573; 529; 285 | Dimethyl dimer |
| 8 | 671 | 509; 491 | Glycosylated diantrone emody |
| 9 | 695 | 543; 525 | Trimeric procyanidine derivative |
| 10 | 709 | 557; 407 | Dimeric procyanidin monogalate |
| 11 | 847 | 685; 523; 505 | Sennoside type B |
| 12 | 879 | 727; 575; 557 | Dimeric procyanidin digalate type A |
| 13 | 997 | 709; 557; 407 | Procyanidine trimer monogalate type A |
| 14 | 1151 | 999; 981 | Procyanidin Tetramer type A |
| 15 | 1153 | 865; 577; 299 | Procyanidin Tetramer type B |
| 16 | 1167 | 1015; 863; 711 | Procyanidin trimer type A digalate |
Table 4: Compounds identified from the Luehea candicans stem crude binder extract by FIA-ESI-IT-MSn.
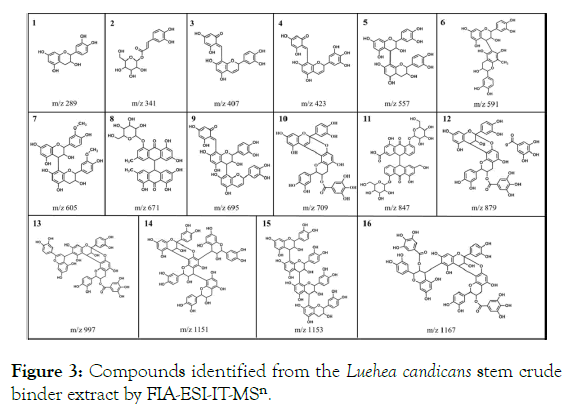
Figure 3: Compounds identified from the Luehea candicans stem crude binder extract by FIA-ESI-IT-MSn.
Evaluation of cellular metabolism by MTT assay
The crude extract, ethyl acetate and methanolic fractions were tested for their putative antitumor effect using the MTT cell metabolism assay. For this, osteosarcoma (OST) and breast cancer tumor cells (TCM) were used. The crude extract (Figure 4A-4C), the methanolic (Figure 4D-and ethyl acetate fractions (Figure 4G-4I) were incubated with OST and TCM cells for 24, 48 and 96 hours. It is possible to observe that, in general, the cellular metabolism increased proportionally to the extract concentration increase. We also observed that cells incubated with DMSO (solvent used to dilute the extracts) did not change in relation to the control cells. Although there is no significant difference between the groups of cells treated (OST × TCM) with 10 μg/mL crude extract of L. candicans, it is worth noting that there was a reduction of metabolism in 54% and 30% in times of 96 (Figure 4C) and 48 hours (Figure 4B), respectively, in relation to control. It is also possible to observe that the ethyl acetate fraction in 10 μg/mL promoted a significant decrease in TCM metabolism by 50% when incubated for 96 h (Figure 4I).
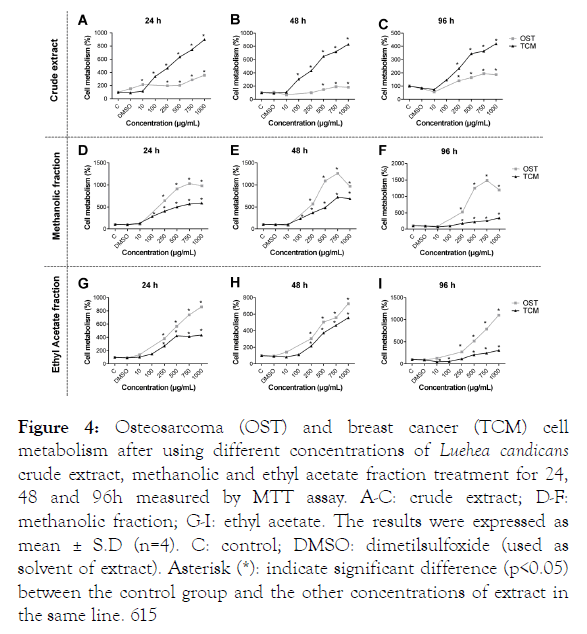
Figure 4: Osteosarcoma (OST) and breast cancer (TCM) cell metabolism after using different concentrations of Luehea candicans crude extract, methanolic and ethyl acetate fraction treatment for 24, 48 and 96h measured by MTT assay. A-C: crude extract; D-F: methanolic fraction; G-I: ethyl acetate. The results were expressed as mean ± S.D (n=4). C: control; DMSO: dimetilsulfoxide (used as solvent of extract). Asterisk (*): indicate significant difference (p<0.05) between the control group and the other concentrations of extract in the same line. 615
Plants are an important molecules source for drug discovery, either as starting materials for laboratory synthesis, as well as for biologically active compounds production model [17]. The plants belonging to the Luehea genus can be seen as a source of new medicinal agents [18,19]. In Brazil, these plants are present in Brazilian Cerrado Bioma and thirteen species are registered in Brazilian herbariums, being the most cited are L. grandiflora and L. divaricata . On the other hands, there are practically no studies involving L. candicans [19]. Thinking to increase the chemical and antitumor effect knowledge, the study of crude hydroethanolic extract of the bare stem parts of Luehea candicans was carried out for the first time. The phytochemical processing is essentially necessary to optimize the components concentration and also to maintain its activities [17]. Based on this, our first step was to evaluate the crude extract and fractions yield. Our results showed that the L. candicans crude hydroethanolic extract, obtained by percolation extraction, had an acceptable yield (~15%). Then, it was submitted to the liquid-liquid fractionation process to obtain the organic phases, using solvent in increasing polarity order. Although the methanolic fraction had a higher yield (40%) than the ethyl acetate fraction (~20%), both had good income. It is known that the solvent polarity is a very important parameter and influence in extracted secondary metabolite yield and type. For example, phenolic compounds highest extract yields were obtained with polar alcohol-based solvents and water addition to ethanol improves the extraction rate [20]. Probably, in our study, the fact that the L. candicans crude extract has a good yield is due to the solvent extractor used (water and ethanol), which extracted a high content of secondary metabolites, mainly polyphenols, as will be discussed below. In relation to the fraction, ethyl acetate was semi polar solvent that could dissolve sterol and alkaloid, on the other hands, methanol can dissolve polar compounds, such as polyphenol [21].
Possibly, the polyphenols present in the crude extract were extracted by methanol, which justifies the higher yield than ethyl acetate fraction. According to our results, the phytochemical prospecting of the L. candicans crude extract profile were composed mainly by polyphenols (flavonoids and hydrolysable tannins). These data were confirmed by mass spectrometry analysis, which showed catechins, procyanidins and their derivatives and anthraquinone class, all belonging to polyphenols group. Besides that, the content of phenolic compounds and flavonoids content showed the large number. Polyphenols are characterized by the presence of one or more aromatic ring bearing one or more hydroxyl moieties, devoid of any nitrogenbased functional group in their most basic structural expression. There are more than 8000 structural variants, being considered the largest family of secondary metabolites generated by the shikimate-derived phenylpropanoid and/or the polyketide pathway. This huge variety of compounds lies in the potential for multiple interactions with other groups, such as sugars, alcohols and acids [22]. In the plants, they are involved in a number of mechanisms, such as defense against ultraviolet radiation, cold temperatures, defend themselves against herbivores, parasites and pathogens [22]. The widest group of phenolic compounds is the flavonoids. The most common flavonoids are usually found in food/plant linked to sugars, acids or alcohols. They can be subdivided into 14 subclasses based on the degree of oxidation of the heterocyclic ring: chalcones, dihydrochalcones, aurones, flavones, flavonols, dihydroflavonols, flavanones, flavanols, flavandiols, anthocyanidins, isoflavones, flavonones and proantocyanidins [22]. Another very common class of phenolic compounds is tannins. The tannins can be divided into four classes: hydrolyzable, condensed, complex and florotannins. Hydrolyzable tannins consist of a monosaccharide nucleus, usually glucose, esterified with gallic acid. Condensed tannins have a flavonoid origin. These are oligo or polymeric proanthocyanidins where the phenolic hydroxyls are wholly or partially esterified with gallic acid. The complex tannins have a molecular structure which can be considered a mixture of hydrolyzable and condensed. Florotanins are a small group of tannins isolated mainly from brown algae [23]. According to Silva et al. [8], the methanolic extracts from the branches and leaves extract from the L. candicans showed that the presence of phenolic groups are able to assist in the identification of chemical markers of this species. In this study, lupeol, betulin, a mixture of steroids, (-)-epicatechin, vitexin and liriodendrin were found [8]. The presence of flavonoids, saponins and tannins has also been described for Luehea divaricata [24]. The presence of flavonoids, characterized as (-)- epicatechin, which belongs to the flavan-3-ol class, has also been isolated from stem’s bark of Luehea divaricata [25]. The ethanolic extracts of L. grandiflora and L. paniculata had the presence of tannins and flavonoids in leaves and bark of both species [26,27]. The skins of L. ochrophylla showed hydrocarbons, aliphatic esters of steroid -sitosterol, pentacyclic triterpenes friedelin and -friedelinol [19]. In addition, the presence of anthraquinone class in species belonging to the family Malvaceae has been described [28,29]. Although there are some studies, little information is known about the chemical constituents present in the genus Luehea [19]. Plants and food products containing polyphenol have been used to treat various human diseases since ancient times [30,31]. The most striking feature of polyphenols is undoubtedly their ability to capture reactive oxygen species (ROS), which include both radical and non-radical oxygen species, as well as oxidative free radicals derived from biomolecules, such as low density lipoproteins (LDL), proteins and oligonucleic acids [32]. This antioxidant ability is frequently associated to the property underlying the prevention and/or reduction of oxidative stress-related diseases [33-35], such as diabetes [30]; cardiovascular diseases [36], neurodegeneration [5], inflammation [33] and carcinogenesis [37-40]. It is known that cancer is characterized by sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis [41]. In most developed nations, cancer is the second leading cause of death, falling only behind cardiovascular diseases [42]. Not only humans are susceptible to neoplasias, being the diagnosis in pe increasingly common. In canine females, mammary neoplasia is one of the most frequent, becoming a problem in veterinary medicine. In addition to this, there is osteosarcoma, which is the most observed primary bone neoplasia in dogs [43]. Multidrug resistance and various adverse side effects have long been major problems in cancer chemotherapy [44]. Based on this, we evaluated the antitumor potential, by cellular metabolism, of L. candicans in canine mammary solid carcinoma (TCM) and osteosarcoma (OST).
Analyzing the results, in general, we can observe a proportional cellular metabolism increase with increasing extracts concentrations, both for TCM and OST. In the study conducted by Silva et al. [8] the antiproliferative activity of the L. candicans crude extract and leaf and stem fractions were evaluated in nine human tumor cells, including mammary adenocarcinoma (MCF-7). The crude extract from the branches inhibited the growth of MCF-7 cells by 50% with a concentration of 37.7 μg/mL. This effect was attributed to the presence of lupeol, which is a triterpene that has been associated with the ability to inhibit the proliferation of a variety of tumor cells [42]. In our study, a minimum amount of lupeol was found, making us infer that this fact did not lead to a decrease in cellular metabolism. In addition, the type of breast neoplasm (MCT) evaluated in our study has more marked malignancy characteristics than MCF-7. A distinct profile was found for the ethyl acetate fraction, which presented a decrease in metabolism with 10 and 100 μg/mL. We can infer that in this case a hormesis process is occurring, which is used to describe a dose-response which a low dose of stimulation or beneficial effect in relation to a high dose of inhibitory or toxic effect [45]. In osteosarcoma there is a reduction of osteoblasts and an increase of osteoclasts, facilitating the process of tumor invasion [46]. It has been described that polyphenols, especially catechins, are capable of activating bone formation, since they promote osteoclasts decrease and osteoblasts increase [38,47]. Although our extract had a high content of polyphenols, there was no decrease in the metabolic activity of the osteosarcoma cells. It is known that the antiproliferative activity of natural compounds is specific for each cell type [48] and that each cell captures polyphenols differently [49]. Possibly, due to the high degree of malignancy of the osteosarcoma cells [50-54] the polyphenols present in the L. candicans extract were not able to exert their function.
In summary, we can highlight that the species Luehea candicans presents a high content of polyphenols, especially flavonoids and terpenes. When its antitumor activity was tested in breast cancer and canine osteosarcoma cells, an increase in cellular metabolism was found, showing that the use of this plant for these tumor types should be careful. However, it is worth mentioning that other tests on other tumor cells must be performed, especially in vivo assays that take into account the complexity of the organism and the different forms of metabolization.
University of São Paulo (USP); Coordination of Improvement of Higher Level Personnel (CAPES); Federal Institute of Maranhão (IFMA).
The authors declare that there is no conflict of interest.
MBPC: performed the characterization of the extract; ACSR: performed the MTT assay and was the lead writer of the article; JB: assisted in cell culture and article writing; FB: assisted in cell culture and MTT assay; RSNB, MAM, FJCC and AJCF supervised all the research and article writing.
Citation: Camara MBP, Rabelo ACS, Borghesi J, Bessa F, da Silva Nunes Barreto R, Angélica Miglino M, et al. (2019) Luehea candicans Increase In vitro Cell Cancer Metabolism Even with High Polyphenols Content. Med Aromat Plants (Los Angeles) 8:329. doi: 10.35248/2167-0412.19.8.329
Received: 12-Feb-2019 Accepted: 18-Feb-2019 Published: 25-Feb-2019 , DOI: 10.35248/2167-0412.19.8.329
Copyright: © 2019 Camara MBP, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.