
Immunotherapy: Open Access
Open Access
ISSN: 2471-9552

ISSN: 2471-9552
Research - (2019)Volume 5, Issue 1
Systemic administration of checkpoint inhibitors alone and especially concurrent with intratumoral administration of oncolytic herpesviruses (oHSV) has a major impact on cancer therapy marred by rare failures of healthy organs. Furthermore, tumors vary with respect to susceptibility to oncolytic effects of oHSV. Here we report the construction and properties of 3 families of oncolytic herpes simplex viruses expressing no immunomodulatory genes (T1 series), murine IL-12 (T2 series) or murine or human IL-12 and anti PD-1 antibody (T3 series). We report that insertion of the gene encoding PD-1 Ab significantly augmented the oncolytic activity of oHSV bereft of immunostimulatory genes (T1 series) or expressing IL-12 alone (T2 series). The T3 oHSV expressed IL-12, PD-1 Ab were restricted to the tumor bed whereas the induced IFN-γ accumulated to high levels both in tumor bed and in blood. Furthermore, the T3 oHSV was superior to systemic administration of IL-12 and antibody to PD-1. This report also shows that the oncolytic activity of T3 oHSV in a relatively resistant tumor was enhanced by concurrent intratumoral administrationof genetically engineered exosomes carrying miRNA targeting CTLA-4 checkpoint.
In the past decade two potentially complimentary cancer therapies made a significant impact on the survival of cancer patients. The first is based on advances in genetic engineering of viruses, particularly herpes simplex virus 1 (HSV-1) that replicate and ultimately kill cancer cells but replicate poorly if at all in normal cells [1-3]. In essence cancer cells frequently lose some features of innate immunity that enables the replication of a genetically debilitated HSV [4-6]. Typically, the oncolytic HSV (oHSV) replicate and kill a fraction of the tumor cells [6,7]. The oncolytic activity of the oHSV hinges on activation of the host innate or adaptive immunity capable of overcoming checkpoints on tumor cell surface. Several generations of HSV oncolytic viruses with varying tropisms and lytic activities against an array of tumor histology have been reported [8-11].
A significant enhancement of cytolytic activity in glioblastomas resulted from the insertion of the gene encoding IL-12 into the genomes of oHSV [12-14]. IL-12 induces the synthesis of IFN-γ and enhances the cytolytic activity of natural killer cells and cytotoxic T lymphocytes [15,16].
The second approach is based on administration to cancer patients of checkpoint inhibitors, particularly antibodies to PD-1, PD-L1, or CTLA-4 that typically block destruction of the tumor cells by immune mechanisms [17-19]. Success of the checkpoint inhibitors in treatment of cancer hinges on the concurrent presence of an activated immune response to the cancer cells [20,21]. The combination of the two approaches, namely the use of oncolytic viruses to trigger innate immunity to the cancer cells concurrently with systemic administration of checkpoint inhibitors proved to be much more effective than either approach alone [22-26]. The disadvantage of systemic administration of checkpoint inhibitors is the rare misdirection of the activated immune response resulting in devastating damage to healthy organs [27].
This report consists of two parts. Foremost we describe the production and properties of a novel genetically engineered oHSV designed to express IL-12 and antibody to PD-1 in the confines of the injected tumor thereby activating the immune system where it is needed most. We also describe the development of an adjunct therapy consisting of exosomes engineered to carry and release into tumor cells miRNAs specifically designed to target mRNAs encoding CTLA-4 checkpoint. We report that concurrent intratumoral administration of T3 oHSV and the genetically engineered exosomes significantly enhanced the oncolytic activity of T3 oHSV in a partially resistant tumor.
Exosomes are extracellular vesicles defined for the purposes of therapeutic applications by size and protein content. They package RNA and protein in cells in which they are produced and deliver the cargo to cells they are exposed. In recent years, numerous studies have shown that RNAs carrying specific nucleotide signatures are preferentially packaged in exosomes [28-31]. An example of the genetic engineering of exosomes is the significant reduction of HSV-1 replication in susceptible cells exposed to exosomes incorporating miRNA targeting the mRNA of an essential viral protein [32].
Viruses and cells
The BAC encoding the HSV-1(F) DNA was reported elsewhere [33,34]. Vero cells were obtained from ATCC. HEp-2 cells were obtained from the American Type Culture Collection and routinely cultured in DMEM (Life Technologies) supplemented with 5% (vol/vol) fetal bovine serum (FBS).
Construction of oHSV recombinant viruses
Constructions of BACs encoding T1012G, T2850, T3855, and T3011 were done as reported elsewhere [33,34]. Plasmid DNAs isolated from BAC-1012G, BAC-2850, BAC-3855, and BAC-3011 were transfected into Vero cells. Plaques obtained on Vero cells were purified three times on Vero cells. Viral DNAs were isolated from individual plaques, and recombinant viruses T1012G, T2850, T3855, and T3011 were sequenced to verify that no adventitious sequences were introduced during assembly of the recombinant viruses.
Antibodies
The antibodies used in this study were anti-His-tag (Cat No. 66005-1-Ig, Proteintech Group), anti-GAPDH (Cat No. #2118, Cell Signaling Technology), anti-CD9 (Cat No. #13174s, Cell Signaling Technology), anti-Flotillin-1 (Cat No. #18634, Cell Signaling Technology) and anti-Calnexin (Cat No. AC018, Beyotime). The anti-human PD-1 antibody (clone C1E1) generated using mouse hybridoma technology was developed and sequenced by Biosion, Inc. (Biosion, Nanjing, CN). The chimeric anti-human PD-1 Fab antibody was developed by fusion with mouse antibody C1E1 IgG viable region and human IgG1 constant region CH1 or κ light chain constant region.
ELISA assays
Human anti-PD-1 antibody concentrations in the samples was measured as follows: 100 ng PD-1/his protein (Sino Biological, Beijing, CN) were coated onto the 96-well ELISA plates (Corning, NY, USA). After removal of the coating solution, nonspecific binding sites were blocked by incubation with 2% bovine serum albumin (BSA). The plates were then rinsed, and exposed to known concentrations of purified anti-PD-1 antibody C1E1-Fab or to samples containing unknown concentrations of the antibody. After a 2 h of incubation, plates were rinsed and then exposed to HRP-conjugated goat anti-human Fab (Sigma- Aldrich, Darmstadt, GER) for an additional 1 h.
Plates were then rinsed again, followed by exposure to tetramethyl benzidine substrate (TMB) for color development. The reaction was stopped by the addition of stop solution. Plates were read on a BioTek microplate reader at a wavelength of 450 nm. The concentration of anti-human PD-1 antibody in the samples was extrapolated from the standard curve.
Secreted mouse anti-PD-1 antibody was measured by the quantitative sandwich ELISA assay with a mouse monoclonal anti-PD-1 antibody (1A1-1-5; Abiocenter Biotechnology Co., Ltd, Beijing, CN) which conjugated by HRP detection antibody (1A4-8-6; Abiocenter Biotechnology Co., Ltd, Beijing, CN). A purified anti-PD-1 antibody (L05; Abiocenter Biotechnology Co., Ltd, Beijing, CN) was used as standards.
IFN-γ
The samples were quantified by ELISA according to manufacturer’s instruction (R&D Systems, MN, USA).
Tumor cell lines
A20 (Murine B Lymphoma) cell was purchased from ATCC. MFC (Murine Forestomach Carcinoma), and MC38 (Murine Colon Carcinoma) cells were kindly provided by JOINN Laboratories, Inc. (Beijing, China), KYSE30 (Human Esophageal Squamous Carcinoma), B16 (Murine Melanoma) was kindly provided by Professor Jun Du (Sun Yat-sen University, Guangzhou, China). SCC7 (Murine Head and Neck Squamous Carcinoma) is a spontaneously arising squamous cell cancer of C3H mice. B16F10-hPD-L1 cell was genetically engineered by Beijing Biocytogen Co., Ltd (Beijing, China) to overexpress human PD-L1 and knock out mouse PD-L1. KYSE30, MC38 and B16F10-hPD-L1 cells were maintained in DMEM (Life Technologies), and MFC, SCC7, B16, A20 cells were maintained in RPMI-1640 (Life Technologies), containing 10% (vol/vol) fetal bovine serum (Gibco). All culture media were supplemented with 100 U/ml penicillin, and 100 μg/ml streptomycin.
Animal models, Human tumors
Nude mice derived from Balb/c were injected subcutaneously into flank with 5 × 106 cells of KYSE30 human tumor cells. The mice bearing tumors averaging volumes stated in Results were randomized and injected with phosphate buffered saline PBS (Control) or oHSV. The tumors and blood were harvested on days 0, 2, 4, 7, 14 and 28 after injection for ELISA assay.
Syngeneic mouse model
The syngeneic mice were Balb/c for A20. 615 for MFC, C57BL/6 for MC38, C3H/HeN mice for SCC7 and C57BL/6 for B16 tumors. The tumors were generated by implantation of 2 × 106 cells subcutaneously into mouse flanks. The procedures were the same as described above.
Humanized PD-1 knock-in mouse model
The mice were purchased from BIOCYTOGEN (Beijing, China). The homozygous humanized PD-1 knock-in mouse contains a chimeric PD-1, in which exon 2 of the mouse Pdcd1 gene was replaced with the human counterpart. In these studies, tumors were generated by injection of 1 × 105 murine B16 tumor cells. Subsequent procedures were the same as described above.
Murine IL-12 and PD-1 antibody
Recombinant murine IL-12 p70 was purchased from Peprotech (Cat. 210-12). The InVivoMAb anti-mouse PD-1 blocking antibody (Clone J43) was purchased from Bio X Cell (Cat. BE0033-2).
Plasmids expressing miR-CTLA-4
The miRNA sequences targeting mouse CTLA-4 were designed as described previously [32]. The synthesized DNA fragments encoding the miRNAs were digested with BamHI and XhoI restriction enzymes and cloned into the corresponding sites of pcDNA6.2-GW/EmGFP-miR-neg control plasmid (Invitrogen). The His-tagged mouse CTLA-4 expression plasmid (mCTLA-4- his) was purchased from YouBio Biotechnology (Changsha, China).
Exosome Isolation
HEp-2 cells (5 × 106) were transfected with 10 μg of plasmids expressing miR-CTLA-4. After 4 h incubation the cells were rinsed three times with PBS to exclude potential contamination of exosome in serum, and the cells were cultured in serum free medium for another 48 h. Exosomes were purified by differential centrifugation processes as reported elsewhere [39]. The pelleted exosomes were then resuspended in 200 μl of PBS and then quantified by a BCA assay as described previously [32].
Analyses of Exosome size and quantifications
Exosome size distribution and quantification were done using the qNano system (Izon, Christchurch, New Zealand) as described in detail previously [32].
Immunoblot assays
Detection of His-tagged CTLA-4, GAPDH and exosome maker proteins by immunoblot assay were measured as described in detail previously [32].
Structure of oHSV used in this study
We produced 3 series of oHSV-1(F) recombinants. In series T1 we deleted 15,091 base pairs of DNA from the inverted repeats of the viral genome. In recombinant T1012G the deleted sequence was replaced by sequences encoding the CMV promoter followed by three stop codons and an open reading frame (ORF) encoding the green fluorescent protein (GFP) driven by SV40 promoter was inserted between UL3 and UL4 ORFs of the viral genome. T1012G was designed as a backbone control to T2 and T3 series. In series T2 we replaced the deleted sequence with an ORF encoding murine IL-12 (T2850) driven by Egr promoter.
Lastly, in series T3 we inserted between UL3 and UL4 of the recombinant T2850 an ORF encoding either the murine scFV (single-chain variable fragment) antibody against PD-1 to generate T3855 or an ORF encoding human PD-1 antibody Fab (antigen-binding fragment) driven by CMV promoter and replaced the deleted sequence with an ORF encoding human IL-12 driven by Egr promoter to generate T3011. The list of recombinants and the inserts are shown in columns 1 and 2 of Supplement Table 1. The protocols for construction of recombinant viruses produced in our laboratories have been extensively documented elsewhere [33,34]. Sources of the ORFs encoding human and murine anti PD-1 antibodies and IL-12 are listed in Materials and Methods.
| Average Tumor Volume (mm3) |
Group (N=6) | D0 | D3 | D7 | D10 | D14 | |
|---|---|---|---|---|---|---|---|
| 1 | Control | 101 ± (6.0) | 336 ± (68) | 725 ± (137) | 1025 ± (212) | 2248 ± (557) | |
| 2 | Anti-PD-1 (10 mg/kg) | 103 ± (7.8) | 281 ± (99) | 464 ± (153) | 871 ± (301) | 2052 ± (852) | |
| 3 | Anti-PD-1 (1 mg/kg) | 109 ± (8.1) | 307 ± (53) | 585 ± (87) | 1027 ± (126) | 1836 ± (200) | |
| 4 | IL-12 (1 µg/animal) | 106 ± (4.6) | 349 ± (74) | 650 ± (95) | 1091 ± (205) | 1929 ± (309) | |
| 5 | IL-12 (0.1 µg/animal) | 103 ± (6.3) | 321 ± (90) | 794 ± (273) | 1228 ± (398) | 2269 ± (715) | |
| 6 | Anti-PD-1+IL-12 | 105 ± (7.4) | 131 ± (42) | 241 ± (106) | 400 ± (179) | 1092 ± (532) | |
| 7 | T1012G | 110 ± (5.7) | 231 ± (79) | 466 ± (212) | 556 ± (240) | 1393 ± (736) | |
| 8 | T3855 | 110 ± (6.8) | 165 ± (32) | 187 ± (85) | 207 ± (138.) | 443 ± (351) | |
| 9 | T1012G+Anti-PD-1+IL-12 | 109 ± (6.4) | 242 ± (44) | 295 ± (107) | 386 ± (184) | 1050 ± (624) |
Table 1. Comparison of anti-tumor efficacy of oncolytic herpes simplex viruses (oHSV), PD-1 antibody and IL-12 protein in A20 tumor model.
Expression of IL-12 and antibodies to PD-1 by the recombinant oHSVs
The purpose of the experiments described below was to assess the production of IL-12 and PD-1 antibody by the oHSV carrying the respective genes. Vero cells grown in T25 flasks containing 2 × 106 cells were mock infected (Mock) or exposed to 1 pfu of T3855, T2850, or T3011 per cell for 1 h. The inoculum was then replaced with fresh medium. The cell culture media (4 ml per flask) were harvested at 48 h after infection. The accumulated levels of human and mouse IL-12 and PD-1 antibodies were analyzed by ELISA assay as described in Materials and Methods. The amounts of IL-12 and PD-1 Ab were calculated based on a standard curve generated with purified IL-12 or PD-1 antibodies (Supplement Table 2). The linear range of both human and mouse IL-12 p70 ELISA assay was 7.8 to 500 pg/ml. The linear ranges of human and mouse PD-1 Ab were 78 to 10,000 pg/ml and 125 to 10,000 pg/ml respectively.
The results of the assays shown in Supplement Tables 2 indicate that the oHSV constructed in this study produced PD-1 Ab and or IL-12 as predicted by their gene contents.
The oncolytic activity of oHSV produced and tested in this study
The purpose of the studies described in this section was to assess the oncolytic activity of the oHSVs described above. In essence as listed in Supplement Table 1 we analyzed the activity of 4 oHSVs in different mouse models implanted with 5 murine and 1 human tumors. Depending on the objective of the studies we used nude mice derived from Balb/c mouse strain (Balb/c Nude), syngeneic mice or syngeneic mice expressing human PD-1. The sources of the mouse strains and tumor cell lines used in these studies are listed in Materials and Methods. The results of the studies may be summarized as follows:
(i) The oncolytic activity of oHSV is incrementally enhanced by the expression of the genes encoding IL-12 or both IL-12 and PD-1 Ab: To compare the oncolytic activity of the oHSV backbone (T1012G), and the oHSV encoding murine IL-12 (T2850) or both murine IL-12 and PD-1 Ab (T3855), we used syngeneic mice implanted with murine tumors (A20 and MFC) shown in preliminary experiments to be relatively resistant to oHSV. In brief, mice were implanted with A20 tumor or MFC tumor. The tumors were injected with 50 μl of PBS (Control) or 1× 107 pfu of T1012G, T2850 or T3855 once the tumor volumes averaged 130 mm3 (Figure 1 Panel A), 110 mm3 (Panel B). In this and all other experiments described in this report the tumor volumes were measured twice weekly. The results (Figure 1A and 1B) show that the reduction in tumor volumes was highest in mice injected with T3855 and lowest in mice injected with T1012G oHSV.
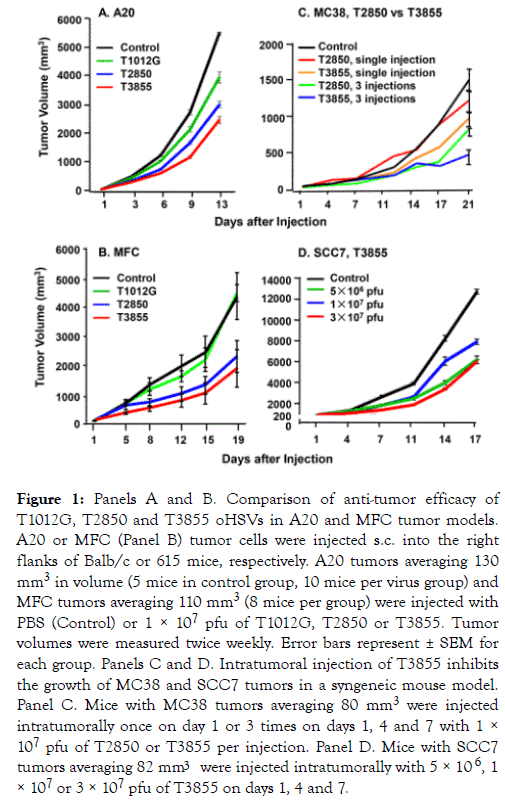
Figure 1. Panels A and B. Comparison of anti-tumor efficacy of T1012G, T2850 and T3855 oHSVs in A20 and MFC tumor models. A20 or MFC (Panel B) tumor cells were injected s.c. into the right flanks of Balb/c or 615 mice, respectively. A20 tumors averaging 130 mm3 in volume (5 mice in control group, 10 mice per virus group) and MFC tumors averaging 110 mm3 (8 mice per group) were injected with PBS (Control) or 1 × 107 pfu of T1012G, T2850 or T3855. Tumor volumes were measured twice weekly. Error bars represent ± SEM for each group. Panels C and D. Intratumoral injection of T3855 inhibits the growth of MC38 and SCC7 tumors in a syngeneic mouse model. Panel C. Mice with MC38 tumors averaging 80 mm3 were injected intratumorally once on day 1 or 3 times on days 1, 4 and 7 with 1 × 107 pfu of T2850 or T3855 per injection. Panel D. Mice with SCC7 tumors averaging 82 mm3 were injected intratumorally with 5 × 106 , 1 × 107 or 3 × 107 pfu of T3855 on days 1, 4 and 7.
(ii) oHSV encoding murine IL-12 and PD-1 Ab (T3855) inhibits the growth of diverse murine tumors in dose dependent manner: In this series of experiments, we compared the efficacy of single injection of 1 × 107 pfu versus triple injections of T2850 delivered on days 1, 4 and 7 encoding IL-12 only with corresponding number of injections of T3855 encoding IL-12 and anti PD-1 antibody. The results (Figure 1 Panel C) show that the reductions in tumor volumes were highest in mice injected with T3855 and lowest in mice injected with T2850 oHSV after either one or 3 injections. Moreover, 3 injections showed a higher anti-tumor efficacy than single injection.
We next tested the oncolytic activity of oHSV encoding murine IL-12 and PD-1 Ab administered to groups of 8 syngeneic mice bearing SCC7 murine tumors with an average size of 82 mm3. The tumors were injected with 50 μl of PBS (Control) or 5 × 106, 1 × 107, or 3 × 107 pfu of T3855 on days 1, 4 and 7. The results (Figure 1 Panel D) are consistent with numerous other reports that tumors vary with respect to susceptibility to oHSV and indicate that the oncolytic activity is dose dependent.
(iii) Comparison of the effectiveness of T2850 and T3855 in syngeneic mice implanted B16 murine melanoma tumors: In this series of experiments B16 mouse melanoma cells were implanted subcutaneously into the flanks of C57BL/6 mice. Tumors averaging 90 mm3 were injected with 50 μl of PBS or oHSV on days 1, 4 and 7. The oHSV dosages administered to mice in groups of 8 were 5 × 106, 1 × 107 or 3 × 107 pfu of T2850 (Figure 2) or T3855 (Figure 2) respectively. The results indicate that the lowest dose of T3855 was more effective than the highest dose of T2850 oHSV.
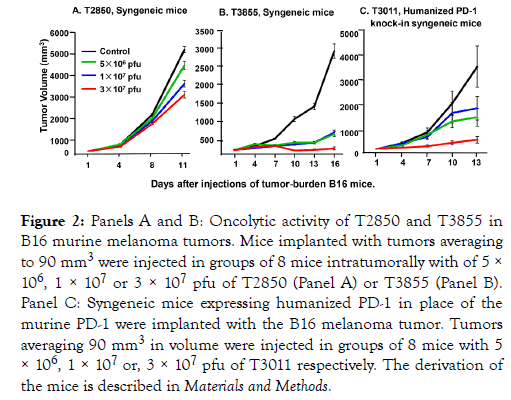
Figure 2. Panels A and B: Oncolytic activity of T2850 and T3855 in B16 murine melanoma tumors. Mice implanted with tumors averaging to 90 mm3 were injected in groups of 8 mice intratumorally with of 5 × 106, 1 × 107 or 3 × 107 pfu of T2850 (Panel A) or T3855 (Panel B). Panel C: Syngeneic mice expressing humanized PD-1 in place of the murine PD-1 were implanted with the B16 melanoma tumor. Tumors averaging 90 mm3 in volume were injected in groups of 8 mice with 5 × 106, 1 × 107 or, 3 × 107 pfu of T3011 respectively. The derivation of the mice is described in Materials and Methods.
(iv) Evaluation of T3011 oHSV in B16 tumors implanted in humanized PD-1 knock-in mice: The objective of this series of experiments was to determine whether T3011 expressing human IL-12 and human PD-1 Ab would replicate in B16 tumors. As in preceding studies the B16 tumors were implanted subcutaneously into flanks of 8 mice. Tumors averaging 90 mm3 were injected with PBS or 5 × 106, 1 × 107 or 3 × 107 pfu of T3011. As shown in Figure 2 panel C, T3011 oHSV effectively suppressed tumor growth. The source and genotype of the mice are described in Materials and Methods.
Accumulation of murine IL-12 and PD-1 Ab in A20 tumor after T3855 oHSV single injection
The purpose of the experiments described below was to assess the accumulation of murine IL-12 and PD-1 antibody in the tumor bed after injection of T3855. Tumors averaging 200 mm3 were injected in groups of 5 with 50 μl of PBS or 1 × 107 pfu of T3855 oHSV. At 4, 7, 14 and 21 days after injection, the tumors were harvested and analyzed as described in Materials and Methods. The results shown in Figure 3A and 3B suggest that both IL-12 and PD-1 Ab accumulated and peaked on the 4th day after infection with T3855 and declined there after (Panel A, B of Figure 3).
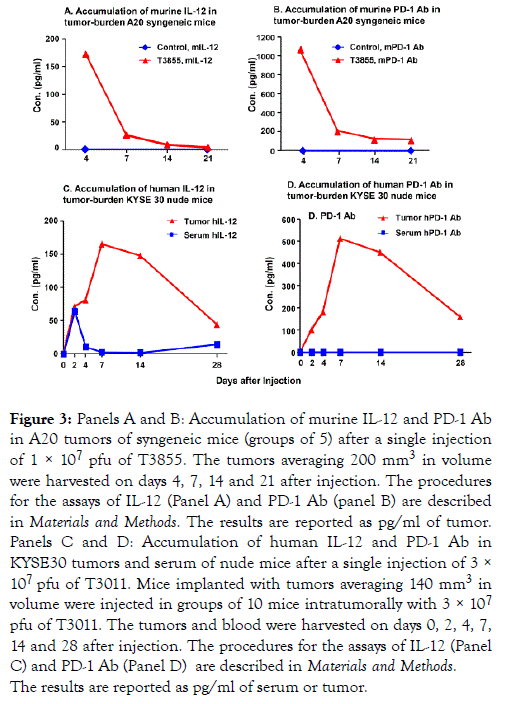
Figure 3. Panels A and B: Accumulation of murine IL-12 and PD-1 Ab in A20 tumors of syngeneic mice (groups of 5) after a single injection of 1 × 107 pfu of T3855. The tumors averaging 200 mm3 in volume were harvested on days 4, 7, 14 and 21 after injection. The procedures for the assays of IL-12 (Panel A) and PD-1 Ab (panel B) are described in Materials and Methods. The results are reported as pg/ml of tumor. Panels C and D: Accumulation of human IL-12 and PD-1 Ab in KYSE30 tumors and serum of nude mice after a single injection of 3 × 107 pfu of T3011. Mice implanted with tumors averaging 140 mm3 in volume were injected in groups of 10 mice intratumorally with 3 × 107 pfu of T3011. The tumors and blood were harvested on days 0, 2, 4, 7, 14 and 28 after injection. The procedures for the assays of IL-12 (Panel C) and PD-1 Ab (Panel D) are described in Materials and Methods. The results are reported as pg/ml of serum or tumor.
The accumulation of PD-1 Ab and IL-12 expressed by T3011 oHSV is restricted in tumors
T3011 oHSV was designed to concentrate anti-tumor effects of IL-12 and PD-1 Ab in tumors and minimize the side effects of PD-1 and IL-12 released into blood. The purpose of the experiments described below was to assess the accumulation of human IL-12 and PD-1 Ab both in serum and tumors of T3011 infected mice. The results (Figure 3C and 3D) were as follows:
(i) Accumulation of both PD-1 Ab and IL-12 increased from day 2 and peaked on days 7 to 14 then declined thereafter but not to the basal level.
(ii) PD-1 Ab was not detected in sera of animals injected with T3011 while IL-12 was detected on Day 2 but rapidly declined to undetectable levels thereafter. The results suggest that PD-1 Ab and IL-12 expressed by T3011 were concentrated in tumors and did not accumulate in significant amounts in blood.
The mechanisms of anti-tumor effects of oHSVs produced and tested in this study
Production of IFNs is a hallmark of immunotherapeutic antitumor activity [35,36]. In the studies reported here we measured the production of IFN-γ in mice implanted with A20 tumor cells and injected with 1 × 107 pfu of T1012G, T2850, or T3855 oHSV.
The experimental design was as described above except that we tested the accumulation of IFN-γ both in serum and tumors of control and infected mice. The results (Figure 4A and 4B) were as follows:
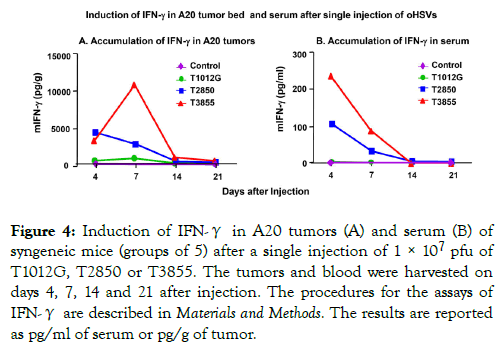
Figure 4. Induction of IFN-γ in A20 tumors (A) and serum (B) of syngeneic mice (groups of 5) after a single injection of 1 × 107 pfu of T1012G, T2850 or T3855. The tumors and blood were harvested on days 4, 7, 14 and 21 after injection. The procedures for the assays of IFN-γ are described in Materials and Methods. The results are reported as pg/ml of serum or pg/g of tumor.
(i) IFN-γ was not detected in sera or tumors of animals injected with T1012G.
(ii) IFN-γ levels increased nearly 4000-5000 folds in tumor bed of mice infected with both T2850 and T3855 by day 4 and declined with T2850 injected tumor. However, IFN-γ levels continuously increased more than 10,000 fold in mice infected with both T3855 by day 7 and thereafter.
(iii) IFN-γ levels increased nearly 100 fold in sera of mice infected with T2850 and more than 200 fold in mice injected with T3855 by day 4 and declined thereafter.
The results suggest that expression of IL-12 by oHSV T2850 is sufficient to induce IFN-γ accumulation and that highest levels of IFN-γ in the tumor bed was induced by T3855 which expresses both IL-12 and PD-1 antibody.
Of particular interest is the observation that the upsurge in the accumulation of IFN-γ in tumors injected with T3855 is not reflected in the corresponding sera. This observation suggests that IFN-γ is either continuously produced or retained in tumors.
Comparison of the effectiveness of intratumoral injection of T3855 oHSV with systemic administration of anti PD-1 Ab and IL-12 alone or in combination with T1012G
The primary objective of the experiments detailed below was to compare the antitumor effectiveness of oHSV expressing both IL-12 and PD-1 Ab with the effect of intraperitoneal injection of IL-12 and or PD-1 Ab. The design of the experiment is illustrated in Table 1. Briefly Balb/c mice in groups of 6 each and bearing A20 tumors averaging 105 mm3 in volume were injected intraperitoneally with (a) mouse PD-1 Ab (10 mg or 1 mg per kg mouse body weight) [37], (b) mouse IL-12 (1 μg or 0.1 μg/mouse) [38] or (c) 1 × 107 pfu of T3855 intratumorally or (d) 1 × 107 pfu of T1012G intratumorally alone or in combination with both IL-12 (1 μg/mouse) and PD-1 Ab (10 mg/kg of mouse body weight) administered intraperitoneally. The tumor volumes were measured on days 0, 3, 7, 10 and 14 after administration of virus, IL-12 or PD-1 Ab. The results (Table 1) were as follows:
(a) Injection of T3855 alone was most effective in reducing tumor volumes.
(b) Slightly less effective were the combination of T1012G with IL-12 and PD-1 Ab or intraperitoneal injection of IL-12 and PD-1 Ab followed by the administration of T1012G alone.
(c) The least effective procedures were administration of PD-1 Ab alone or IL-12 alone.
Production and characterization of exosomes containing miR-CTLA-4 (miR-CTLA-4 exo)
The procedures for production, purification and employment of exosomes containing microRNAs (miRNAs) targeting specific mRNAs employed in this laboratory were described elsewhere in detail [32]. Briefly: The first step was to design a series of 10 miRNAs targeting CTLA-4 and carrying published sequences that result in preferential packaging of the miRNAs into exosomes (Panel A of Figure 5) [28,30]. The sequences of the 10 miRNAs are shown in Panel B of Figure 5. The 10 plasmids contain the sequences encoding the miRNAs were transfected in HEp-2 cells along with a plasmid encoding His-tagged-CTLA-4.
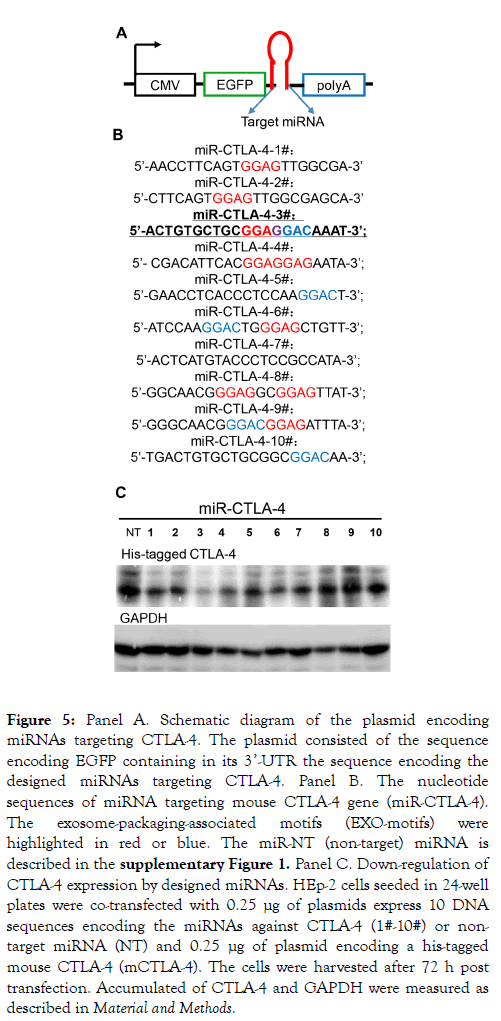
Figure 5. Panel A. Schematic diagram of the plasmid encoding miRNAs targeting CTLA-4. The plasmid consisted of the sequence encoding EGFP containing in its 3’-UTR the sequence encoding the designed miRNAs targeting CTLA-4. Panel B. The nucleotide sequences of miRNA targeting mouse CTLA-4 gene (miR-CTLA-4). The exosome-packaging-associated motifs (EXO-motifs) were highlighted in red or blue. The miR-NT (non-target) miRNA is described in the supplementary Figure 1. Panel C. Down-regulation of CTLA-4 expression by designed miRNAs. HEp-2 cells seeded in 24-well plates were co-transfected with 0.25 μg of plasmids express 10 DNA sequences encoding the miRNAs against CTLA-4 (1#-10#) or nontarget miRNA (NT) and 0.25 μg of plasmid encoding a his-tagged mouse CTLA-4 (mCTLA-4). The cells were harvested after 72 h post transfection. Accumulated of CTLA-4 and GAPDH were measured as described in Material and Methods.
After 72 h the cells were harvested, solubilized, electrophoretically separated in in denaturing gel and probed with antibody to His. As shown in Figure 5 panel C several miRNAs reduced the accumulation of CTLA-4. We selected miR-CTLA-4 No. 3 for further studies. In the next step we produced exosomes encoding the selected miR-CTLA-4 (miRCTLA- 4 exo). Thus HEp-2 cells were transfected with the plasmid encoding the No. 3 miR-CTLA-4. After 48 h the extracellular medium was harvested and the exosomes were purified as described in detail elsewhere [39] and analyzed with respect to protein content and size distribution. The results of these analyses are shown in Panels A and B of Supplementary Figure 1. As expected they show that the exosomes contain CD9 and Flotilin-1 but not Calnexin, and that they average 100-200 nm in diameter.
Concurrent administration of exosomes carrying miRCTLA- 4 and oHSV into MFC implanted tumors enhances the oncolytic activity of T3 but not T1 or T2 oHSVs
The 4 panels of Figure 6 illustrate the results of a single experiment. In brief, 8 groups of 8 C57BL/615 mice were each implanted with MFC tumor cells. When the tumors reached an average of 80 mm3 they were injected with 1 × 107 pfu of T1012G (Panel B), T2850 (Panel C) or T3855 (panel D) alone or in combination with 10 μg of miR-CTLA-4 exosomes. The results were as follows.
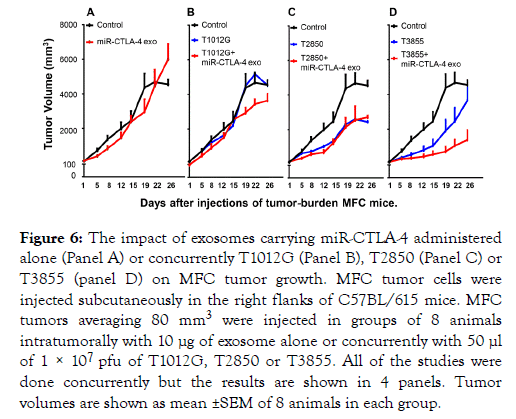
Figure 6. The impact of exosomes carrying miR-CTLA-4 administered alone (Panel A) or concurrently T1012G (Panel B), T2850 (Panel C) or T3855 (panel D) on MFC tumor growth. MFC tumor cells were injected subcutaneously in the right flanks of C57BL/615 mice. MFC tumors averaging 80 mm3 were injected in groups of 8 animals intratumorally with 10 μg of exosome alone or concurrently with 50 μl of 1 × 107 pfu of T1012G, T2850 or T3855. All of the studies were done concurrently but the results are shown in 4 panels. Tumor volumes are shown as mean ±SEM of 8 animals in each group.
The miR-CTLA-4 exosomes made no impact on the growth of uninfected tumors (Panel A), tumors injected with T1012G oHSV (Panel B) or tumors injected with T2850 oHSV (Panel C). miR-CTLA-4 significantly reduced the growth of tumors injected with T3855 (Panel D).
As noted in the introduction immunotherapy involving systemic administration of immune modulators and antibodies to checkpoints plus concurrent intratumoral injection of oncolytic viruses have led to significant improvements in the lifespan of cancer patients. These advances are marred by two problems. First, rare misdirection of the activated immune response results in devastating damage to healthy organs. Second, human tumors vary with respect to susceptibility to oncolytic viruses including the new generations of oncolytic viruses that incorporate genes encoding immune modulators or antibodies to checkpoints. Treatment of resistant tumors requires adjunct therapies.
We report on the properties of a family of novel oncolytic HSV (oHSV). The family consists of mutants without inserts, mutants incorporating mouse or human IL-12 alone or in addition murine or human genes encoding single chain antibody against PD-1 (PD-1 Ab). We also report on an adjunct therapy effective in tumors resistant to the oHSV. The key results of the studies presented in this report show the following: (i) Insertion of the gene encoding PD-1 Ab significantly augmented the oncolytic activity of oHSV bereft of immunostimulatory genes (T1 series) or expressing IL-12 alone (T2 series). In syngeneic mice, the T3 series oHSV expressing murine IL-12 and PD-1 Ab was effective against a variety of murine tumors. (ii) Concurrent with enhanced cytolytic activity, the T3 oHSV induced the production and significant intratumoral accumulation of IL-12, PD-1 Ab and IFN-γ. (iii) We compared the oncolytic effects of a single intratumoral injection of oncolytic viruses alone, in combination with anti PD-1 antibody and or IL-12 administered intraperitoneally or intraperitoneal administration of IL-12 or anti PD-1 antibody alone. The most effective oncolytic effect resulted from the administration of T3855 expressing both IL-12 and anti PD-1 antibody. The least effective were single intraperitoneal administration of IL-12 or PD-1 antibody. (iv) We compared the effectiveness of intratumoral injection of T3 oHSV with systemic administration of anti PD-1 Ab and IL-12 alone or in combinations with T1. Intratumoral injection of T3 oHSV was most effective against murine tumors in comparison with either systemic administration of anti PD-1 Ab and IL-12 or intratumoral injection of T1 in combinations of systemic administration of anti PD-1 Ab and IL-12. (v) Lastly we identified a murine tumor relatively resistant to the oncolytic activity of murine T1, T2 and T3 series of oHSV. The adjunct therapy we selected consisted of exosomes carrying genetically engineered miRNAs targeting CTLA-4 mRNA. The results presented in this report showed that a single injection of exosomes expressing miR-CTLA-4 administered concurrently with the murine T3 oHSV impacted on the growth of tumors. Administration of miR-CTLA-4 exosomes concurrently with the T1 or T2 had no effect on the growth of the implanted tumor. Relevant to the results presented in this report are the following: IL-12 is a cytokine with potent antitumor effects. Thus IL-12 induces a TH-1 type immune response which may provide a durable antitumor effect [39,40]. IL-12 has been reported to have in vivo anti-angiogenic activity, which may also contribute to its antitumor effects. Lastly IL-12 has been reported to stimulate the production of high levels of IFN-γ which has multiple immunoregulatory effects including the capacity to stimulate the activation of CTLs natural killer cells and macrophages and to induce/enhance the expression of class II MHC antigens. IFN-γ plays a significant role in the process of inducing T-cell migration to tumor sites. Increases in the intratumoral levels of IFN-γ correlated with a decrease in the size of the tumor burden [41-49].
PD-1 Ab releases the biological “ brake ” that prevents the immune system from besieging and destroying tumor cells. Clinically, administration of PD-1 Ab resulted in impressive and often durable responses in patients with a number of advanced stage malignancies [50]. The downside of systemic administration of PD-1 Ab is very rare devastating damage to healthy organs [27]. A key advantage of the T3 recombinants is that IL-12, and PD-1 Ab were concentrated in the tumors in which they were produced rather than dispersed systemically. The greater efficacy of the T3 series reflects the conversion of the infected cell into a virus factory propagating immunogenic cell death. The life of the “factory” can be extended by repeated injection of the oHSV as needed. In the studies presented here the intraperitoneal administration of IL-12 and anti PD-1 antibody did not yield results superior to those of oHSV administered intratumorally.
Lastly as noted earlier in the text exosomes are extracellular vesicles that carry RNA and proteins from the producer cells to recipient and are an important form of intercellular communication. Recent advances in the studies led to the identification of nucleotide sequences of RNAs preferentially packaged into exosomes. We have used this information to construct miRNAs and package into exosomes that specifically target mRNAs encoding specific proteins [28-31]. In the studies reported here we targeted mRNA encoding the CTLA-4 checkpoint. The results suggest that the immunotherapy of MFC tumors was significantly enhanced by the concurrent inhibition of both PD-1 and CTLA-4 checkpoints.
This work was supported by National Major Scientific and Technological Special Project for Significant New Drugs Development during the Thirteenth Five-year Plan Period under Grant 2018ZX09733002 to Immvira Co., Ltd and Shenzhen Overseas High-Caliber Peacock Foundation under Grant KQTD2015071414385495 to Shenzhen International Institute for Biomedical Research.
Citation: Yan R, Zhou X, Chen X, Liu X, Tang Y, Ma J, et al. (2019) Enhancement of Oncolytic Activity of oHSV Expressing IL-12 and anti PD-1 Antibody by Concurrent Administration of Exosomes Carrying CTLA-4 MiRNA. Immunotherapy (Los Angel) 5:154. doi: 10.35248/2471-9552.19.5.154
Received: 21-Jul-2019 Accepted: 29-Jul-2019 Published: 07-Aug-2019 , DOI: 10.35248/2471-9552.19.5.154
Copyright: © 2019 Yan R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This work was supported by National Major Scientific and Technological Special Project for Significant New Drugs Development during the Thirteenth Five-year Plan Period under Grant 2018ZX09733002 to Immvira Co., Ltd and Shenzhen Overseas High-Caliber Peacock Foundation under Grant KQTD2015071414385495 to Shenzhen International Institute for Biomedical Research.