Journal of Proteomics & Bioinformatics
Open Access
ISSN: 0974-276X
ISSN: 0974-276X
Research Article - (2019)Volume 12, Issue 3
Long-acting human growth hormone (hGH) is intended to improve compliance, adherence and efficacy for patients with growth hormone deficiency or other growth disorders. There is poor patient compliance with daily dosing and novel strategies for improving pharmacokinetic half-life and potency are under development. Subcutaneously and intramuscularly administered recombinant human growth hormone (aka somatropin) has a short half-life of just a few hours. Growth hormone is cleared by glomerular filtration based on its size and through receptor mediated uptake. By engineering a multimeric hGH, the clearance via glomerular filtration may be reduced and the receptor binding improved through multiple points of contact. A synthetic form of hGH was created by linking multiple hGH proteins together through bi-functional PEG linkers. In addition a recombinant form of hGH was created by expressing three hGH proteins tethered together by a repetitive amino acid linker sequence. These engineered proteins were evaluated for structure and function. The tethered hGH proteins were potent and increased weight gain in hypophysectomized rats.
Growth hormone; PEGylation; Protein engineering; Transglutaminase; Biotethering; Somatropin; Ligand valency
Human growth hormone (hGH) is a peptide of 191 amino acids (22 kDa) and was introduced to the clinic in its recombinant form in 1985 and successfully used in children and adults for its growth promoting and metabolic effects [1]. Growth Hormone is secreted from the pituitary and acts on most cells and tissues. A single monomer of GH binds its cognate receptor, which forms a dimer and triggers a signal transduction cascade [2]. The effects of GH are indirectly mediated by upregulation of insulin-like growth factor (IGF-1), which is expressed following GH treatment [3]. Recombinant human growth hormone, delivered by daily subcutaneous injections has been shown to improve growth velocity, lean body mass, increased muscle performance and decreased adipose in children and adults [1]. Approved indications include Growth Hormone deficiency, chronic kidney disease, turner syndrome, idiopathic short stature and other disorders. Since 1985 many recombinant forms of hGH (somatropin) have been approved. These include Humatrop, Nutropin, Norditropin, and Genotropin. The original hGH products require uncomfortable and burdensome daily subcutaneous dosing. Upwards of 50% of pediatric patients failed to adhere to GH treatment recommendations [4]. More recently, second generation, long lasting growth hormone products have entered clinical trials [5,6]. These forms of hGH impart improved pharmacokinetics by including PEGylation, addition of lipid or protein linkers, or other modifications to improve half-life [7-10]. The premise of these next generation protein therapeutics is to increase the size or bulk of growth hormone via a permanent modification to decrease renal clearance. The addition of a linker that associates the hGH protein with circulating serum albumin or otherwise protects the protein from clearance or degradation have also been employed [7]. These protein engineering strategies attempt to balance the need for increased circulating half-life while maintaining receptor binding and also to avoid immunogenicity or other adverse response to the engineered therapeutic. These experimental therapeutics include ACP-001, MOD- 4023, VRS-317, and NNC0195-0092, to name a few examples [7]. The market for growth hormone is greater than $3 Billion USD worldwide and has attracted many novel follow on therapeutics. However, many of these have failed to show improvement in safety or efficacy versus the approved products (Table 1). No long lasting modified form of hGH has been approved in Europe or the US. PEGylation has been used successfully to modify small therapeutic proteins [11-13]. One such example is Pegfilgrastim (trade name Neulasta) a 20 kDa PEGylated form of recombinant granulocyte colony-stimulating factor (GCSF/ filgrastim), with a total size of 39 kDa. Pegylation increase the size of filgrastim so it becomes too large for renal clearance and the clearance of the protein is based on receptor mediated effects. Biosimilars of PEGfilgrastim have also successfully reached the market [14]. However, high MW PEG can cause cell vacuolation, reduced protein activity, poor tissue bioavailability, solubility issues and high viscosity [13]. Mono- PEGylation may not provide enough protection against protease/peptidase. For these reasons we engineered multimeric, PEGylated hGH with increased protein size to reduce renal clearance efficiently, but maintained minimal PEG content, while improving ligand-receptor interaction. Tethering or linking hGH proteins together endows higher activity than the monomeric protein due to multivalency and improved receptor binding [15]. Less drug is needed with longer intervals between doses, thus providing for an improved pharmaceutical profile. These design principles can be applied to other protein therapeutics.
| Drug Name | Manufacturer | Approval Date or Status |
|---|---|---|
| Humatrope | Lilly | 1987 |
| Nutropin AQ | Roche | 1993 |
| Norditropin | NovoNordisk | 1995 |
| Zomacton | Ferring | 1995 |
| Genotropin | Pfizer | 1995 |
| Saizen | Merck Serono | 1996 |
| Omnitrope | Sandoz | 2006 |
| TV-1106 | Teva | Phase 2 |
| ACP-001 | Ascendis | Phase 3 |
| MOD-4023 | Opko/Pfizer | Failed Phase 3 |
| VRS-317 | Versartis | Failed Phase 3 |
| NNC0195-0092 | NovoNordisk | Phase 3 |
Table 1: Human Growth Hormone Drugs and Status.
Materials
Recombinant human growth hormone (rhGH) produced in E. coli is a single non-glycosylated polypeptide chain containing 191 amino acids. It is biologically active and has a molecular mass of 22.1 kDa as analyzed by SDS-PAGE (GeneScript, Piscataway, NJ). Recombinant, trimeric hGH expression construct was cloned by Blue Heron BioTech (Bothell WA). Mono and bi-functional PEG was purchased from Creative PEGWorks (Durham, NC). Recombinant microbial transglutaminase catalyzes the post translational modification of proteins by inserting an isopeptide bond within or between polypeptide chains. The mTGase was purchased from Zedira GmbH (Darmstadt, Germany).
Analysis of synthetic and recombinant hGH multimers by SDS-PAGE
Samples from the synthetic and recombinant hGH multimers were analyzed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) to determine the molecular weight and yield (BioRad, Hercules, CA). Protein reactions or extracts were analyzed with a molecular weight standard. SDS denatures the secondary, tertiary, and quarternary structures by binding to hydrophobic protein regions and it confers a net negative charge on the proteins, which results in a constant charge to mass ratio.
Bioanalysis of synthetic and recombinant hGH multimers by ELISA
Sprague Dawley rat blood was collected individually on day 11 by cardiac puncture under 2.5% isoflurane anesthesia. The blood is collected into K2-EDTA anticoagulant containing tubes and processed into plasma. The human growth hormone immunoassay is a solid phase ELISA designed to measure rhGH in biological matrices (R&D Systems (Minneapolis, MN).
Chromatography
GE AKTA lab-scale protein purification systems were used for purification of hGH multimers (GE Healthcare Life Sciences, USA). Analysis of hGH multimers utilized Agilent HPLC systems (Santa Clara, CA) with TOSOH TSK gel column to separate based on macromolecular size.
TOF-MS
Synthetic PEGylated hGH preparations were analyzed by Waters MALDI/ESI QTOF. This high resolution mass spectrometry with ion mobility separation is used to determine the mass of multimeric hGH a the Scripps Center for Metabolomics (La Jolla, CA). A sample at 1.43 mg/mL in phosphate buffered saline was prepared in sinapinic acid matrix and analyzed using MALDI-TOF. The scan range was 1000 to 150000.
Animal bioactivity study
Synthetic and recombinant multimeric tethered hGH were reconstitute in 0.1 M ammonium bicarbonate pH 8.0 and prepared for 2 ug/rat/day dosing. Male Sprague Dawley rats were hypophysectomized at 25 to 30 days of age (HYPOX1 rats, Charles River, Wilmington, MA). Rats are weighed when they are 37 to 44 days old and only healthy rats retained. Rats were randomized and divided into test groups, each group containing 6 rats. Each day for 10 days rats are injected subcutaneously with 0.1 mL of the control solution and test solutions. Body weights of each animal were recorded at the start of the test and following the 10th injection. The change in body weight for each rat during the 10 day period was measured (Explora Labs, San Diego, CA). On day 11 plasma is collected for bioanalysis.
Engineering multimeric hGH
Modification of proteins with hydrophilic polymers and increasing protein molecular weight is an effective strategy to improve pharmacokinetic profile. The synthetic hGH multimer was engineered by chemically linking rhGH together via bi-functional PEG. The sites of PEGylation were controlled by using microbial transglutaminase (mTgase). The transglutamination reaction creates gamma-amides of glutamic acid and ammonia [16,17]. Human growth hormone amino acid residues Gln40 and Gln141 are targeted by mTgase and can be linked to bi-functional amino derivative PEG (NH2-PEG-NH2). Bi-functional PEG (5.5 kDa) is added to rhGH in order to drive the reaction. The resulting hGH has two bi-functional PEG moieties attached at Gln40 and Gln141. In the second reaction, hGH is added to generate the synthetic trimer that is tethered together with PEG. The expected molecular weight is approximately 76 kDa (Figure 1A). The recombinant trimeric hGH is prepared by creating an expression construct that includes three hGH ORFs linked together by a repetitive sequence encoding an amino acid linker (Figure 1B). The construct is expressed in E. coli and purified using the 6 His affinity tag and other means. The expected molecular weight based on the sequence is 86 kDa.
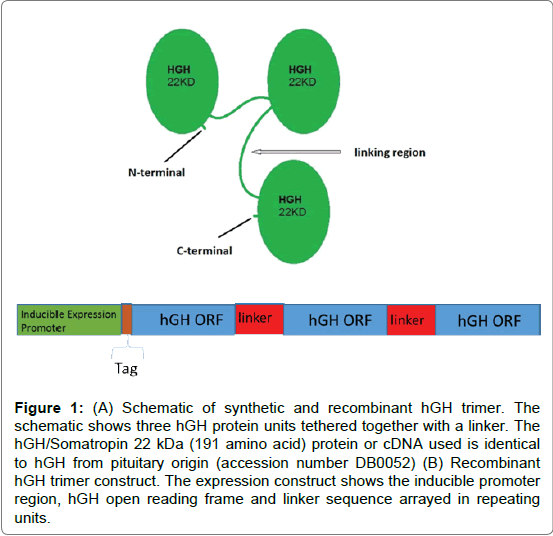
Figure 1: (A) Schematic of synthetic and recombinant hGH trimer. The schematic shows three hGH protein units tethered together with a linker. The hGH/Somatropin 22 kDa (191 amino acid) protein or cDNA used is identical to hGH from pituitary origin (accession number DB0052) (B) Recombinant hGH trimer construct. The expression construct shows the inducible promoter region, hGH open reading frame and linker sequence arrayed in repeating units.
Analysis of multimeric hGH
The engineered hGH proteins were analyzed by SDS-PAGE and HPLC-SEC to separate and examine synthetic reaction products or E. coli expression products. SDS-PAGE was used to visualize tethered proteins based on size. The recombinant trimeric hGH is readily expressed in E. coli and can be processed through a Ni-NTA affinity procedure (Figure 2). The observed MW of the primary product is consistent with the expected MW of 86 kDa. The synthetic trimeric hGH had multiple reaction products that needed to be separated (data not shown). In addition, PEG causes changes in the electrophoretic mobility of proteins. We found that the synthetic hGH multimers did not migrate exactly as predicted, this is consistent with other publications showing proteins moving behind the PEG front show greater mobility and those moving ahead of the front lower mobility than in the absence of PEG [18].
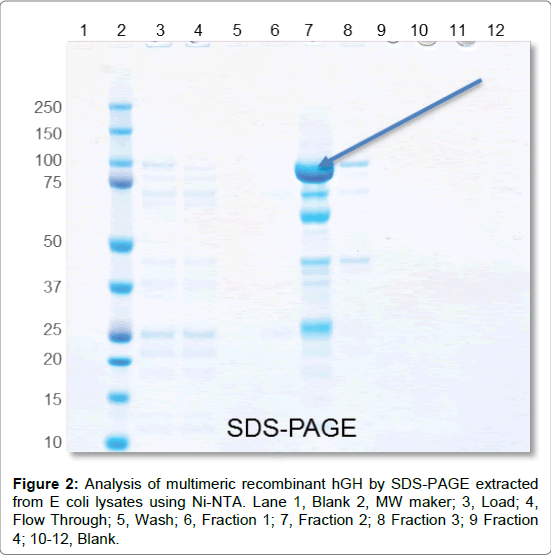
Figure 2: Analysis of multimeric recombinant hGH by SDS-PAGE extracted from E coli lysates using Ni-NTA. Lane 1, Blank 2, MW maker; 3, Load; 4, Flow Through; 5, Wash; 6, Fraction 1; 7, Fraction 2; 8 Fraction 3; 9 Fraction 4; 10-12, Blank.
MALDI-TOF-MS is a useful tool to measure PEGylation products and has been used elsewhere on PEGylated hGH because the spectra is less complex and TOF-MS is able to measure large MW entities [19]. Multimeric hGH reaction products were cleaned up using Q-Sepharose dialized against borate buffer and concentrated using 10 kDa MWCO filter prior to analysis with MALDI-TOF-MS. The TOF-MS results show the stepwise increase in MW with PEGylation of hGH (Figure 3). The addition of 5 kDa bi-functional PEG to rhGH increases the mass to 27, 34, and 39 kDa. This indicates that the first step in the reaction generated hGH with one, two and three PEG moieties. The hGH with two PEG moieties per monomer located at residues Gln40 and Gln141 is desired and used for the final reaction step. The synthetic rhGH trimer could be separated and analyzed by size exclusion chromatography (SEC-HPLC). The synthetic hGH trimer is bigger and elutes from the size exclusion column sooner than monomeric hGH (Figure 4).
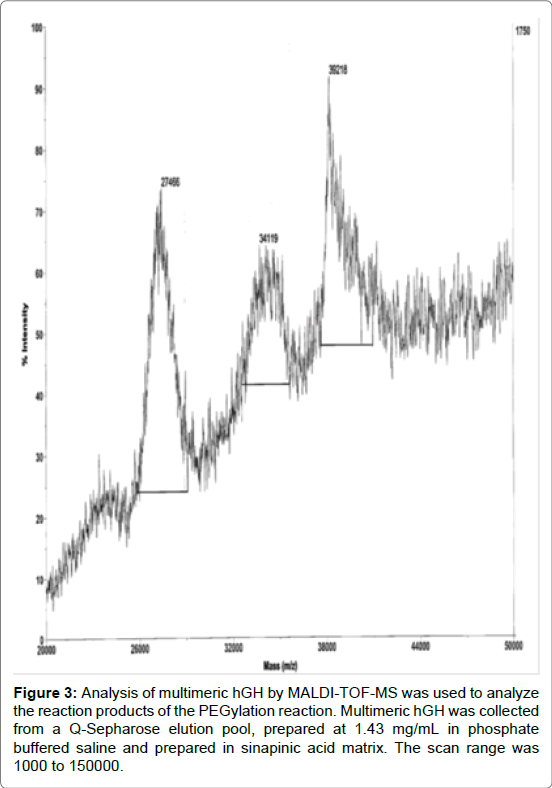
Figure 3: Analysis of multimeric hGH by MALDI-TOF-MS was used to analyze the reaction products of the PEGylation reaction. Multimeric hGH was collected from a Q-Sepharose elution pool, prepared at 1.43 mg/mL in phosphate buffered saline and prepared in sinapinic acid matrix. The scan range was 1000 to 150000.
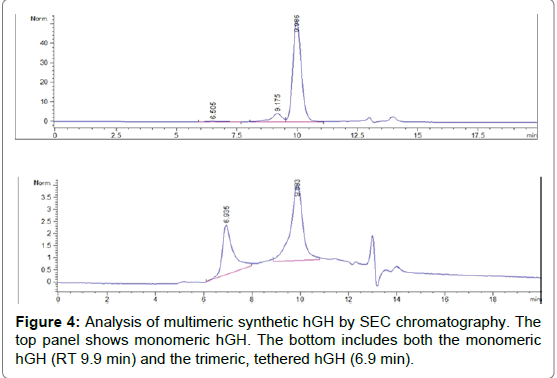
Figure 4: Analysis of multimeric synthetic hGH by SEC chromatography. The top panel shows monomeric hGH. The bottom includes both the monomeric hGH (RT 9.9 min) and the trimeric, tethered hGH (6.9 min).
BioAssay of engineered hGH
In order to measure the bioactivity of the engineered synthetic hGH multimer and recombinant hGH multimers a weight gain study in rats was conducted. The rat weight gain study is an integral part of the quality control and determination of potency of marketed rhGH products. The study design is adapted from the established USP Bioidentity Assay. Hypophysectomized rats were given daily doses of vehicle, synthetic hGH trimer and recombinant hGH trimer for 10 days. Weight gain was evaluated and expressed as a fold increase compared to vehicle group (Figure 5). The synthetic hGH trimer had an increase in weight about 1.6-fold versus vehicle. The recombinant hGH trimer had an increase in weight gain of about 1.3-fold versus vehicle.
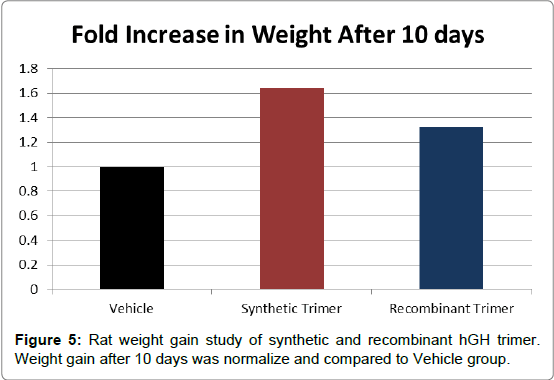
Figure 5: Rat weight gain study of synthetic and recombinant hGH trimer. Weight gain after 10 days was normalize and compared to Vehicle group.
Blood was collected after Day 10 to show exposure and accumulation. Hypophysectomized rats were dosed daily for 10 days with vehicle, synthetic trimeric hGH, and recombinant trimeric hGH. Animals were sampled to show exposure. The plasma from rats dosed with synthetic and recombinant hGH trimeric proteins showed exposure and had steady levels of the synthetic and recombinant multimeric forms of hGH out to the 24 hr time point. However, the plasma from rats dosed with synthetic hGH trimer had lower absolute signal. This is likely due to the PEGylation of hGH and potential masking of epitopes by PEG, and could interfere with the immunoassay (data not shown). Both the synthetic and recombinant forms of multimeric hGH have bioactivity and stimulate growth in the rat weight gain model. The engineered hGH trimers were detected in rat plasma and stayed in circulation for at least 24 hrs.
In this work multimeric engineered hGH proteins were evaluated for structure and function. The tethered hGH proteins were potent and increased weight gain in hypophysectomized rats. Therefore, making synthetic and recombinant forms of multimeric hGH is feasible. This gives biopharmaceutical drug developers manufacturing options. These design principles can be applied to other protein therapeutics. In some cases small proteins can be synthesized and tethered by PEG through entirely chemical means. An approach using recombinant technologies may be preferred, or a mix of both as we describe here. Recombinant expression and purification of hGH is well established and described in the literature [20]. But monomeric recombinant, unmodified hGH has a short half-life and requires daily subcutaneous injections. This dosing regime is a burden to patients and decreases adherence and therefore has driven innovative drug development strategies. Site directed PEGylation has been used to improve hGH half-life and reduce immunogenicity [21-23]. Our design strategy used targeted PEGylation to control the placement of bi-functional, low molecular weight PEGs on Gln40 and Gln140 residues of hGH. As an alternative strategy, we used a recombinant approach, with three hGH protein units tethered together with an encoded amino acid sequence. The increase size of our engineered hGH proteins from the 22 kDa monomeric form to 77 kDa for our synthetic hGH trimer and 84 kDa for our recombinant hGH trimer was chosen to reduce renal clearance but avoid poor bioavailability to target tissue. Another important and novel attribute of the engineered hGH proteins presented here is their multivalency. The tethered trimer design improves receptor interactions and likely allows for lower and less frequent drug administration. Multivalent ligands can cause receptors to cluster on the cell surface. This can lead to robust activation of signaling pathways. In addition, multivalent ligands display higher local concentrations of binding epitopes, which can result in higher apparent affinities [15]. Overall the data presented here supports the notion that tethered, multimeric hGH will have improved half-life and bioactivity. These engineered GH proteins join a growing list of products that have been developed with improved pharmaceutical profiles. Ultimately these products will improve patient adherence to GH therapy and clinical outcomes.
The authors declare that they have no competing interests. This study was funded by BioTether Sciences Inc.
EF participated in the design, coordination, testing, and analysis, TW participated in the design and analysis, BZ participated in the design, testing, and analysis. All authors read and approved the final manuscript.
We would like to acknowledge the vendors, Blue Heron, Aragen, and Explora Labs for contributions to this study.
Citation: Wang T, Zhao B, Foehr ED (2019) Engineering a Long Lasting Tethered, Multimeric Human Growth Hormone Protein to Improve Pharmacokinetic Half-Life and Potency. J Proteomics Bioinform 12: 056-060. doi: 10.35248/0974-276X.19.12.497
Received: 22-Mar-2019 Accepted: 15-Apr-2019 Published: 24-Apr-2019 , DOI: 10.35248/0974-276X.19.12.497
Copyright: © 2019 Wang T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.