Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2016) Volume 7, Issue 4
In the present work, pistachio shell, local agriculture waste, has been successfully used for the preparation activated carbon (AC) by sulphuric acid. This sulphuric acid based activated carbon (ACS) was used for the removal of two cationic dyes viz., methylene blue (MB) and Brilliant green (BG) from aqueous solutions. The structure and physical and chemical properties of ACS are investigated using FTIR and SEM analysis and pH surface and Boehm titration. The effect of the differential experimental parameters controlling the adsorption of dyes onto ACS, was thoroughly investigated, such as the effect of pH, initial dye concentration, contact time and adsorbent dosage in batch mode. Employment of equilibrium isotherm models for the description of adsorption capacities of ACS explores the good efficiency of Langmuir model for the best presentation of experimental data with maximum adsorption capacity of 208.333 and 151.515 mg/g for MB and BG dyes. The kinetic data were fitted to the pseudo-secondorder kinetic model with cooperation with interparticle diffusion model. Thermodynamic parameters were evaluated to predict the nature of adsorption. These results point out the endothermic and spontaneous nature of the sorption process. The results demonstrate that ACS is effective in the removal of MB and BG dyes from aqueous solutions and can be used as an alternative to the high-cost commercial adsorbents.
Keywords: Pistachio shell; Activated carbon; Cationic dyes; Adsorption
The dyes and pigments usage in last two decades (more than 107 kg/year) in human life and industries like textile dyeing, dermatological agent, a biological stain, paper, carpet and printing wastewater widely was increased [1]. Dyes, which usually have a synthetic origin, are characterized by complex aromatic molecular structures that supply stabilities of thermal, physicochemical and optical [2]. Dyes can be classified as cationic (basic dyes), anionic (direct, acid, and reactive dyes) or non-ionic (disperse dyes) [3]. Cationic dyes such as Methylene Blue (MB) can be applied to leather, silk, paper wool, plastics, in addition to for the production of ink, copying paper and cotton mordant with tannin [4]. In spite of low toxicity of MB, it can cause harmful effects such as vomiting enhance in heart rate, cyanosis, diarrhea, shock, jaundice, quadriplegia and human tissue necrosis [5] Brilliant green (BG) is applied as dermatological agent, veterinary medicine and as inhibitor of mold propagation and following contact with skin and eye, inhalation and ingestion generates toxic to the lungs and other tissues and lead to target-organ damage [6].
A vast number of physicochemical, chemical and biological methods have been used for removing dyes from wastewater e.g., ozonation [7], adsorption [8], electrochemical techniques [9], coagulation and flocculation [10] biological treatment [11]. Among these methods, adsorption has gained favour in recent years due to proven efficiency in the removal of pollutants from effluents. Activated carbon (AC) is known to be a very efficient adsorbent because of its large surface area, highly developed porosity, changeable characteristics of surface chemistry, and the high degree of surface reactivity [12]. Activated carbon, as an adsorbent has been widely investigated for the adsorption of dyes, but its high cost limits its commercial application. In the last two decades, there has been growing interest in finding inexpensive and effective alternatives to carbon, such as jute sticks [13], acorn [14], Lantana camara [8], cocoa shell [15], aegle marmelos [16], Pea shells [17], sugarcane bagasse [18], Rice Husk [19], Waste Weed [20].
The objective of the present study is the preparation of pistachio shell based-activated carbon (ACS). Pistachio shell is considered as waste matter in the environment and has low cost. Similar to other agro-residues, pistachio shell is chiefly comprised of lignin, hemicellulose, and cellulose. Such composition makes pistachio shell a good raw material for the output of new adsorbents for processes of water treatment.
In this investigation, the pistachio shell is simultaneously carbonized and activated chemically by sulphuric acid [21] to the removal of methylene blue (MB) and Brilliant Green (BG) from aqueous solution and some water samples. The various analytical factors affecting the removal of dyes are investigated viz. pH, the dose of activated carbon, the initial concentration of dye, the temperature and the contact time. Also, the adsorption kinetic, thermodynamic and isotherm studies were determined to foresee the sorption behavior.
Adsorbates and materials
Methylene Blue (MB), basic blue 9, C.I. 52015; chemical formula, C16H18N3ClS, and molecular weight 319.85 g/mol, λmax: 662 nm, (Figure 1a), supplied by Merck. A 1000 mg/L stock solution of MB dye was prepared by dissolving the required amount of dye powder in bidistilled water.
Brilliant green dye, also called Basic Green 1 (C.I.: 42040, chemical formula, C27H34N2O4S, FW: 482.62 g/mol, λmax: 623 nm), supplied by Titan Biotech Limited, Bhiwadi, India (75% dye content) (Figure 1b), to prepare a 1000 mg/L stock solution appropriate amount of the dye was dissolved in DDW.
Calibration curves were constructed in the concentration ranges 1.0-20.0 mg/L and the concentrations of the investigated dyes were determined spectrophotomterically at λmax=662 and λmax=623 nm for MB and BG, respectively.
Apparatus
The laboratory measurements of pH were performed using HANNA pH-meter model Hi 931401 (Portugal), The concentrations of MB and BG dyes were determined using UV-Vis spectrophotometer (Chrom Tech., Ltd., USA).
Pistachio shells were collected from local markets, cleaned with tap water hardly and rinsed with distilled water for several times, then dried in at 373 K for 12 h. Sulphuric acid based activated carbon (ACS) was prepared by mixing 150 mL of 13 mol/L sulphuric acid with 30 g of pistachio shell. The mixture was put in furnace whose temperature was kept about 423-430 K for 90 min with occasional stirring. After cooling, the resulting black residue was filtered using a Buchner funnel under vacuum. Activated carbon was washed for some time with distilled water until pH value became 5-6 and dried at 373 K.
Adsorption studies of dye
To perform batch adsorption experiments, 0.025 grams of ACS with 25 mL aqueous solution of dye were introduced into 250 mL Erlenmeyer flasks, shake well in a temperature controlled water bath shaker using variable concentrations of dye between 10 and 400 mg/L of BG and between 50 and 500 mg/L of MB, pHs (between 3-10 for BG and 2-12 for MB), temperatures (between 32°C and 50°C), doses of ACS (between 0.005 and 0.0625 g) and ionic strength (between 0.005 and 0.2 mole/L) and shaking at a constant rate of 200 rpm. The concentrations of the non-adsorbed in the solution, was determined spectrophotometrically at 623 and 662 nm for BG and MB, respectively.
The capacity of adsorption of dye removed by adsorbent (q), and the removal percentage (R %) of cationic dyes are determined by the following equations. (1) and (2):


Where q is the adsorption capacity of dye (mg/g), Co and Ce are the initial and equilibrium state concentrations of dye (mg/L), respectively. The term m is the mass of the adsorbent used (g), and V is the solution volume of the dye (mL).
Desorption of dyes
100 mg of ACS adsorbents were shaken separately with 0.1 L of 10 mg/L of the dye solution. The adsorption capacity of dyes was determined. Thereafter, the dye-loaded ACS was desorbed using 0.05, 0.1 and 0.2 mol/L HCl solutions.
The quantity of the desorbed dye was calculated from the following dependence:
qd=(Cd–Ca)/m
Where: qd − the quantity of dye desorbed, Cd − concentration of dye after desorption, Ca − concentration of dye before desorption (after adsorption), m − ACS mass
The percentage of desorption of dye % was calculated by using the following equation:
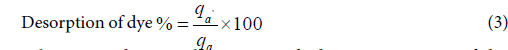
Where qd and qa are the amount of adsorption capacity of dye (mg/g) of desorption and adsorption, respectively.
Characterization of adsorbents
A measurement of specific surface area of the activated carbons produced from the Pistachio shell (ACS) was made by N2 adsorption (at 77 K), using a surface analyzer (QUANTACHROME – NOVA 2000 Series). The value by this method is 58.03 m2/g and the total pore volume with diameter less than 25.8 nm at P/Po=0.30019 is 1.735E-02 cc/g. While specific surface area value of ACS by DH method cumulative adsorption and desorption are 77.37 and 77.58 m2/g, respectively. Table 1 shows a comparison of the results of the present study by the previously reported studies. Marked enhancement in the surface area and adsorption capacity was obtained upon using the proposed ACS in ftThe presenft sftudy [21-27].
| Parameters | Present study | |||||||
|---|---|---|---|---|---|---|---|---|
| SBET (m2/g) | aSSH1 (at 25°C) 10.35 |
bPNS1 (at 303K) 25.34 |
cAC1 8.8 |
- | - | dBC400 2.8 |
72.00 | eACS 58.03 |
| SSH2 (at 80°C) 18.19 |
PNS2 (at 333K) 25.34 |
AC2 240.02 |
BC700 367 |
|||||
| SSH3 (at 100°C) 21.06 |
PNS2 (at 353K) 25.34 |
AC3 114.77 |
- | |||||
| qe (mg/g) | SSH1 (100 ppm) 34.00 |
PNS1 78.74 |
AC1 (at 25°C) 10.21 |
277.80 | 279.72 | - | 32.30 | 205.42 (149.00) |
| SSH1 (100 ppm) 66 |
PNS2 93.45 |
AC2 (at 25°C) 16.43 |
||||||
| SSH1 (100 ppm) 52 |
PNS3 125.00 |
AC3 (at 25°C) 15.80 |
||||||
| Sources | Sunflower Seeds hull |
Pistachio Nut Shell | Sunflower Oil Cake |
Lapsi seeds |
Biomass plant |
Bamboo Charcoal |
Peanut Shell |
Pistachio Shell |
| Adsorbate | Acid Violet 17 | Acid Violet 17 | Methylene Blue |
Pb2+ | Pb2+ | NH3 | Se(IV) | MB (BG) |
aSSH=Sunflower Seeds Hull; bPNS=Pistachio Nut Shell; cAC=Activated Carbon; dBC=Bamboo Charcoal and eACS=Sulphuric acid based-activated carbon
Table 1: Comparison of surface area values and adsorption capacities of the proposed ACS with different adsorbents previously reported.
Scanning electron microscopy (SEM) analyses showed that the sulphuric acid-treated activated carbon (ACS) had rough areas containing various irregular-shaped particles and macropores (Figures 2a and 2b).
The FTIR spectrum (Figure 3a) of the activated carbon ACS shows several bands at 3566, 3447 and 3422 cm-1 assigned to γ(OH) phenolic [23], νas(NH2) and νs(NH2) vibrations [28], respectively. Also, the bands observed in the 1797-2022 and 2929-3065 cm-1 ranges are attributed to the existence of hydrogen bonding in the activated carbon [29]. Moreover, the bands at 1703, 1683, 1649 and 1627 cm-1 are attributed to the free ν(C=O), ν(C=O; hydrogen bonded), ν(C=N) and ν(C=C) phenolic vibrations [30], respectively.
On other hands, Figure 3b shows FTIR spectrum of the activated carbon ACS loaded with methylene blue dye (MB). The results show new bands in the 1648-1465 cm-1 which are assigned to the C=C and C=N bands which are absent in the spectrum of the activated carbon ACS alone (Figure 3a). Also, the bands in the 1319-1165 cm-1 region are assigned to the C-O bands which have existed in the spectrum of ACS. The band at 3424 cm-1 in the case of AC attributed to ν(OH) vibration is shifted to lower wave number and observed at 3449 cm-1 (Figure 3b). This suggests the involvement of this group in bonding between ACS and MB.
Figure 3c shows the FTIR spectrum of the activated carbon ACS loaded with Brilliant Green dye (BG) several bands in the 1757-1266 cm-1. These bands are obscured in the spectrum of the activated carbon ACS alone (Figure 3a). The bands observed at 1757 cm-1 is assigned to the quinoid structure. Also, the band at 1473 cm-1 is attributed to the C=C groups. Moreover, the two bands at 1347 and 1266 cm-1 are mainly due to the ν(C-O) vibration of the lactonic and/or the phenolic groups existed in the ACS. All these observations suggest that BG is attached to activated carbon ACS via the C-O group.
The pH of the slurry of the ACS substance in aqueous solution provides a favorable pointer of the surface groups on carbon. The pH of the carbon slurry reflects the surface acidic groups which, in this case, for ACS are mainly carboxylic groups with only a very slight addition from sulfonic groups due to the low concentration of sulfur in the sorbent [31]. As the concentration of such functional groups increases on the carbon surface, the pH of the carbon decreases. The large quantities of the acidic functional groups on the surface of ACS result in increased cation exchange capacity and more adsorption of the dyes onto ACS surface. Boehm titration and surface acidity and basicity results are shown in Table 2. The synoptic number of the basic sites of the surface was calculated in all cases is smaller than the synoptic number of the acidic sites of the surface. The pHSUS is in agreement with this result, which is also acidic, and point of zero charge pHPZC.
| Acidic groups (mmol/g) |
Basic groups (mmol/g) | Surface pH | Point of zero charge | Moisture content % | Ash content % | |||
|---|---|---|---|---|---|---|---|---|
| Carboxylic | Lactonic | Phenolic | Total | |||||
| 3.670 | 1.940 | 2.400 | 8.010 | 0.330 | 2.93 | 3.02 | 2.62 | 0.56 |
Table 2: Chemical and physical Parameter of ACS adsorbent.
Adsorption of MB and BG
The effect of pH on the dye adsorption: The pH of the dye solution plays a significant role in the whole sorption process and specifically on the adsorption capacity. The effects of initial pH of sample solution on the removal percentage of MB and BG dyes using ACS adsorbent were evaluated within the pH range 2–12. Figure 4 shows that the adsorption process of BG dye onto ACS adsorbent does not depend on the change in the pH values of the solution of the dye. This finding agrees with the FTIR results discussed above, which suggests that the sorption mechanism should not be electrostatic Otherwise, the adsorption should take place by the interaction of the dye with the aromatic rings of the activated carbon [32]. This behavior is very significant from the analytical point of view as there is no need for pH adjustments for removing the BG dye using ACS adsorbent.
For MB, on the other hand, the removal percentage increases gradually upon increasing the pH from 2 to7. Then, the removal % of MB remained nearly constant over the pH range 7-12. The decreased removal % noticed at low pH values, may be due to competition between the protonated H+ ions and the cationic dye molecules for the active sites on the adsorbents When the solution pH increased, the surface of ACS may get negatively charged due to adsorption of hydroxyl groups, and the functional groups got deprotonated producing negatively charged adsorption sites, which improved the interaction between the adsorbent and the cationic dye molecules. For both MB and BG, the subsequent experiments were carried out at ambient pH.
The effect the initial concentration of dyes: The effect of the initial concentration of MB and BG dyes on the capacity of adsorption of these dyes using ACS at normal ambient pH had been studied. Figure 5 shows that the capacity of adsorption of the ACS adsorbent increase sharply when the initial concentration of the dyes increased. Then the capacity of adsorption gradually increases with further increase of the concentration of the dyes. At higher concentrations of the dyes, the equilibrium is reached. At higher MB and BG dyes concentrations, adsorption capacity reached a plateau indicating saturation of the available binding sites on the adsorbent. The sharp increase in the adsorption capacity in the early phase can be attributed to the great driving force of the concentration gradient at the solid-liquid interface causing an increase of the amount of MB and BG dyes adsorbed on the adsorbent [33]. When the initial concentration of MB and BG dyes increases from 50 to 500 mg/L and 10 to 400 mg/L at 25°C, the amount of dye adsorbed at equilibrium (qe), the increase from 49.94 to 205.42 mg/g and from 9.998 to 125.00 mg/g, for MB and BG, respectively.
Effect of adsorbent dosage: The effect of the amount of adsorbent ACS on the removal % of the investigated dyes is illustrated in Figure 6. As it can be noticed the removal % of each dye increased gradually upon increasing the dose of the adsorbent. Increasing the dose of adsorbent would provide more functional sites on the adsorbent capable of binding more dye molecules and thus increasing the removal %. The removal % of MB and BG dyes adsorbed increased from 18.55% to 99.83% and 60.63% to 98.93% as the adsorbent dose increased from 0.005 to 0.0625 g, respectively.
The effect of interferents: The interference of some foreign cations such as Ca2+, Mg2+, Na+, K+ and Na(I) as well as anions (F−, Cl−, acetate and oxalate) on the process of MB and BG dyes adsorption from aqueous solutions was studied and estimated by ACS. Estimation of the possible interference of foreign ions was performed on the basis of 10 mg/L of dye versus other interfering species. The results presented in Table 3, show that the presence of anions and cations do not affect the adsorption percentage of MB and BG dyes on the ACS adsorbent, especially Ca2+, Mg2+, Na+, K+ at the concentration of 200 mg/L.
| Foreign ion(200 mg/L) | Removal percentage, R% | |
|---|---|---|
| Methylene Blue | Brilliant Green | |
| F- | 98.43 | 93.66 |
| Cl- | 98.72 | 94.78 |
| Acetate | 98.74 | 98.14 |
| Oxalate | 94.83 | 96.27 |
| Mg2+ | 94.84 | 94.41 |
| Ca2+ | 93.71 | 92.17 |
| Na+ | 98.43 | 97.60 |
| K+ | 99.86 | 98.85 |
Table 3: Effect of interferents on the removal percentage of Methylene Blue and Brilliant Green.
The effect of contact time: The contact time between the dye and the ACS adsorbent is important in the dye removal from the solution by the adsorption process. The effect of contact time is studied by batch adsorption processes. Figure 7, shows that the adsorption capacity of MB and BG dyes increased with time and reached equilibrium at 540 minutes for MB dye and 480 minutes for BG dye.
Adsorption kinetics: The linearized form of the pseudo-first-order kinetic model [34] can be expressed by equation 4:
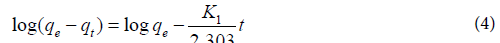
Where qe and qt correspond to the amount of dye adsorbed per adsorbent unit mass (mg/g) at equilibrium and at time t (min), respectively, k1 are the constant of equilibrium (min-1) and qe which were obtained from the slopes and intercept of the linear plots of log (qe-qt) versus t (Figure 8a).
The pseudo-second-order model of kinetic studies can be expressed by the following equation:

Where, qe and qt represent the amount of dye adsorbed per adsorbent unit mass (mg/g) at equilibrium and at time t (min), respectively. qe and k2 can be calculated from the slope and intercept of plot t/qt versus t (Figure 8b) [35].
To determine the best-fit model for adsorption of MB and BG on the ACS, the linear correlation coefficient (R2) was investigated. The results are shown in Table 4. The correlation coefficient (R2) of pseudo-second-order (R2 2 ≥ 0.9977) which is close to 1 was much higher than that of pseudo-first-order (R1 2 ≤ 0.9847). The theoretical q2 value calculated from pseudo-second-order was much closed to the experimental value qexp (Table 4) for both dyes MB and BG indicating that the pseudo-second-order model fits well for the system.
| Dye | qmax (mg/g) | ||
| 32°C | 40°C | 50°C | |
| MB | 205.42 | 226.44 | 257.16 |
| BG | 149.00 | 154.59 | 162.11 |
Table 4: Parameters of kinetics for the adsorption of Methylene Blue and Brilliant Green onto ACS.
The intra-particle diffusion model is an empirical found functional relationship, supposing that the adsorption capacity varies almost proportionally with t0.5 [36,37] this model is expressed by the following equation:

Where kint (g/mg min1/2) is that the adsorption constant, the intercept is C, both C and kint are calculated from a plot qt versus t0.5 (Figure 8c). Two distinct linear trends (MB1 and MB2) and (BG1 and BG2) are present in intra-particle diffusion model. The first trend (MB1) and (BG1) represents rapid surface adsorption (<240 min) of MB and (<180 min) of BG molecules via boundary layer diffusion, in which dye molecules move from bulk solution to the external surface of ACS particles and tend to cover mesopores of ACS surface. The MB2 and BG2 trend shows the attainment of equilibrium stage (>240 min) and (>180 min), respectively. The plot in Figure 8c does not pass through the origin implying that dye adsorption is partly controlled by intraparticle diffusion. The large value of boundary layer (Cint of BG >85.592 mg/g) implies that adsorption is mainly controlled by boundary layer adsorption. The similar mechanism has been reported for BG adsorption on activated carbon [38].
The kinetic data were further investigated using the Boyd [39] equation kinetic expressed by equation (7):

Where F(t) is the equilibrium fractional at different times t, and B(t) is mathematical function of F, n is an integer that defines the infinite series solution and F(t) is the fractional achievement of equilibrium at time (t) and is determined by the following equation:

Where q(e) and q(t) are the adsorbent capacity of dye adsorbed at equilibrium and the time t, respectively. Reichenberg [40] succeeded to obtain the following approximations:
For F values>0.85;
B (t)=-0.4977-ln (1-F) (9)
And for F values<0.85
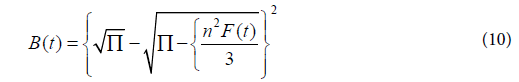
The plots of Boyd's equation (Figure 8d) didn’t go through the origin denoting that film diffusion is that the rate-limiting process of sorption for the sorption of dyes onto ACS.
The ionic strength effect: The effect of ionic strength on the removal of MB and BG dye as shown in Figure 9. The capacity of adsorption is reduced as the the ionic strength increased. Similarly, the concentration of NaCl ions increased from 0.01 to 0.20 mol/L, the capacities of adsorption is reduced from 95.23 to 93.01 mg/g, from 90.75 to 86.34 mg/g for MB and BG, respectively.
Effect of solution temperature and thermodynamic studies: The effect of temperature on the adsorption capacities of dyes on ACS was investigated at various temperatures (32, 40 and 50°C). The capacities of adsorption of dyes were represented in Table 5. Figures 10a and 10b show that the capacity of adsorption of MB and BG increases from 205.42 mg/g to 257.16 mg/g and 149.00 mg/g to 162.11 mg/g as the temperature increases from 32 to 50°C, respectively. Increasing temperature could also enhance the rate of diffusion of the dye molecules across the external boundary layer and internal pores of peat which reduces the viscosity of BG solution [6].
| Model | Kinetic parameter | Methylene Blue | Brilliant Green |
| qe, exp (mg/g) | 205.42 | 149.00 | |
| Pseudo-First-order Equation |
qe1 (mg/g) | 131.079 | 51.676 |
| k1 (min-1) | 0.00673 | 0.0071 | |
| R21 | 0.9847 | 0.9746 | |
| Pseudo-second-order Equation |
qe2 (mg/g) | 208.3333 | 136.99 |
| K2 (g/mg.min) | 0.00014 | 0.00074 | |
| R22 | 0.9977 | 0.9985 | |
| Intra-particle diffusion equation | Kint (mg/g.min1/2) | 0.03479 | 2.3227 |
| C | 4.8821 | 85.592 | |
| R2int | 0.9606 | 0.9663 | |
| Boyd equation | Intercept | -0.0657 | 0.4321 |
| R2 | 0.9847 | 0.9749 |
Table 5: Effect of temperature on adsorption capacities of Methylene Blue and Brilliant Green by ACS activated carbon.
The thermodynamics studies of MB and BG dye adsorption onto ACS were performed to find the energy dependent mechanism of the adsorption process. The standard free energy change (ΔG°), enthalpy change (ΔH°) and entropy change (ΔS°) associated with the adsorption processes are calculated using the following equations:


Where, Kd is the distribution coefficient (Langmuir constant), R is gas constant (8.314 J/mol K) and T is the solution temperature (K), respectively. The (ΔS°) and (ΔH°) values are obtained from the intercept and slope of ln Kd versus 1/T plot (Van’t Hoff) for the adsorption of MB and BG onto ACS at different temperature (Figure 11). The parameters of thermodynamic values are given in Table 6. The negative (ΔG°) values suggested that the adsorption process is spontaneous and more favorable at low temperature [41]. Moreover, the endothermic nature of adsorption process confirmed by the positive value of (ÄH°) and positive (ΔS°) value point out the increased randomness at the solid/ liquid interface during the adsorption of dyes onto ACS [42].
| Dye | ∆Hº (KJ/mol) |
∆Sº (KJ/mol) |
∆Gº (KJ/mol) |
||
|---|---|---|---|---|---|
| 305ºK | 313ºK | 323ºK | |||
| Methylene Blue | 27.8594 | 0.0924 | -0.3159 | -1.0681 | -1.8706 |
| Brilliant Green | 6.7885 | 0.0252 | -0.8832 | -1.0922 | -1.2634 |
Table 6: Parameters of thermodynamics for the adsorption of Methylene Blue and Brilliant Green on ACS.
Adsorption Isotherms: In the present investigation, the isotherm data were analyzed using the Langmuir and Freundlich isotherm equations (Figure 12).
Langmuir model is represented by the following equations [43]:

Where, qe the amount was adsorbed at equilibrium (mg/g), qm is the theoretical maximum adsorption capacity (mg/g), Ce is the equilibrium concentration of dye (mg/L) and kL is Langmuir adsorption constant (L/mg) related to the energy of adsorption. A plot of Ce/qe against Ce (Figure 12a) gave a straight line graph with a slope 1/qm and intercept of 1/kLqm. Values of qm and kL are calculated from the graph and reported in Table 7. The fundamental characteristics of the Langmuir isotherm can be expressed by a dimensionless separation factor, RL, defined by:
| Dye | Langmuir parameters | Freundlich parameters | |||||
|---|---|---|---|---|---|---|---|
| qm (mg/g) | KL (L/mg) | RL | R2 | n | KF | R2 | |
| MB | 208.333 | 0.2449 | 0.06372 | 0.9986 | 5.166 | 83.994 | 0.9830 |
| BG | 151.515 | 0.2734 | 0.0103 | 0.9993 | 4.039 | 47.196 | 0.9609 |
Table 7: Langmuir and Freundlich isotherm Parameters.
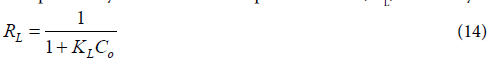
Where KL is Langmuir constant and C0 is the highest initial dye concentration (mg/L). The RL parameter indicates the type of the isotherm as follows: (RL>1), unfavourable; (RL=1), linear; (0
Isotherm model of Freundlich is expressed as equation [44]:

Where kF and n are Freundlich constants, the characteristic of the system, kF and n are the indicator of adsorption capacity and adsorption intensity, respectively (Figure 12b).
The parameters of Langmuir and of Freundlich isotherm models are given in Table 7. Examination of Table 7 reveals that the values of R2 acquired from Langmuir model are closer to unit than that of the model of Freundlich. A finding that denotes that the isotherm of Langmuir fits better with the adsorption both MB and BG dyes onto ACS.
Effect of desorption: The regeneration studies were conducted using three different concentrations of hydrochloric acid (0.05, 0.10 and 0.20 mol/L) revealed a maximum ability of desorption of 99.30 and 98.10% with 0.20 mol/L of HCl for MB and BG, respectively. At lower pH the greater numbers of H+ ions present will compete with dye cations for the same binding sites, resulting in greater desorption of dye cations; the data are presented in Table 8.
| (Dye) | qe Adsorbed (mg/g) | Desorption of dye using 0.05 mol/L HCl | Desorption of dye using 0.1 mol/L HCl | Desorption of dye using 0.2 mol/L HCl | |||
|---|---|---|---|---|---|---|---|
| qe desorbed (mg/g) | Desorption, % | qe desorbed (mg/g) | Desorption, % | qe desorbed (mg/g) | Desorption % | ||
| MB | 10 | 8.97 | 89.70 | 9.475 | 94.75 | 9.93 | 99.30 |
| BG | 10 | 8.387 | 83.87 | 9.054 | 90.54 | 9.81 | 98.10 |
Table 8: Desorption % of Methylene Blue and Brilliant Green from ACS.
Analytical applications
By spiking known concentrations of MB dye the ability of application of the ACS for uptake of the MB dye from different samples of water was investigated. The Table 9 shows the recovery percentage (R %) is more than 99.18% with less than 1% of relative standard deviation (RSD %).
| Sample | MB dye added (mg/L) |
MB dye removed (mg/L) |
R, %a | RSD, %b |
|---|---|---|---|---|
| Bi distilled water | 5 | 4.99875 | 99.98 | 0.05 |
| 10 | 9.99153 | 99.92 | 0.0245 | |
| 15 | 14.98058 | 99.87 | 0.026435 | |
| Underground water (Mit Gammr) | 5 | 4.96602 | 99.32 | 0.0797 |
| 10 | 9.91869 | 99.19 | 0.04686 | |
| 15 | 14.98762 | 99.18 | 0.04214 | |
| Domiat sea water | 5 | 4.984224 | 99.69 | 0.002427 |
| 10 | 9.963592 | 99.64 | 0.002802 | |
| 15 | 14.94782 | 99.65 | 0.00243 | |
| Alexandria sea water | 5 | 4.979952 | 99.54 | 0.093388 |
| 10 | 9.959952 | 99.60 | 0.024368 | |
| 15 | 14.94053 | 99.60 | 0.031115 |
aR, %=Removal % of methylene blue;
bRSD, %=Relative standard deviation
Table 9: Recovery (R %) of Methylene Blue from different samples of water using ACS adsorbent (n=4).
The removal of MB and BG dye by the proposed ACS activated carbons is in good comparison with the results of the previously reported studies [13,14,45-52] (Table 10). The prepared low cost material such as pistachio shell activated carbon ACS is beneficial to the one high-cost commercial activated carbon.
| Adsorbent | Dye | Adsorption capacity (mg/g) | Reference |
|---|---|---|---|
| Pistachio shell Activated carbon (H2SO4) (ACS) | MB | 205.42 | Present study |
| Pistachio shell Activated carbon (H2SO4) (ACS) | BG | 149.20 | Present study |
| Strychnos Potatorum Seed (AC) | MB | 100.00 | [45] |
| Jute fiber-based activated carbon | MB | 225.64 | [46] |
| Coconut husk-based activated carbon | MB | 434.78 | [47] |
| E. strobilacea char (ESC) | MB | 31.152 | [48] |
| ESC impregnated with 95% H3PO4 (ESP) | MB | 21.929 | [48] |
| ESC impregnated with 85% ZnCl2 (ESZ) | MB | 37.037 | [48] |
| Wood waste activated carbon | MB | 4.937 | [49] |
| Loofa activated carbon (Zn Cl2/ H3PO4) (AC1) | MB | 33.7496 | [50] |
| Loofa activated carbon (HNO3) (AC2) | MB | 32.9992 | [50] |
| Jute sticks charcoal | BG | 52.00 | [13] |
| Jute sticks steam activated carbon (ACS) | BG | 150.00 | [13] |
| Jute sticks chemical activated carbon (ACC) | BG | 286.00 | [13] |
| ACORN (AC) | BG | 02.01 | [14] |
| Bagasse fly ash | BG | 116.00 | [51] |
Table 10: Adsorption capacities of different adsorbents previously reported for the removal of MB and BG compared with ACS.
Activated carbon prepared from pistachio shell (ACS) is identified to be an effective adsorbent for the removal of two dyes methylene blue and brilliant green from aqueous solution. The adsorption is highly dependent on various operating parameters, like; temperature, adsorbent dose, pH, initial dye concentration, contact time, ionic strength. The kinetic studies indicated that follows pseudo-secondorder beside intra-particle diffusion and equilibrium in the adsorption of MB and BG on ACS were attained within 540 and 480 min, respectively. The equilibrium data were studied by Langmuir and Freundlich models, the adsorption equilibrium can be best represented by the Langmuir isotherm model, with maximum monolayer adsorption capacity of 208.333 and 151.515 mg/g for MB and BG on the adsorbent at 32°C. The results of thermodynamic indicated that the (- ÄG°) as expected for a process spontaneously under the optimum conditions. The synthesized ACS was successfully applied for the removal of the MB dye from samples of natural water. Desorption of the both dyes from ACS could easily be achieved by using 0.2 mol/L HCl. Finally, this work shows the possibility of using this technique widely in different applications.