Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2022)Volume 10, Issue 7
A novel method for the encapsulation of probiotics has been developed using a supercritical carbon dioxide (scCO2)-based Particles from Gas Saturated Solutions (PGSS) process. The excipients used in microencapsulation using scCO2 are usually pharmaceutical-grade polymers that need Food and Drug Administration (FDA) approval. This study aimed to evaluate the suitability of two new food-grade materials with Generally Recognized as Safe (GRAS) status as candidate excipients for encapsulation of probiotic cultures. Compritol E 472 ATO and Vegetal BM 297 ATO were used to encapsulate Bifidobacterium lactis Bb12 using scCO2 as a plasticizer in the PGSS process. The resulting microparticles were characterized based on morphological properties, particle size distribution as well as encapsulation efficiency. Compritol E 472 ATO was found to be unsuitable due to the poor survival of probiotic cultures during the encapsulation process. Vegetal BM 297 ATO was found to be suitable as a carrier. The resulting microparticles were found to have an encapsulation efficiency of 97% and a mean particle size of 31 µm. Vegetal BM 297 ATO has potential for use in the food and pharmaceutical sectors for controlled release and protective barrier function applications as it has a high bacterial load and produces microparticles small enough to not affect the texture of the food products.
Microencapsulation; Probiotics; Bifidobacteria; Supercritical; Microparticles
The human gastrointestinal tract plays host to many cells, more so microorganisms than human [1]. The human gut microbiome is very diverse in terms of the sheer number of organisms, about 1013, but also the diversity of species, about 500 [2]. The balance between the good or probiotic bacteria and pathogenic bad bacteria play a lifelong role in overall health [2]. Good gut health is important in terms of resistance to diseases, especially in impoverished areas as it prevents many conditions that require expensive and sometimes dangerous treatments. Maintaining good gut health and consuming a healthy and diverse diet prevents certain illnesses which saves governments substantial amounts of money spent that would have been spent on healthcare needed to treat avoidable problems.
The probiotic microorganisms inhabiting the human gastrointestinal tract offer a range of benefits which include detoxification of gut metabolites, increases in non-specific immune response, aid in carbohydrate digestion, deactivation of pro-carcinogenic compounds, and the synthesis of vitamins [2-4]. On the other hand, the pathogenic bacteria lead to disturbances collectively termed dysbiosis which include irritable bowel syndrome, digestive disturbances, diarrhea, production of carcinogens, alterations in endocrine system functioning, hypervigilant immune responses, weight gain due to changes in carbohydrate and fat metabolism, hypercholesterolemia, fatigue, electrolyte imbalances, atopic dermatitis, depression, anxiety, allergies, and metabolic syndrome [5-9]. The pathogens that cause these disturbances are believed to be curbed by probiotics either by inhibition, competitive exclusion, or inhibitory factors [10,11].
Probiotic cultures have short shelf lives in refrigerated products due to low temperatures and low viability over time in dried products [12,13]. During storage and processing, they undergo stress when exposed to light, moisture, and oxygen. In addition to these outside factors, there are harsh conditions in the human body, mainly the low pH of the stomach and the presence of bile salts, which result in a loss of live cells [14]. This results in a decrease in the number of live cells in the small intestine and colon where adhesion needs to be achieved. If fewer cells are present at the adhesion site, the chance of adhesion decreases, leading to a lower chance of successful colonization, which needs to occur for health benefits to be realized. If the cells could be protected against these harsh environments and reach the adhesion sites in higher numbers, fewer cells would have to be added to the initial foodstuff to account for losses, which would result in a significant cost saving for the consumer.
Microencapsulation is a technique that involves entrapping microbial cells or bioactive compounds in a semi-permeable either organic or inorganic polymeric gel structure to protect it from some factor that might decrease the efficacy or viability of its contents such as light, moisture, oxidation, and acids [15]. This allows for the targeted delivery of microorganisms as well as increasing shelf-life thereby yielding more viable cultures to colonize the gut. The current methods used in microencapsulation are plagued with difficulties such as the use of toxic organic solvents, high temperatures, low production capacity, large microcapsule diameters, and low encapsulation efficiency. The different coating materials must meet FDA standards before they may be used as a coating material and even then many of the polysaccharides are not permitted in certain foodstuffs [16]. The pharmaceutical nature of the polymers, therefore, presents problems. A shift needs to be made toward food-grade coating materials that are Generally Regarded as Safe (GRAS) and do not need FDA approval, together with the use of novel encapsulation methods.
The use of supercritical fluids to create particles from gas-saturated solutions for the microencapsulation of probiotics has recently been investigated and shown to have great promise [17]. It allows for the use of carbon dioxide which is a stable, inexpensive, non-toxic gas, thereby negating the use of solvents or surfactants, it produces microcapsules of a desirable diameter for use in food applications and does not harm sensitive bioactives like probiotic cells.
There has been a recent increase in the public interest in probiotic cultures and the benefits they offer. Probiotic cultures are already common additions to many products used daily in most households and already have a strong market for themselves in the pharmaceutical industry with a reported market value of $ 19.6 billion in 2013. More research needs to be done to evaluate the suitability of food-grade excipients for use in the encapsulation of probiotics due to a current lack of knowledge on the subject. This research should be done in order to expand the current market for probiotics and improve current techniques used in targeted-delivery systems.
The main aim of this study was to assess the suitability of Compritol E 472 ATO and Vegetal BM 297 ATO as food-grade coating materials for the encapsulation of bifidobacteria using a PGSS method with supercritical carbon dioxide as a solvent.
The specific objectives were to successfully encapsulate B. lactis Bb12 in both materials and to determine the characteristics of the resulting microparticles. The particles were then tested for stability during simulated gastrointestinal transit and shelf-life studies.
Bacterial cultures
Bifidobacterium lactis Bb12 (CHR-Hansen) was obtained in freeze-dried form. The cultures were stored at -4°C.
Excipients
Biogapress Vegetal BM 297 ATO and Compritol E 472 ATO (Gattefosse, France) were obtained in powder form.
Differential Scanning Calorimetry (DSC)
The respective melting temperatures of Compritol and Vegetal were determined by Differential Scanning Calorimetry (DSC) at the Council for Scientific and Industrial Research (CSIR). A DSC Q2000 (TA Instruments), calibrated with indium and zinc, was used to perform the DSC analysis. A heating rate of 10°C/min was used in a nitrogen atmosphere, with a flow rate of 25 mL/min. The temperature range was 0 to 120°C. Aluminum sample pans were used. The sample masses, which were accurately determined on an analytical balance, ranged between 2 mg and 5 mg.
Encapsulation of bacteria
Encapsulation was accomplished using the PGSS technique (Separex Equipments, France). All equipment was wiped with 70% alcohol and allowed to dry before contact with materials. 8 gm of Vegetal BM 297 ATO and Compritol E ATO were weighed off respectively and added to the freeze-dried B. lactis Bb12 culture (2 gm). The blend was then mixed and immediately transferred to a pre-heated 1-liter mixing chamber (50˚C). The chamber was sealed, flushed, and pressurized with sterile filtered CO2 (99.995% purity, air products) up to a pressure of 300 bar, with the temperature being adjusted to 50°C for Vegetal and 70°C for Compritol. The material was left to equilibrate for 3 hrs with intermittent stirring (200 rpm), being activated after 1 hr. The CO2 was then removed via depressurization and the encapsulated product was collected directly from the mixing chamber.
Determination of viable numbers
Vegetal and Compritol microparticles (100 µg) were suspended in 900 µl of absolute ethanol (Illovo) and vortexed for 30 sec. The mixture was spun down in a microcentrifuge and the supernatant was removed. The pellet was then resuspended in ¼ strength Ringers solution up to a final volume of 1 ml. A serial dilution was then performed and plated out on De Man, Rogosa, and Sharpe (MRS) agar (Merck) supplemented with 0.05% v/w cysteine hydrochloride using the pour plate method. The plates were incubated in anaerobic jars at 37°C for 72 hrs with Anaerocult A gaspaks (Merck). Anaerocult strips were used to confirm the absence of oxygen.
Determination of encapsulation efficiency
The Encapsulation Efficiency (EE %) was measured by determining the number of bacteria entrapped into the microparticles. 900 µl of anhydrous alcohol (Illovo) was added to 100 µg of entrapped bacterial cells in an Eppendorf tube (1.5 ml). The mixture was vortexed for 15 sec and centrifuged at 5000 rpm for 30 sec. The supernatant was removed and the pellet was resuspended in 900 µl ¼ strength Ringers solution up to a final volume of 1 ml. A serial dilution up to 10-9 was performed and 100 µl aliquots were plated out in Petri dishes containing De Man, Rogosa and Sharpe (MRS) medium (Merck) supplemented with 0.05% cysteine hydrochloride using the pour plate method. Plating was carried out in triplicate. The plates were incubated at 37°C for 72 hrs in anaerobic jars with Anaerocult A strips (Merck) for indication of anaerobic condition.
Encapsulation efficiency was calculated using the equation:
Encapsulation Efficiency (%)=Viable probiotics released from microparticles/Viable probiotics added to encapsulation mix × 100
Particle distribution
Particle size distribution and mean particle size were determined using a Malvern Mastersizer (Mastersizer 2000, Malvern Instruments, UK). Two identical samples of the Vegetal microparticles created at 50°C with a ratio of 3 gm B. lactis to 7 gm Vegetal BM 297 ATO were analyzed in and the results were recorded. The particles were dispersed in water before measurement at 25˚C. Each sample was run 3 times.
Scanning electron microscopy
The encapsulated bacterial powder (100 µg) was sputtered with gold under an argon atmosphere (Emitech K550X, Ashford, UK). The particles were transferred to the aluminum pins using double-sided adhesive tape. Viewing was done using a JSM-840 microscope (JEOL, Tokyo, Japan).
Transmission electron microscopy
The microparticles (100 µg) suspended in 1/4 strength Ringers solution were spun down at 5000 rpm for 2 mins (Minispin). The supernatant was removed and the pellet was then fixed in 2.5% glutaraldehyde solution in a 0.075 M phosphate buffer (pH 7.4). The pellet was then rinsed three times in phosphate buffer and fixed in 0.5% osmium tetroxide. The sample was rinsed with distilled H2O and dehydrated alcohol in steps ( 50,70,90,100%) before being infiltrated with Quetol epoxy resin (30% for 1 hr,50% for 1 hr,100% for 2 hr) and allowed to polymerize for 39 hr at 60ᵒC. Ultrathin sections were cut and stained with aqueous uranyl acetate. A counterstain with lead acetate was performed and the sections were rinsed in the H2O. Then 0.5 µm monitor sections were cut and stained in Toluidine blue. The preparations were then viewed under a JEOL JEM-2100F (JEOL, Tokyo, Japan).
The encapsulation conditions for the two excipients were determined by taking into account the melting temperature of the compound, the conditions that would harm the probiotic cultures, and the conditions needed for CO2 to reach a supercritical state. CO2 reaches a supercritical state at a pressure of 72 bar and 31˚C [18]. For the excipient to fully liquefy in the scCO2, the temperature must be equal to or higher than its melting temperature in the supercritical CO2 medium. The higher the temperature inside the reactor chamber causes greater the loss in the number of viable probiotics. It is thus desirable to have an excipient that has a low melting temperature so that the encapsulation conditions are less harsh on the probiotic organisms. Pressure affects melting temperature [19].
In the case of CO2, an increase in pressure causes the increased density of the CO2 which causes more CO2 to be absorbed into the compound. The more CO2 absorbed into the compound, the greater the depression of melting temperature. This effect lasts until a critical pressure is reached after which the compressive force becomes the overriding effect. When this occurs the melting temperature will start to increase again (P.W. Labuschagne, personal communication). Bifidobacteria can withstand immense pressures with 104 bar leading to a 2 log cycle reduction in the number of live organisms [20].
The encapsulation pressure was therefore chosen as 300 bar, which is the maximum pressure the PGSS equipment can withstand. The melting temperature at atmospheric pressure (1.014 bar) for Compritol E 472 ATO was determined to be 75˚C and 60˚C for Vegetal BM 297 ATO. The temperatures needed to liquefy the excipients at a pressure of 300 bar were determined to be 70˚C and 50˚C for Compritol and Vegetal respectively.
Preliminary viability testing
The higher mixing chamber temperature of 70˚C needed to liquefy the Compritol E resulted in an 8.5 log cycle decrease in the numbers of live B. lactis cultures (Figure 1). On the other hand, Vegetal that liquefied at a lower temperature of 50˚C resulted in only a 4 log cycle reduction in viable numbers of probiotic organisms. This loss in the number of viable cultures was considered too high. The conditions that would result in fewer losses of live probiotic organisms were determined.
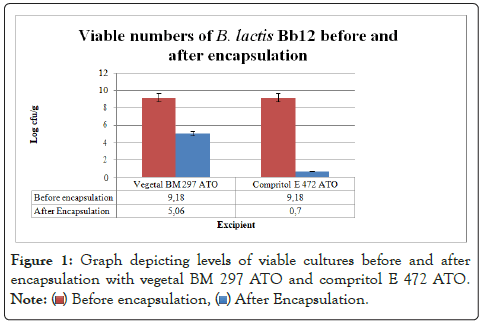
Figure 1: Graph depicting levels of viable cultures before and after
encapsulation with vegetal BM 297 ATO and compritol E 472 ATO.
A decrease of less than 1 log cycle of viable numbers of B. lactis is desirable as the higher the number of cells that survive the encapsulation process, the higher the EE% will be. The reduction in viable numbers caused by the high encapsulation temperature conditions needed to liquefy Compritol was too high to warrant further testing. Attempts to use mixing chamber temperature conditions resulted in poor plasticization and hence poor encapsulation of bacteria. This indicated that a higher temperature was critical to the successful utilization of this material as an encapsulation coat using supercritical conditions. Therefore Vegetal BM 297 ATO which resulted in less reduction of live organisms was used in further optimization tests.
Optimization of encapsulation conditions using Vegetal BM 297 ATO
It was decided that different formulations of bacteria to excipient could yield better results. A ratio of 3 gm of bacteria to 7 gm of Vegetal was chosen. The ratio was chosen on the basis that the fewer bacterial cells needed in the final formulation, the less expensive the food additive would ultimately be as probiotics are expensive and unlikely to be covered by medical insurance in the near future [21]. It has been suggested that at higher bacterial loading the bacteria are better protected against harsh environments due to cell-cell interactions [22].
The product obtained directly from the mixing chamber with encapsulation done at 50˚C with an 8:2 ratio of Vegetal to bacteria was a solid light yellow mass with a slight buttery odor. When ground in a mortar and pestle, yellow grains with uniform color and consistency were observed. The product formed by encapsulation done at 48˚C was a fine white powder containing solid yellow clumps. After grinding in mortar and pestle there was a fine pale yellow powder with larger yellow granules present. Uniform texture is desirable in a food additive as differences could affect the sensory properties of the food [14]. The properties of the product obtained at 50˚C process temperature are more desirable, due to its even texture. The viable counts in both formulations before processing were ~9.2 log cfu/gm (Figure 2).
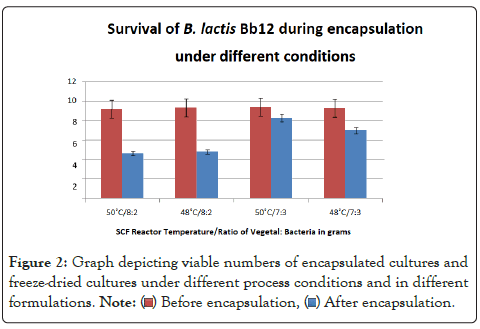
Figure 2: Graph depicting viable numbers of encapsulated cultures and
freeze-dried cultures under different process conditions and in different
formulations.
The decrease in numbers of viable cultures was 4.54 and 4.6 log cycles for reaction products obtained at 48˚C and 50˚C respectively. An EE of 49% and 51% were achieved respectively. This shows similar losses at both temperatures.
The visual characteristics of the two products suggest that poor plasticization occurs at 48˚C as some of the Vegetal powder did not dissolve. This implies that testing of process temperatures lower than 48˚C is unnecessary as a higher temperature is needed for complete plasticization. The need for testing of process temperatures above 50˚C is not necessary as the material completely liquefies at this temperature. Higher temperatures will expose the probiotic organisms to harsher conditions which will lead to the loss of viable cells. It was decided that a change in reactor temperature was not advisable as 50˚C is the minimum needed for complete plasticization of the excipient.
This ratio proved successful enough that the addition of more bacteria would have been unnecessary. The new encapsulation mixture was plasticized at both 48˚C and 50˚C to compare the properties of the products obtained from the mixing chamber to the previous formulation. The results were similar. The product obtained from the encapsulation at 50˚C was a solid brittle yellow mass that was milled into a fine yellow powder. The product obtained from the mixing chamber at 48˚C contained undissolved Vegetal powder with clumps of brittle yellow granules.
The numbers of viable cultures present in the mixture before processing was ~9.3 log cfu/gm as shown in Figure 2. The decrease in numbers of viable cells was 2.3 and 1.1 log cycles for the processing done at 48˚C and 50˚C respectively. Encapsulation efficiency of 75% and 88% was achieved as shown in Figure 3. The microparticles obtained from the higher process temperature of 50˚C showed improved survival over the product obtained at 48˚C. The particles obtained from the encapsulations done at 50˚C with a ratio of 3 gm of bacteria to 7 gm of Vegetal performed showed the highest numbers of viable bacteria after processing with an encapsulation efficiency of 88% (Figure 3). This method was used to manufacture the microparticles to be characterized.
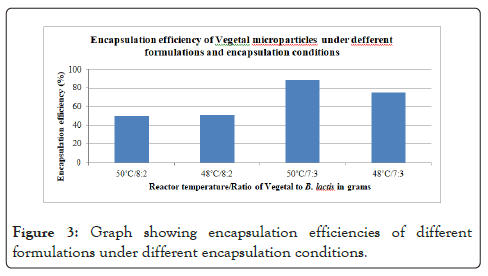
Figure 3: Graph showing encapsulation efficiencies of different formulations under different encapsulation conditions.
Particle distribution and encapsulation efficiency
The microparticles of the samples processed at 48˚C and 50˚C showed similar bell-curve distribution patterns with a clear peak at 30-32 µm as shown in Figure 4. Both also produced microparticles with an average D50 of 31.66 which mean 50% of the particles were smaller than 31.66 µm. The average D90 of 108.36 also shows that only 90% of the particles were smaller than 108.36 µm. Inversely, it shows that only 10% of the particles are larger than 108.36 µm.
The raw data shows that only 13% of the particles were larger than 100 µm. The particles had a mean diameter of 31 ± 21 µm (Mean ± standard deviation, n=6). This is desirable as smaller particles have a smaller chance of affecting the foods texture and will be more palatable to the consumer [23,24]. It has been suggested that particle size should ideally be below 350 µm [25] but newer research suggests particle sizes below 100 µm for stability, easier handling and storage of cultures and limited effects on food texture as larger microparticles can affect mouthfeel (Figure 4) [26,27].
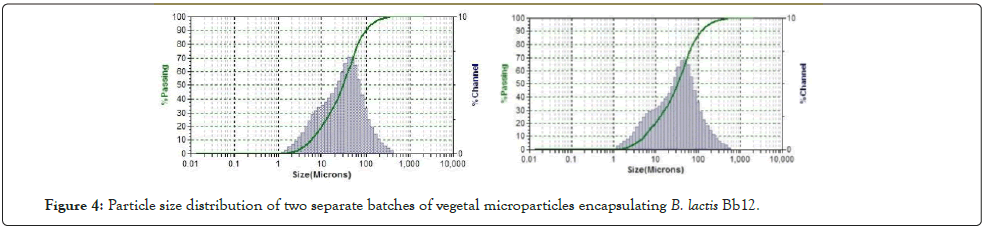
Figure 4: Particle size distribution of two separate batches of vegetal microparticles encapsulating B. lactis Bb12.
Other studies investigating microencapsulation of probiotics using supercritical carbon dioxide reported larger particle sizes. A study [28] reported microparticles with a mean size of 166 µm when using an interpolymer complex of poly-(vinylpyrrolidone)-poly-(vinyl acetate-co-crotonic acid). The particles were reported to be porous. This is thought to be caused by the escape of CO2 from the microparticles during the depressurization process or hydrophobic interactions in the matrix [29-31]. These pores are undesirable as they will expose the entrapped probiotic cells to unfavorable environments [14]. The presence of pores could also interfere with the release of the bacteria [32].
Other methods of encapsulation of probiotics also report microparticles larger than what was found in this experiment. Alginate encapsulation of 3 bifidobacterial strains has resulted in mean particle sizes of 250 µm [33], 500-1000 µm [34], 2000-3000 µm [35] and 200-300 µm [36]. Various strains of Bifidobacteria species were encapsulated using denatured whey protein solutions by Picot and Lacroix [16]. The generated microparticles were hydrophobic and had a comparable size range of 3-75 µm.
EE% was also measured for both samples and shown to be 88.1% as shown in Table 1. This value is lower than the 96% reported by Mamvura [28] using an interpolymer matrix excipient under similar encapsulation procedures. A high EE is desirable as it indicates minimal loss and complete release of the probiotic cells. In a study by Annan et al. [37] B. adolescentis was encapsulated in small alginate-coated gelatin microparticles. The microparticles had a comparable size of 50 µm. An EE of 41-43% was obtained. The strong stability of the covalently cross-linked gels was stated as a possible reason for a lowered EE% due to the incomplete release of cells from the matrix. The incomplete release of the cells from the matrix will result in an underestimation of the number of viable cells. This will in turn lead to a lower EE% value. Encapsulation with Vegetal produced a lower EE than similar studies using scCO2 but far higher than other encapsulation methods (Table 1).
| Mixing chamber temperature | D10 | D50 | D90 | Mean size (µm) | Particle shape | Encapsulation efficiency (EE%) |
|---|---|---|---|---|---|---|
| 50˚C | 5.62 | 30.38 | 98.62 | 30.38 | Irregular | 88.10% |
| 50˚C | 5.24 | 32.94 | 118.1 | 32.94 | Irregular | 88.00% |
Table 1: Particle characteristic criteria of vegetal microparticles.
A study by Moddler and Villa-Garcia [38] revealed that encapsulation of probiotics in butterfat afforded no protective measure when stored in yogurt. Dispersion of probiotic cells in lipid-based microparticles has been described as a difficult technological task due to premature melting of the capsule in elevated temperatures and uneven dispersion of probiotic cells in oil [16,38]. As the melting point of Vegetal is 60°C it is a solid powder under normal conditions and will not melt. The fact that is solid also means that no separation problems due to different densities or phases will occur. Phase separation was listed as a concern, [39] stating that lipid-based microparticles may be limited to solid foods. The higher melting temperature and solid nature of the Vegetal microparticles are therefore desirable as these listed problems will not arise.
Particle characterization by scanning and transmission electron microscopy
Scanning Electron Microscopy (SEM) revealed that the Vegetal microparticles containing B.lactis Bb12 had an irregular shape and a smooth outer coating as shown in Figures 5B and 5C. The smooth coating and particle size are said to be a product of the supercritical carbon dioxide encapsulation process [40]. Non-spherical microparticles created using scCO2 have been reported [41,28]. The use of other encapsulation techniques resulted in spherical microparticles [42-45]. The irregular shape is undesirable as it will affect the dispersion of microparticles in the final food product. Non-spherical particles are reported to be more prone to uneven distribution in a foodstuff [45]. The particles reported in the literature were consistently larger than the Vegetal microparticles containing B. lactis and had a lower encapsulation efficiency. The high encapsulation efficiency and small size of the Vegetal microparticles containing B. lactis warrant further testing (Figure 5).
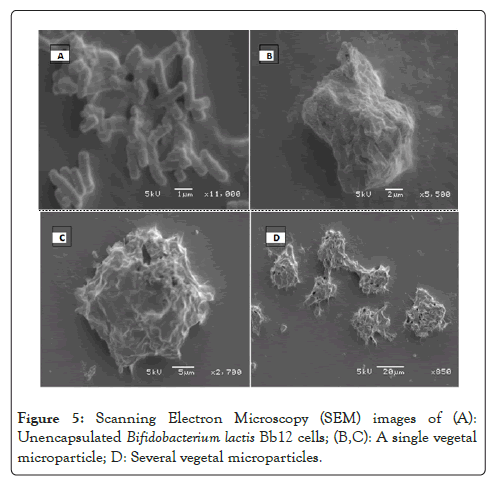
Figure 5: Scanning Electron Microscopy (SEM) images of (A): Unencapsulated Bifidobacterium lactis Bb12 cells; (B,C): A single vegetal microparticle; D: Several vegetal microparticles.
To study the release of B. lactis from the Vegetal microparticles they were exposed to Simulated Intestinal Fluid (SIF) (pH=6.8) and viewed under the SEM. The particles were found to dissolve quicker in a high pH environment which resulted in the release of B. lactis entrapped within the matrix. A close-up of the released material was taken to show that it contains B. lactis cultures. These cultures show characteristic Bifidobacterial morphology indicating that there was no observable change.
To confirm this, an ultrathin cross-section of a group of microparticles was taken using a transmission electron microscope as shown in Figure 6A. Visual examination shows that the microparticles are filled with high numbers of bacteria as shown in Figure 6B. Probiotic organisms are 1-5 µm in size [46-49]. The Transmission Electron Microscopy (TEM) image shows that the B. lactis organisms are tightly packed, which suggests that a high bacterial load was achieved (Figure 6).
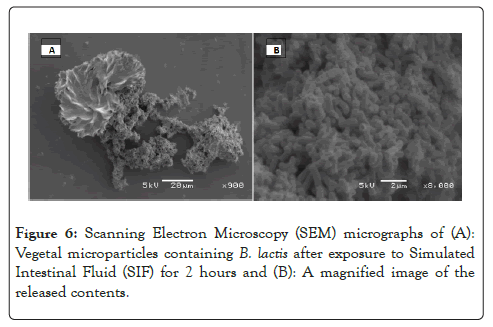
Figure 6: Scanning Electron Microscopy (SEM) micrographs of (A): Vegetal microparticles containing B. lactis after exposure to Simulated Intestinal Fluid (SIF) for 2 hours and (B): A magnified image of the released contents.
The average diameter of the Vegetal microparticles was 31 µm as shown in Table 1. Taking an average particle diameter of 30 µm the estimated volume is 4/3 π (15 µm)2=942 µm3.
The average volume of a B. lactis cell (cylindrical cell where radius=0.5 µm and length=2 µm, [16] is 1.6 µm3. This suggests that an average Vegetal microparticle will contain 177 B. lactis cells if the total volume of the particle is 70% Vegetal assuming even distribution throughout the matrix. This even distribution throughout the matrix can be observed as shown in Figure 7B (Figure 7).
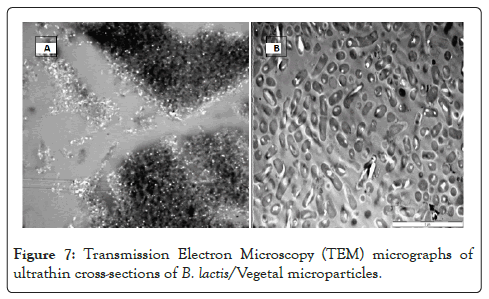
Figure 7: Transmission Electron Microscopy (TEM) micrographs of ultrathin cross-sections of B. lactis/Vegetal microparticles.
The results of this study show that the Compritol E 472 ATO cannot be used for the production of microparticles containing B. lactis Bb12 as it requires the use of temperatures that are detrimental to the survival of the probiotic bacteria. However, the results indicate the suitability of Vegetal BM 297 ATO as a suitable lipid-based excipient for use in microencapsulation applications due to its low melting point which does not result in a significant decrease in the number of viable probiotic cells. Furthermore, the Vegetal microparticles have a small size and high EE%, both of which are desirable properties for microparticles used in food applications. These results warrant more research into the performance of Vegetal microparticles in protecting the enclosed probiotics during exposure to a simulated gastrointestinal environment as well as during storage of products.
I would like to thank Dr. Mapitsi Thantsha for her expertise and support during the conduct of this research. I would also like to thank Dr. Phillip Labuschagne and Andrea Chioma Amakiri for their technical support and assistance.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Citation: Labuschagne MC, Thantsha MS (2022) Encapsulation of Probiotics in Lipid-based Microparticles Using Supercritical Fluid Plasticization. J Prob Health. 10:281.
Received: 24-Jun-2022, Manuscript No. JPH-22-18068; Editor assigned: 27-Jun-2022, Pre QC No. JPH-22-18068 (PQ); Reviewed: 11-Jul-2022, QC No. JPH-22-18068; Revised: 18-Jul-2022, Manuscript No. JPH-22-18068 (R); Published: 26-Jul-2022 , DOI: 10.35248/2329-8901.22.10.281
Copyright: © 2022 Labuschagne MC, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.