Poultry, Fisheries & Wildlife Sciences
Open Access
ISSN: 2375-446X
ISSN: 2375-446X
Research Article - (2015) Volume 3, Issue 1
Okobaba hub of Lagos Lagoon is a water body that is presented to direct impact of sawmilling movement and other local wellsprings of contamination of the water body. The physic-concoction parameters, substantial metals and also disintegrated supplements for both water and silt tests of Lagos tidal pond at Okobaba were considered for a time of six month, tests were gathered from March to August as part of the arrangement of studies for the determination for the levels of tainting of the oceanic environment. Five stations were considered and an aggregate of thirty examples were gathered for both water and dregs tests each. Four out of the five stations extended along the water bank of Okobaba while the last (control) is taken after third terrain span. Profundity of water ranges between 0.6 m-2.2 m, temperature ranges somewhere around 240°C and 300°C for air and 250°C and 350°C in water. Complete strong in water was seen to have a scope of 7010-21104. Saltiness of water body ranges from 5.7-22.8 mg/l. Scope of qualities for DO, BOD, alkalinity and pH qualities are given as 3.5-7.4 mg/l, 2106-12104 mg/l, 36.5-105.2 mg/l and 6.7-7.9 mg/l individually. Results got demonstrated that with the exception of Chloride, suspended strong, depth and transparency, all other physic-compound parameters and broke down supplements are not altogether diverse (p>0.05). Centralization of overwhelming metals, for example, Chromium, Nickel, Zinc and Iron demonstrated noteworthy distinction (p<0.05). It was thusly presumed that the levels of sawmill waste contamination from Okobaba has brought about a contrarily noteworthy effect on the water body.
<Keywords: Aquatic pollutants, Wood pollutants; Biodiversity loss, Sustainability, Contamination, Environmental degradation
Aquatic ecosystems have been suffering significant changes due to anthropogenic activities which eventually produce biodiversity losses as a direct consequence. Ajao [1] identified sand mining, sand filling, industrial effluent discharge, oil wastes, domestic waste, sewage discharges among others as human related activities capable and presently destroying the sensitive coastal environment of Nigeria species. According to Ajao [1] pollution is a natural or induced change in the quality of water which renders it unusable or dangerous as regards food, human and animal health, industry, and agriculture, fishing, or leisure pursuits. Basically, pollution is induced by those human activities which cause pollutants to enter natural waters.
Pollution of water ways by organic discharges in Nigeria is perhaps a serious threat posed to the Nigerian inland waters. Sources of pollution of the inland waters of Nigeria are well known. The most notable point source arises from the dumping of untreated or partially treated sewage into the River [2], brewery effluents into the river. discharge of bio-degradable wood wastes from sawmill located along the lagoon [3]. Wood shavings and leachates are sources of inert solids as well as toxic pollutants that directly clog fish sill (FAO 1991) and indirectly reduce light penetration which limits productivity contamination of the aquatic environment, makes aquatic organism vulnerable (FAO 1991), fish immune system in particular are weakened leading to increased incidence of parasites. Manufacturing operations that produces raw wood, such as sawmill, paper mills and furniture manufacturers are the major source of pollution in the Nigeria water ways. Others include Agricultural and domestic wastes which find their ways into the river body [4]. Wood residue can negatively impact the environment, contaminate and destroy fish habitat. Wood residue leachate is produced when water percolates, or flows, through wood residue; storing wood waste in pits where it has contact with groundwater creates another source of leachate (FAO) [5].
Typically, pure wood residue leachate is a black liquid with petroleum like odour that causes foaming water. Wood residue decomposition is a slow process that can result in decades of leachate production. During periods of prolonged substances found naturally in wood, such as resin acids, lignins, terpenes, fatty acids and tannins, dissolved from these concentrations. Wood wastes deposited into or near water course can alter, disrupt or destroy fish habitat. In fact, it can smother spawning ground areas, decreasing fish variety and abundance (FAO, 1991).
The fish and the fisheries of the Lagos lagoon has been documented by authors like Fagade; FAO, Fagade and Olaniyan, Udolisa and Solarin, Solarin, Solarin and Kusemiju, Emmanuel et al. [6-9].
Over the years the Lagos lagoon has continued to be under intensifying pressure from pollution. According to Ajao et al. [10] coastal waters can be contaminated from both natural and anthropogenic sources of pollution. The anthropogenic sources include sand mining, industrial and domestic effluents, logging and timber transportation by water. The anthropogenic pollutants/waste has an attendant effect on the biodiversity of aquatic resources. Reduction in the composition and density of algae, zooplankton, benthic invertebrates, fish and fisheries resources has been linked to the increasing menace over the years of aquatic pollution in the Lagos lagoon, Nigeria [9,11-13].
The importance of fish in the society cannot be over emphasized, hence there is the need to know the influence of sawmill wood wastes on the water quality and distribution of fish.
Water pollution
Contamination is a characteristic or incited change in the nature of water which renders it unusable or perilous as respects sustenance, human and creature wellbeing, industry, and agribusiness, angling, or recreation interests. Essentially, contamination is actuated by those human exercises which cause contaminations to enter characteristic waters. The fundamental driver of water contamination is the release of strong or fluid waste items containing contaminations on to the area surface, or into surface or beachfront waters. Direct release of untreated local squanders, for example, kitchen squanders, excrement and pee into the Lagos tidal pond frameworks undermine the oceanic biological system from numerous points of view. These incorporate bringing about an increment in the microbial load in these water bodies, supplement enhancement, contamination of the dirt and amphibian situations [14] and in addition the accessibility of substratum for bacterial development. It additionally causes a decrease in broke up oxygen level, diminishment in the appropriation and differing qualities of life forms and lessening in straightforwardness because of the vicinity of non-disintegrated solids and eutrophication [15].
The amassings of heavy metals in the water may be raised provincially by releases from numerous mechanical techniques, and in silt they may get to be high. They may be discharged into the water from residue aggravated by digging, or by changes in pH or redox potential. Watchful checking of these metals is clearly crucial in any territory where lethal metal contamination of fish’s is plausibility.
The Lagos Harbor, in the same way as other beachfront Harbors, serves as a seaport, community for recreational cruising and a sink for transfer of local and modern squanders. A scarcity of data exists on the degree of contamination of the harbor and henceforth its general wellbeing ramifications.
The brooks and tidal ponds of south-western Nigeria, aside from their more biological and financial essentialness, serve as sink for the transfer of an expanding exhibit of waste sorts. Sewage, wood waste, refine oil, waste warmth, city and mechanical effluents among others discover their path unabated into prompt seaside waters through courses, for example, tempest water channels, streams, springs and tidal ponds [16].
Ecological unsettling influences from such squanders are known to incite changes to the structure and capacity of natural frameworks. Accordingly, scientists throughout the years have endeavored to judge the degree and seriousness of contamination by dissecting changes in organic frameworks [17].
The amphibian environment has a characteristic capacity for purging toward oneself. Physicochemical and biologic exercises including microbiologic activity achieve the breakdown of little amounts of natural materials. Essentially, debased waterway, for example, a stream can be rinsed through self-purification forms [18]. It is the point at which the kind of waste is non-biodegradable e.g. plastics or the measure of natural data surpasses the conveying limit of the environment that there radiate undesirable conditions that evoke negative effects on the biological system. This is the stage that there is ecological contamination.
Heavy metals pollution: Heavy metals are viewed as an essential wellspring of contamination, not only in light of the fact that they are dangerous at generally low fixation, additionally in light of the fact that they are not subject to corruption thus upon presentation they get to be perpetual expansion into the environment. The uptake and capacity of these metals by oceanic organic entities when presented over and again to sub lethal amassing of these poisons likewise prompts the life form’s tissue convergence of substantial metals to turn into a few requests of size higher than that of control creatures, a sensation known as bioaccumulation. This increment in the tissue’s metal focus happens as a consequence of the net pick up in metal fixation because of the low discharge rates of the metals from the creature’s body and the ensuing impelling of metal detoxification frameworks, for example, the establishment of metal granules and blend of low- :atomic weightprotein e.g. metallothionien [19].
Lead is an environmental poison known to bring about harm to human wellbeing, influencing exceptionally the focal sensory system, conceptive organs, the insusceptible framework and kidney [20]. It is viewed as an aggregate toxic substance to warm blooded animals, symptomatically, intense lead harming generally influences the gastrointestinal tract or the nervous framework and incidentally it influences both, There can be a sweetish metallic taste, blazing in the mouth, serious anorexia, sickness, extreme migraine and spewing. For these and different manifestations, researchers created enthusiasm for concentrating on the danger of the metal. From the point of view of propagation, lead influences both men and ladies. Reported impacts in ladies incorporate fruitlessness, unsuccessful labor, preeclampsia, pregnancy hypertension and unexpected labor [21]. Lead may instigate engraving instrument [22], bringing on relentless changes in uterine estrogen receptors and ovary LH receptors following perinatal introduction.
In Mytilusedulis (a mollusc), examines on the uptake of lead demonstrated that the kidneys contained 50-70% of the aggregate lead and were the tissues which picked up and lost it most promptly [23]). Ireland and Wootton [24] reported that in both Littorina and Thais, the convergence of lead in the shell was far higher than the fixation in the delicate body; they further specified that in both species the lead substance of the shell represented around 80% of the aggregate lead content. While the lead quality reported by [25] was somewhat higher in Patella shell than ody, yet it was impractical for them to know whether it was altogether diverse since no correlation was made in the middle of body and lead values. Lead was likewise found in the tissues of different mammalian species to be moved in the bone [26-30].
Robert et al. [31] on their examination on the intense danger and bioaccumulation of lead and cadmium in sea-going spineless creatures reported that lead was intensely poisonous to amphipods and brought on more noteworthy than 50% mortality at amassings of 136 ugfi or more following 4 days of introduction. They further reported that lead exposures with stoneflies, caddies flies and snails proposed that some oceanic irwertebrates were generally insensitive to this metal, even following 28 days presentation.
Merlini et al. [32] explored the aggregation of lead by an eatable freshwater fish at pH 7.5 and pH 6.0; as per their examination, they discovered that t the lower pH the sunfish focused just about three times more lead than at the higher pH. They additionally inferred that when lead was added to Lake Maggiore water as a sak just 8% stayed in the ionic state and as being what is indicated was grabbed by fish; this they said demonstrated that lead aggregation by fish would rely on upon its chemicophysical state, which thusly, rely on upon the water nature of the oceanic environment.
Mathis and Kevem [33] expressed that the component was very poisonous to oceanic creatures; with fish being the most touchy gathering. They found that the mixture sunfish in Wintergreen Lake had a mean of 0.98 ± 0.110 ppm of lead whilst flesh eating fish from the Illinoids River were reported to have 0.57 ppm (Mathis and Cumming, 1973).
Patrick et al [34] thought about the amassing of lead in tiny fish tests from Ulliswater with focuses in tests from Bassenthwaite Lake and Mockerkin Tarn. They discovered that Melosiraand Asterionella sprouts from ullswater contained up to 772 ugP bg-1 natural dry weight, whilst tests from alternate lakes contained 29-169 ugpbg-1, and that the algal blossoms contributed 1-2 mg Pbm-2 to the silt Fergusson et al. analyzed lead in human hair and discovered the mean lead substance of the head hair of 203 nationals of Christchurch, New Zealand to be 10.4ugg-1 while the mean level for i 6 workers of a neighborhood battery plant was 363uge and that of 65 individuals from representatives families was 67 ugg-1. They further reported that the high mean for the relatives (both grown-ups and kids) was essentially diverse (P<0.001) from the mean for the city study, which uncovered an issue emerging from workers exchanging lead tidy home, likely in their working garments.
Simpson and Hunt [35] indicated lead harming because of the ingestion of lead angling shot as the reason for death of various quiet swans (Cygnus olorgmelin). They reported that the territory in which they were bolstering was vigorously debased with angling shot. They further reported that the kidneys of the dead flying creatures contained from 350 to 6650 ugg-1 DM of lead and blood lead levels in the rest of the winged creature were significantly lifted, ascending to 3290 ug/10 Ornl.
As per Joosse et al. [36] high lead fixations in the sustenance of litter-staying Collembola showed up not to have any hurtful impacts, and that the vast majority of the lead stayed unabsorbed and was moved in the defecation, while of the retained lead 30% was put away in intestinal epithelium cells and discharged by occasional remodel of the intestinal epithelium, which happened at every shedding. Nuclear retention spectrophotometric examination was directed by Braharn on fissues tests taken from 19 organs in the California sealion, Zalophuscalifornianus to focus the conveyance and amassing of the overwhelming lead metal. He discovered that lead was gathered in altogether higher focuses in hard tissues, bone and teeth, than in delicate tissue, for example, fat and muscle.
EIA on the Lagos Lagoon: In a community oriented study with Oyenekan and Nwankwo, the effects of natural toxins in the Lagos tidal pond on the green growth, microscopic organisms and benthic spineless creatures were researched [11]. Sawdust brought about silting and oxygen lacking water. Natural matter substance of the tidal pond had a direct connection with the coliform and heterotrophic bacterial populaces yet was contrarily connected with benthic fauna. Organic toxins in Lagos tidal pond forced turbidity that diminished profundity of light entrance with resulting decline in photosynthetic exercises including disintegrated oxygen focus. In this manner, contamination has negative effects on essential and auxiliary generation of the oceanic environment.
Wood processing industry in Nigeria: the sawmill
A sawmill can be characterized as a wood handling industry outfitted with different wood preparing machines. These machines incorporate band saw and round saws. In sawmill industry, the wood must be changed over into different sizes that will boost benefit furthermore fulfill the interest of the individuals.
The sawmill business remains the foundation of furniture industrial facilities which has supported numerous Nigerian families and has given venture opportunities on account of its high productivity because of the plenitude of its crude materials. This has prompted the change of the financial improvement and strengthening of its laborers [37,38].
Sawmilling industry started in Nigeria with the foundation of the first pit-sawing office in 1782 [39]. From that point forward, more sawmills have been made as the interest for timber (sawn wood) keeps on expanding. As at 1991, there were around one thousand six hundred and seventeen (1617) sawmills in Nigeria, larger part of which are situated in the backwoods rich ranges including the Western piece of the nation [40]. Sawmill industry in Nigeria is to a great extent described by little size administrators who more often than not handle timber with the CD arrangement of sawing machine (i.e. CD4, CD5, CD10, machines and so on). Expansive amounts of waste in type of pieces, sawdust and bark are frequently produced in sawmills.
Lumber recuperation component has been characterized as the rate or a degree of volume of sawn wood yield to that of the volume info of logs handled in the sawmill, paying little heed to the sorts and sort of handling hardware embraced and types of wood included [41]. It is a measure of sawmill effectiveness. Lumber recuperation component is a sign of the effectiveness with which sawmill is being run.
Lumber recovery is determined by many factors. These factors include:
1. Log diameter, length, taper and quality.
2. Kerf width of the sawing machines.
3. Sawing variation, rough green-lumber size, and size of dry dressed lumber.
4. Product mix.
5. Decision making by sawmill personnel.
6. Condition and maintenance of sawmill equipment.
7. Sawing method.
Wood waste produced in sawmills can’t however be totally disposed of yet can be fundamentally decreased by enhancing the preparing systems in the sawmill [41]. Various variables have been recognized as the component impacting the wood recuperation degree from logs amid transformation in sawmill. These incorporate log shape (slanted, decrease, forked and straight), log sizes (circumference and length), machine upkeep society and accessibility of machine parts and experience of the administrator. Badejo is of the assessment that, the sizes of log being transformed, paying little mind to the species, have imperative impact on timber recuperation.
Larger part of the saw plant commercial enterprises is situated in the wood delivering precipitation woods zones of the Country in which the western states are among. The biggest centralization of sawmills are in Lagos, Ekiti, Osun Cross River, Ondo, Oyo, Imo, Edo, Delta and Ogun States. Together, they represented more than 90% of the saw processing exercises in the Country. This showed that ensured log supply is a central point in the area of sawmills in the Country.
Dangers/hazards in the sawmill business incorporate; ecological risks as an aftereffect of poor ranger service practices and administration, poor strong waste administration and dangerous emanations to air, clamor, perils because of hardware utilization, and ergonomic dangers coming about because of lifting of overwhelming burdens, coming to for articles, dull work, and poor work carriage [42]. Specialists are likewise inclined to harm when expelling scrap or completed pieces from the Tables 1 and 2, Kickback wounds as a consequence of off base sharpened steel tallness and so forth. As a consequence of the abnormal state of human (manual taking care of) associations in sawmilling operations, laborers are presented to more elevated amounts of dangers connected with log taking care of and machine operation, ecological risks, work related body wounds, and in amazing cases, passing.
| Stations | Description of study area | Co-ordinates | |
|---|---|---|---|
| Longitude | Latitude | ||
| 1 | Okobaba Water Shore (Nearest to the Sawmill) | 30 231 34.2011E | 60 291 25.5711N |
| 2 | Way to Iddo, Right of Okobaba Sawmill | 30 231 36.8011E | 60 291 21.6911N |
| 3 | Between Makoko and Okobaba | 30 231 40.6011E | 60 291 42.6611N |
| 4 | Close to Makoko | 30 231 50.1111E | 60 291 47.0511N |
| 5 | Beyond Third Mainland Bridge | 30 240 00.6911E | 60 291 21.6311N |
Table 1: Description of co-ordinates of sampling areas.
| Month | Values (mm) |
|---|---|
| MARCH | 28.7 |
| APRIL | 161.0 |
| MAY | 221.1 |
| JUNE | 476.7 |
| JULY | 250.2 |
| AUGUST | 10.4 |
| TOTAL | 1148.1 |
Table 2: Record of rainfall measured at 06 Gmt in Lagos State.
Human components, which follows up on the working limit and the day by day generation productivity, incorporate the individual qualities, for example, sex, age, body-size, physical wellness, dietary and condition of wellbeing [43]. It has likewise been watched that mental, social, monetary, innovative and authoritative elements additionally follow up on man’s working limit and generation.
`Okobaba sawmill: The Okobaba Sawmill Industry is situated at Ebute Meta, in the Mainland of Lagos State of Nigeria, near to the Third Mainland Bridge that unites the Mainland to the Lagos Island. The sawmill sources its wood from the different woods in and around Lagos and are transported by rafting through the Lagos tidal pond to the sawmill for preparing to sawn wood, plywood and so forth. Monstrous measure of sawdust are created as an aftereffect of the sawmill exercises and to lessen the pile of this sawdust, the mill operators resort to consistent blazing which causes awesome situations effect on the occupants inside its region. These incorporate contamination by particulate matter from sawdust, thick overwhelming smoke from blazing of the sawdust, the foul scent from logs of wood submerged in the tidal pond anticipating transforming, wood shaving that finds some way or another to the adjacent water body and so on (Figures 1-3).
Investigations on sawdust pollution of Lagos lagoon (Okobaba area): As indicated by akpata, At EbuteMetta, Okobaba, there are sawmills situated on the bank of the tidal pond. Logs of timber are drifted on the tidal pond at this station. Sawdust created from processing the logs is stored adjacent to the tidal pond. The stores were washed by water amid wave activity and ebb and flow into the tidal pond. At this station, the event and periodicity of organisms in Lagos tidal pond were contemplated with a perspective to comprehension the environment of parasites and their parts in the biodegradation of the plant material dirtying the tidal pond. Interestingly, the organic poison at Iddo station was of creature inception and our examinations were mostly on the bacteriological status of the tidal pond (Table 3).
| Parameters | Station 1 | Station 2 | Station 3 | Station 4 | Station 5 | F.values | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | Mean ± SD | Min | Max | 1 | ||||
| Air Temperature | 26.50 ± 1.378 | 25 | 29 | 26.33 ± 2.422 | 24 | 30 | 26.75 ± 1.332 | 24.5 | 28 | 26.667 ± 2.5033 | 24 | 30 | 26.4167 ± 1.25 | 24 | 29 | 0.049 |
| Water Temperature | 28.00 ± 1.897 | 26 | 30 | 28.1667 ± 2.228 | 25 | 30 | 29.0833 ± 2.2003 | 25 | 32 | 29.500 ± 2.8167 | 26 | 34 | 28.5833 ± 1.855 | 26 | 31 | 0.476 |
| Conductivity | 1.006 ± 0.0031 | 1.002 | 1.01 | 1.0065 ± 0.0028 | 1 | 1.01 | 1.0058 ± 0.0042 | 1 | 1.01 | 1.0142 ± 0.0177 | 1.003 | 1.05 | 1.00767 ± 0.00216 | 1.01 | 1.01 | 1.023 |
| Transparency | 0.211 ± 0.083 | 0.08 | 0.28 | 0.345 ± 0.108 | 0.24 | 0.55 | 0.395 ± 0.20724 | 0.2 | 0.6 | 0.475 ± 0.2812 | 0.15 | 0.83 | 0.682 ± 0.335 | 0.28 | 1.01 | 3.594 |
| Depth | 0.667 ± 0.216 | 0.64 | 0.69 | 1.038 ± 0.32 | 0.78 | 1.6 | 1.2367 ± 0.1161 | 1.02 | 1.34 | 1.382 ± 0.286 | 1.06 | 1.88 | 1.8217 ± 0.2573 | 1.43 | 2.15 | 20.508 |
| pH | 7.51 ± 0.23 | 7.25 | 7.9 | 7.43 ± 0.243 | 7.13 | 7.71 | 7.272 ± 0.395 | 6.78 | 7.82 | 7.338 ± 0.1639 | 7.11 | 7.61 | 7.358 ± 0.2822 | 7.01 | 7.74 | 0.668 |
| Total Solid | 52225.667 ± 0.00018 | 17250 | 69800 | 41152.33 ± 0.00037 | #### | #### | 32607.167 ± 83555.5 | 18960 | 41185 | 31241 ± 0.000155 | 7040 | #### | 11725 ± 4798.28 | 7521 | 20840 | 7.695 |
| Suspended Solid | 9232.0 ± 1658 | 7010 | 12104 | 5631.5 ± 821.9 | 4236 | 6580 | 7829.3 ± 133.6 | 6540 | 10315 | 6746.5 ± 2001.92 | 3800 | 9141 | 4835.3 ± 2074.7 | 2106 | 8460 | 6.757 |
| Chloride | 14279.16 ± 2490.19 | 10524 | 17510 | 13342.83 ± 2250.8 | 9876 | #### | 11935.67 ± 1964.34 | 9786 | 15211 | 13001.67 ± 3403.28 | 9042 | #### | 8826.67 ± 2200.3 | 5263 | 11450 | 4.206 |
| Salinity | 18.193 ± 2.734 | 15.3 | 22.8 | 17.095 ± 2.7446 | 14.2 | 21.8 | 15.65 ± 4.8656 | 10.6 | 22.08 | 16.628 ± 3.083 | 12.5 | 19.8 | 11.928 ± 5.303 | 5.7 | 20707 | 2.266 |
| Alkalinity | 64.55 ± 23.04 | 43.2 | 101.4 | 49.98 ± 13.58 | 36.5 | 70.2 | 59.7 ± 15.004 | 38.4 | 84.1 | 58.517 ± 14.402 | 44.8 | 84.4 | 66.95 ± 19.808 | 52.7 | 105.2 | 0.836 |
| NO3 | 0.794 ± 0.71 | 0.3 | 2.201 | 0.571 ± 0.626 | 0.15 | 1.81 | 0.454 ± 0.288 | 0.265 | 1.022 | 0.655 ± 0.43 | 0.133 | 1.32 | 0.684 ± 0.166 | 0.51 | 0.937 | 0.408 |
| SO4 | 9.56 ± 6.19 | 2.038 | 16.7 | 9.35 ± 5.88 | 2.72 | 17.4 | 10.97 ± 7.77 | 1.36 | 18.8 | 8.8 ± 7.11 | 0.697 | 18.7 | 10.42 ± 4.77 | 5.14 | 18.9 | 0.11 |
| PO4 | 0.274 ± 0.357 | 0.017 | 0.86 | 0.138 ± 0.158 | 0.35 | 0.4 | 0.093 ± 0.757 | 0.008 | 0.184 | 0.068 ± 0.105 | 0 | 0.28 | 0.148 ± 0.150 | 0.01 | 0.31 | 0.989 |
| DO | 5.567 ± 1.01 | 4.22 | 6.74 | 5.405 ± 1.19 | 3.84 | 6.54 | 5.22 ± 1.12 | 3.45 | 6.51 | 5.973 ± 0.911 | 4.34 | 7.14 | 5.675 ± 1.050 | 4.53 | 7.42 | 0.43 |
| BOD | 100.21 ± 40.3 | 64.2 | 161 | 104.3 ± 39.65 | 70.6 | 163 | 127.08 ± 63.58 | 86.8 | 247 | 111.73 ± 110.86 | 54.2 | 337 | 137.53 ± 107.09 | 68.2 | 348 | 0.24 |
| Cu | 0.013 ± 0.004 | 0.006 | 0.018 | 0.0115 ± 0.00698 | 0 | 0.02 | 0.0095 ± 0.0061 | 0 | 0.019 | 0.0125 ± 0.063 | 0.05 | 0.02 | 0.0185 ± 0.118 | 0.01 | 0.42 | 1.198 |
| Cr | 0.00267 ± 0.00216 | 0 | 0.006 | 0.001 ± 0.0013 | 0 | 0 | 0.00067 ± 0.0012 | 0 | 0.003 | 0.0067 ± 0.00497 | 0 | 0.01 | 0.00050 ± 0.00084 | 0 | 0.002 | 6.073 |
| Pb | 0.0 ± 0.0 | 0 | 0 | 0.0 ± 0.0 | 0 | 0 | 0.0 ± 0.0 | 0 | 0 | 0.00117 ± 0.002 | 0 | 0.01 | 0.00017 ± 0.000408 | 0 | 0.001 | 1.788 |
| Ni | 0.0048 ± 0.00299 | 0.01 | 0.008 | 0.00167 ± 0.00137 | 0 | 0 | 0.00017s ± 0.000408 | 0 | 0.001 | 0.00033 ± 0.00052 | 0 | 0.01 | 0.00 ± 0.00 | 0 | 0 | 10.969 |
| Zn | 0.568 ± 0.07 | 0.009 | 0.172 | 0.123 ± 0.087 | 0.01 | 0.21 | 0.0513 ± 0.247 | 0.018 | 0.083 | 0.00917 ± 0.0015 | 0.008 | 0.01 | 0.0073 ± 0.007 | 0 | 0.021 | 5.015 |
| Fe | 0.0998 ± 0.246 | 0.072 | 0.13 | 0.1665 ± 0.0903 | 0.05 | 0.31 | 0.2092 ± 0.458 | 0.125 | 0.253 | 0.266 ± 0.0834 | 0.184 | 0.38 | 0.2670 ± 0.1175 | 0.16 | 0.487 | 4.738 |
Table 3: Summary of physico-chemical, heavy metal and dissolved nutrient conditions of water of the stations; mean ± standard deviation, minimum and maximum values and anova f-values.
Microorganisms and growths are significant gatherings of microorganisms in the sea-going environment. Tests of water were gathered aseptically from assigned stations on the tidal pond and investigated for organisms utilizing distinctive society media. Some physicochemical highlights e.g. profundity, turbidity, temperature, pH, aggregate suspended solids, broke up oxygen and saltiness of the tidal pond were measured amid test accumulation to check whether there were connections with the occurrence of parasites. Some growths happened during the time though others showed up regularly [18]. Individuals from the class Mucor happened amid the stormy season or at stations having low saltiness as recorded at mid-tidal pond. The Lagos tidal pond was shallow at the sawdust station; values for turbidity and suspended solids were high though broken down oxygen level were low. Saltiness differed with season, being higher in the dry season, lower amid the downpours and at the mid-tidal pond control station. The ramifications of these outcomes were clarified via completing some research center studies.
Microfungi involved in wood-waste: Waste cellulosic materials which may be delegated rural, mechanical or civil squanders are delivered in gigantic amounts in all aspects of Nigeria. These strong squanders are generally disposed of aimlessly or dumped at different destinations where they are blazed, covered of left to break down in this manner bringing about huge natural contamination with genuine wellbeing outcomes [44,45]. The saw-plants situated at Okobaba, Ebute- Metta, Lagos, on the western piece of Lagos Lagoon introduce a significant wellspring of point contamination. The wood-wastes (sawdust, wood-shavings and leachates) are kept on the shores of the tidal pond from where they inevitably discover some way or another into the waters bringing about substantial natural tainting at the saw-plant site of the tidal pond. This has been found to diminish biodiversity, especially, of diatoms, which could adversely influence the amphibian sustenance web [16].
These waste materials could be transformed from liabilities into resources. Living beings which use cellulosic materials for their carbon and vitality source could be abused for the transformation of these squanders into items that are helpful to man. Case in point, waste cellulosic materials give perfect substrates to the development of mushrooms while the growths can thus debase the stringy squanders and add parasitic protein to the spent substrates [46-48]. Recuperation and re-utilization of waste cellulose has been a subject of far reaching examinations as a method for allaying vitality and sustenance deficiencies [49,50]. Cellulose is a renewable carbon source and records for the aging capability of the Ligno-cellulose materials. It can be corrupted by various microscopic organisms and growths. These living beings can deliver a direction battery of extracellular chemicals fit for corrupting cellulose and in addition some other plant celldivider polymers [51]. Bioconversion, especially, enzymatic hydrolysis of cellulosic materials to helpful items has extraordinary potential. Of expanding hobby is the creation of modern compounds, for example, cellulases, xylanases, glucose oxidases and so on from creatures become on these materials [45].
The accessibility of tremendous measure of cellulosic materials in Nigeria underlines the need to investigate the possibilities of the characteristic decomposers of the plant cell-divider polymers for the change of these squanders into helpful items. Strains of the practical life forms could be utilized for the generation of cellulases and other celldivider hydrolyzing chemicals required for modern saccharification of cellulosic materials (Tables 4 and 5). Despite the fact that microscopic organisms and growths can create cellulolytic proteins, the contagious catalysts are typically favored on the grounds that they are extracellular, versatile and normally emitted in extensive amounts (up to 2% by weight) amid development in submerged clump fluid fermenters. This is in sharp differentiation to numerous bacterial catalysts which exist as tight multi-enyme buildings, frequently layer bound, from which it is hard to recoup singular dynamic protein species [52,53]. Conseqently, our attention is on cellulolytic microfungi. We have evaluated the possibilities of a portion of the microfungi separated from deteriorating wood-squanders from Okobaba saw-factories, EbuteMetta, Lagos for cellulose generation.
| STATION 1 | STATION 2 | STATION 3 | STATION 4 | STATION 5 | F-values | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± | Std. Dvtn | Min | Max | Mean ± | Std. Dvtn | Min | Max | Mean ± | Std. Dvtn | Min | Max | Mean ± | Std. Dvtn | Min | Max | Mean ± | Std. Dvtn | Min | Max | ||
| pH | 6.876667 ± | 0.46457 | 6.01 | 7.23 | 6.918333 ± | 0.59677 | 5.82 | 7.56 | 6.725000 ± | 0.34616 | 6.12 | 7.03 | 6.705000 ± | .3970264 ± | 5.9 | 6.92 | 6.635000 ± | 0.32886 | 6.04 | 6.98 | 0.453 |
| Moisture_Content_(%) | 5.378333 ± | 0.30453 | 4.98 | 5.78 | 4.943333 ± | 0.62905 | 3.96 | 5.52 | 3.170000 ± | 0.49084 | 2.75 | 4.05 | 4.575000 ± | .4582030 ± | 3.97 | 5.12 | 4.078333 ± | 0.13615 | 3.85 | 4.22 | 22.669 |
| Total_Organic_Carbon_(%) | 1.935000 ± | 1.13412 | 1.11 | 4.17 | 1.713333 ± | 1.22443 | 0.76 | 4.11 | .253333 ± | 0.20877 | 0.05 | 0.53 | .891667 ± | .5228161 ± | 0.12 | 1.42 | .916667 ± | 0.5487 | 0.22 | 1.76 | 4.095 |
| Total_Organic_Matter_(%) | 3.501667 ± | 2.33385 | 1.92 | 8.15 | 2.963333 ± | 2.11892 | 1.31 | 7.11 | .438333 ± | 0.36526 | 0.09 | 0.92 | 1.543333 ± | .9046694 ± | 0.21 | 2.46 | 1.590000 ± | 0.94712 | 0.38 | 3.05 | 3.817 |
| NO3_(Mgkg-1) | 1.608333 ± | 0.43356 | 0.92 | 2.17 | 1.208333 ± | 0.51909 | 0.27 | 1.72 | 1.998333 ± | 0.5011 | 1.08 | 2.58 | 2.686667 ± | 1.4389394 ± | 1.04 | 4.16 | 1.706667 ± | 1.66381 | 0.85 | 5.08 | 1.639 |
| PO43-_(Mgkg-1) | 49.258500 ± | 31.0364 | 1.381 | 82.19 | 55.236667 ± | 31.6551 | 3.89 | 86.15 | 22.920167 ± | 14.8124 | 0.201 | 38.19 | 35.103667 ± | 20.7197191 ± | 0.022 | 62.14 | 29.934000 ± | 16.9566 | 0.084 | 46.12 | 1.87 |
| Cu_(Mg_Kg-1_) | 1.070333 ± | 0.52183 | 0.053 | 1.522 | 1.127833 ± | 0.64387 | 0.098 | 2.131 | .706833 ± | 0.28085 | 0.412 | 1.12 | .751833 ± | .2559198 ± | 0.432 | 1.124 | .632000 ± | 0.57833 | 0.23 | 1.768 | 1.307 |
| Cr_(Mg_Kg-1_) | 0.013333 ± | 0.01953 | 0 | 0.052 | .320500 ± | 0.17818 | 0.035 | 0.511 | .321017 ± | 0.18219 | 0.0211 | 0.502 | .005833 ± | .0124325 ± | 0 | 0.031 | .091833 ± | 0.21959 | 0 | 0.54 | 6.673 |
| Pb_(Mg_Kg-1_) | 0.325000 ± | 0.14035 | 0.084 | 0.447 | .030333 ± | 0.01917 | 0 | 0.058 | .004833 ± | 0.00854 | 0 | 0.021 | .031833 ± | .0282022 ± | 0 | 0.062 | .071167 ± | 0.11182 | 0 | 0.295 | 15.643 |
| Ni_(Mg_Kg-1_) | 0.001167 ± | 0.00183 | 0 | 0.004 | .362833 ± | 0.09212 | 0.282 | 0.536 | .417833 ± | 0.15183 | 0.126 | 0.536 | .050333 ± | .1218272 ± | 0 | 0.299 | .195167 ± | 0.14451 | 0 | 0.393 | 15.139 |
| Zn_(Mg_Kg-1_) | 0.709000 ± | 0.37896 | 0.324 | 1.21 | 1.174500 ± | 0.4791 | 0.611 | 2.003 | .891833 ± | 0.2543 | 0.512 | 1.254 | .286000 ± | .2477192 ± | 0.086 | 0.768 | .502000 ± | 0.80555 | 0.092 | 2.14 | 3.082 |
Table 4: Summary of sediment conditions of the stations; mean ± standard deviation, minimum and maximum values and anova f-values.
| AIR TEMPERATURE | WATER TEMPERATURE | CONDUCTIVITY | TRANSPARENCY | DEPTH | pH | TOTAL SOLID | SUSPENDED SOLID | CHLORIDE | SALINITY | ALKALINITY | NO3 | SO4 | PO4 | DO | BOD | Cu | Cr | Pb | Ni | Zn | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AIR TEMPERATURE | 1 | |||||||||||||||||||||
| WATER TEMPERATURE | .452* | 1 | ||||||||||||||||||||
| CONDUCTIVITY | .406* | .268 | 1 | |||||||||||||||||||
| TRANSPARENCY | .416* | .500** | .348 | 1 | ||||||||||||||||||
| DEPTH | -.273 | -.115 | .098 | .355 | 1 | |||||||||||||||||
| pH | .391* | -.028 | .178 | .086 | -.291 | 1 | ||||||||||||||||
| TOTAL SOLID | -.286 | -.204 | -.243 | -.552** | -.645** | -.069 | 1 | |||||||||||||||
| SUSPENDED SOLID | -.101 | -.192 | -.012 | -.396* | -.423* | .217 | .558** | 1 | ||||||||||||||
| CHLORIDE | -.064 | -.151 | -.147 | -.409* | -.601** | .097 | .825** | .441* | 1 | |||||||||||||
| SALINITY | .513** | .227 | .203 | .072 | -.581** | .450* | .236 | .296 | .501** | 1 | ||||||||||||
| ALKALINITY | .565** | .441* | .090 | .411* | -.075 | .146 | -.493** | -.212 | -.323 | .387* | 1 | |||||||||||
| NO3 | .669** | .149 | .207 | .144 | -.146 | .400* | -.384* | -.131 | -.196 | .277 | .567** | 1 | ||||||||||
| SO4 | .716** | .558** | .384* | .553** | -.175 | .349 | -.362* | -.125 | -.197 | .562** | .626** | .504** | 1 | |||||||||
| PO4 | .122 | -.109 | -.128 | -.021 | -.214 | .051 | -.063 | -.088 | -.110 | -.022 | .238 | .272 | -.024 | 1 | ||||||||
| DO | -.665** | -.392* | -.110 | -.387* | .242 | -.343 | .350 | .108 | .263 | -.557** | -.692** | -.409* | -7.370E-1 | -.224 | 1 | |||||||
| BOD | .454* | .334 | -.007 | .516** | .020 | .061 | -.469** | -.258 | -.270 | .446* | .776** | .287 | 5.245E-1 | .202 | -.725** | 1 | ||||||
| Cu | .523** | .481** | .211 | .592** | -.003 | .149 | -.365* | -.326 | -.168 | .370* | .633** | .252 | 4.701E-1 | .010 | -.412* | .626** | 1 | |||||
| Cr | .311 | .216 | .367* | .006 | -.195 | .022 | .206 | .065 | .327 | .311 | .034 | .226 | .085 | -.276 | .138 | -.170 | .084 | 1 | ||||
| Pb | .368* | .159 | -.002 | .102 | -.045 | -.072 | .118 | -.137 | .345 | .160 | .054 | .297 | -.006 | -.113 | .116 | -.023 | .122 | 6.565E-1 | 1 | |||
| Ni | .255 | .035 | -.044 | -.307 | -.692** | .439* | .324 | .247 | .267 | .483** | .374* | .420* | .313 | .176 | -.304 | .064 | .108 | .153 | -.094 | 1 | ||
| Zn | .410* | .170 | -.022 | -.132 | -.443* | .318 | .039 | -.025 | .043 | .415* | .265 | .294 | .369* | .158 | -.561** | .170 | .052 | -.111 | -.171 | .485** | 1 | |
| Fe | .555** | .421* | .420* | .752** | .397* | .034 | -.672** | -.348 | -.400* | .171 | .465** | .247 | .456* | .016 | -.435* | .634** | .582** | .141 | .156 | -.364* | -.039 | 1 |
Table 5: Correlation matrics for physico-chemical parameters, heavy metals and dissolved nutrients of okobaba waters (March- August 2012).
Effects of sawdust on germination of fungal spores in Lagos lagoon: The impacts of wood concentrates on parasitic spore germination and germ tube lengths were measured. Data was gotten on the sorts of wood species transported on the tidal pond. Fluid concentrates were arranged of sawdust of wood species specifically; Khaya ivorensis (iroko), Mitragyna ciliate (abura) and Triplochiton scleroxylon (obeche). Research center investigations were planned such that spores of distinctive organisms were hatched in wood extricates. Aliquots of the way of life were analyzed with the magnifying instrument and quantitative information was acquired on number of spores sprouted. Additionally, lengths of germ tubes were measured with a balanced eyepiece micrometer embedded in a magnifying instrument. Length of germ tube and number of spores sprouted expanded essentially (p>0.05) for Aspergillus flavus in the concentrate of K. ivorensis. This added to the number of inhabitants in the parasite in the amphibian biological system.
Microscopic examination of the architecture of conidiophores of Aspergillus giganteus” One of the growths disengaged from the sawdust contamination site was Aspergillus giganteus that has exceptionally tall and thin conidiophores. The interior structure of the conidiophores was inspected minutely utilizing birefringence and dichroism. The learning could be helpful to or affect decidedly on the building design and designing configuration of thin and tall structures. A piece of this work was done in the research facility of Graham Gooday at the Department of Biochemistry and Molecular Biology, Marischal College, Aberdeen, UK.
Studies on bioconversion and degradation of waste materials in coastal environments: The capacity of organisms to corrupt cellulose, a real segment of wood was tried in lab tests. The discoveries explained the parts of growths in the tidal pond biological community. Case in point, some growths took an interest in the disintegration of wood squanders in Lagos tidal pond. An unidentified basidiomycotina developed and delivered fruiting body on sawdust. Additionally, Aspergillus species and Trichoderma aureoviride breakdown channel paper cellulose in research center examinations. Growths consequently partake in the biogeochemical cycling of substances in nature. Cellulose debasing growths created catalyst cellulases that may be helpful in different commercial ventures. Another biodegradation study was completed utilizing chitin, part of the shell of shrimp, squid and different creatures, a copious waste in the oceanic environment.
Dissimilar to cellulose which is an aliphatic polymer of glucose units, chitin is a fragrant polymer of N-acetylglucosamine units and the vicinity of a benzene ring makes the atom more steady and hard to assault by microorganisms. Bioconversion of chitin by some estuarine microscopic organisms was researched with a perspective to discovering a characteristic generation of chitosan that is of interest in pharmaceutical and different businesses [11]. Brutal concoction systems are utilized as a part of the generation of business chitosan.
Project objective
This project study is a product of a six months ecological study of the physical and chemical characteristics and heavy metal of Okobaba area of Lagos lagoon. The objective of the project work includes:
1. To determine the physic-chemical parameters of the okobaba waters and also to know how the values of this parameters have deviated from the normal parameters of the Lagos lagoon.
2. To examine the heavy metal contents in the sediment and also in the water body of the area.
3. To attempt a general environmental assessment on the study stations based on the pollution activities around the area.
Description of the study area
Tidal ponds are unmistakable hydrological highlights along the West African Coast [54]. They are naturally and monetarily imperative amphibian environments in South-western Nigeria. Also, they are vital for sustenance particularly angle, in water transportation, vitality era, abuse and investigation of some mineral assets including sand [16,55,56].
Lagos tidal pond has bolstered many years of little scale fisheries which have hinted at over-abuse. The angling apparatuses and artworks utilized incorporate gillnet, stownets traps, liftnets, longline, wicker container traps and so on [6,8,9,13].
Lagos lagoon: The Lagos tidal pond is situated in Lagos State, Nigeria and is one of the nine tidal ponds in South-western Nigeria [57,13]. The tidal pond complex extends from Cotonou in republic of Benin to the edges of the Niger delta in Nigeria along its 750 km course [57]. The tidal pond is bolstered by different waterways, for example, Lekki, Marjidun, Ogun and Epe Lagoons. The Lagos tidal pond purges into the Atlantic Ocean by means of a harbor. The southern edge of the Lagos tidal pond is limited by five Cowrie River. The eastern edge by the palaver island and the northern outskirt by Ikorodu. It has a profundity running from 1.5-3.0 m and made up of sloppy and sandy base. The focal assemblage of the tidal pond is found between 30231 and 3041 and latitude 60221 and 60281. The tidal pond is an open, shallow and tidal pond, with a surface region of 208 km2 (FAO, 1969). It gives the main opening to the ocean for the nine tidal ponds of South Western Nigeria. Owing to the flow of waterway inflow and seawater invasion, the Lagos tidal pond encounters bitter condition that is more discernable in the dry season. In the wet season, the expanded stream inflow makes freshwater and low bitter conditions in different parts of the tidal pond. The harmattan, a short season of dry, dusty North-East Trade winds are experienced now and again in the middle of November and January in the area decreasing perceive ability and bringing down temperatures [58]. The Lagos Lagoon is the biggest of the four tidal pond frameworks of the bay of guinea [57] (Figures 4 and 5).
Study sites
Five stations were selected and sampled from the 31st of March to the 25th of august, 2012. The stations were marked with the aid of global positioning system (GPS) and recorded as co-ordinates of the stations.
The stations are:
Station 1: Okobaba water shore (nearest to the sawmill)
Station 2: way to iddo, right of Okobaba sawmill
Station 3: between Makoko and Okobaba
Station 4: close to Makoko
Station 5: beyond third Mainland Bridge
Station 1: Infront of Okobaba sawmill: The location is on longitude 30 231 34.2011E and latitude 6° 291 25.5711N. It is close to the water bank were wood wastes are deposited. The color is silver black, depth is 0.67 m and transparency is 0.08 m.floating vegetation is water hyacinth. Settlement is concentrated around the water bank and the major occupation is transportation of people and goods from the bank to other parts of the lagon sediment type is clayish (dark) in texture.
Station 2: Between Okobaba and Iddo: The station is located on longitude 3°231 36.8011E and latitude 6° 291 21.6911N. It is to the right of Okobaba sawmill. The depth of the water body is 1.61 m and transparency is 0.48 m, colour of water is clear greenish yellow, water hyacinth is the only floating vegetation. There is no settlement in the area and the major activity is the transportation of logs of wood across the water body. Soil type is silty.
Station 3: Between Makoko and Okobaba: It is located on longitude 3° 231 40.6011E and latitude 6° 291 42.6611N. The depth of the water body is brown. The major occupation of people in that area is fishing and sand mining, there is also a clustered settlement pattern on the water body. The soil type is sandy/silt.
Station 4: Close to Makoko (left of Okobaba): It is located on longitude 3° 231 50.1111E and latitude 6° 291 47.0511N. The depth of water is about 1.43 m, transparency is 0.19 m and the colour of water is brown. Settlement is dispersed and scanty in the area. Water hyacinth is the major floating vegetation and the soil type is sandy.
Station 5: Beyond third Mainland bridge in Okobaba (control): The lagoon is part of the Lagos lagoon (in the Okobaba axis), located vertically away from the sawmill in Okobaba (on longitude 3° 241 00.6911E and latitude 6° 2911 21.6311N). The location is in the open lagoon and has such has no settlement and the only activity carried out in the area is fishing. Floating vegetations are rare to find, water is transparent brown (transparency is 0.3), dept is about 2.15 m. Sediment type is sandy.
Sampling operation
Five stations were selected and sampled monthly between March and August, 2012. The stations were marked with the aid of global positioning system (GPS) and the readings on the GPS were recorded as coordinates of the sampling stations. Samples were collected every last Friday of the month between 7.30 am and 10.00 am on sample collection days. Sampling operations were carried out on board (using an engine boat) (Figures 6 and 7).
Collection of samples and measurement of physico-chemical parameters and heavy metals
Collection of water sample: Gathering of water tests was completed first in all the examining stations. Water test were gathered for examination by plunging 1L plastic compartments beneath the water surface at the diverse examining stations. The diverse plastic jugs were named fittingly with the dates of when the specimens were gathered and the name of the station where the examples were gathered from. Water tests were put away in the cooler for time of 2–3 days after which they were taken to the research center for examination and qualities for physico-concoction parameters and heavy metals were gotten.
Collection of sediment and benthic organisms: Benthic examples were taken at each of the assigned stations with the support of Van Veen get. 2-3 snatch pulls were taken at every station relying upon the amount of every pull acquired. The silt was sieved through a 0.5mm cross section strainer. The held materials were saved in 4% formalin and kept in marked holders which were taken to the research facility for sorting and ID. A bit of dregs was additionally gathered in a marked polythene pack for protection in the profound cooler for 2-3 days prior to they were taken to the research facility for the examination of substantial metal substance in them (Figure 8).
Sediment analysis: Five sampling stations were selected and identified (as station 1,2,3,4 and 5) based on sediment type. Samples of sediments were collected monthly with a Van-Veen grab. Some portions of the sediments were later sun dried before they were taken to the laboratory for physico-chemical parameters and heavy metal analysis.
Fauna analysis (benthic organisms): Preserved animal samples collected in the field were washed with tap water through a 0.5 mm sieve to remove preservative and any remaining sediment. The animals were sorted and classified into taxonomic groups (Phyla, class, species) where close members of a family exists, the specimen were examined using a hand lens and a low powered microscope. The number of identified organisms were counted and recorded.
Measurement of some physico-chemical parameters
Colour: The color of the sampling stations were determined visually and recorded.
Air and water temperature (°C): The water temperature influences numerous advantageous uses including modern, household water supplies and amusement. The impacts of temperature on amphibian life are of most concern, nonetheless, the water quality criteria were produced to shield the touchiest oceanic living being from anxiety connected with lifted temperatures. Air temperatures were taken by suspending a mercury-in-glass thermometer noticeable all around for around 2-4 min and the readings were recorded, water temperatures of the different stations were likewise taken by plunging the mercuryin- glass thermometer at ever station inspected. The thermometer was permitted to stay in the water for around 3-6 min preceding taking the readings. The qualities were recorded likewise for every station inspected all through the time of testing (Figures 9 and 10).
pH (hydrogen ion concentration): This is a measure of the hydrogen particle movement in a water test. The pH of common waters is a measure of the corrosive base harmony attained to by the different broke down mixes, salts and gasses. The vital synthetic framework controlling pH in characteristic waters is the carbonate framework. The Hanna’s instrument was utilized to focus the pH by dunking the test into 1 liter of water test and perusing off the qualities from the meter.
Salinity (%): The salinity of the water samples was measured by using a refractometer. This was done by putting drops of water at the transparent end of the refractometer and readings on the instrument were recorded for the five sampling stations.
Conductivity (mScm-1): This is simply defined as the amount of ions present in water. The conductivity of the water was determined by using the refractometer. The left side of the refractometer measures the conductivity. Drops of water were placed on the refractometer and the corresponding values on the instrument were recorded.
Total dissolved solids (TDS) (mg/l): The total dissolved solid (TDS) was determined by using the Cole Parmer TDS meter (wide range). The meter was calibrated using calibration standards which were obtained from commercially prepared solution. The probe was immersed into the water sample and the reading noted. Whatman GF/C grade glass fiber disc was used in the determination of the total dissolved solids. The disc was washed with 20 ml of distilled water. A clean evaporating dish was heated at 105°C in an oven for hour. The heated evaporating dish was reweighed and the difference in weight represented the TDS (Figure 11).
Nitrates (mg/l): The determination of nitrate content was carried out using the method outline in APHA (1995). 1 ml of freshly prepared 0.5% sodium salicylate was mixed with 20 ml of the samples. The solution was then evaporated on a water bath and allowed to cool. 2 ml of sulphuric acid was added. 25 ml of water was used to wash the solution into a calorimeter cylinder or cuvette and 7 ml of alkali reagent was added. After 10 minutes reaction time, the solution was made up to the 50 ml mark with distilled water. Finally an yellow colour emerged and was matched with prepared standards while the absorbance was read from a spectrophotometer at the wavelength of 500 nm.
Phosphates (mg/l): The investigation of the phosphate content in the specimens gathered was completed utilizing the Vanadomolybdate phosphoric corrosive colorimetric system. 10 ml of Vandatemolybdate reagent was added to 35 ml of tests and weakened to 50 ml with refined water. Ten minutes response time was permitted after which another specimen containing 35 ml of refined water was arranged, Vandatemolybdate reagents, the absorbance of the water test and the clear was measured at a wavelength of 470 nm. The absorbance estimation of the example was changed over into equal phosphate as parts every million (ppm) from the standard adjustment bend.
Dissolved oxygen (mg/l): This is the measure of oxygen disintegrated in water that is accessible for natural exercises by seagoing organic entities. Broken up oxygen was resolved synthetically by utilizing the Winkler’s strategy. Water test to be broke down was gathered utilizing 25 ml glass plug bottles. 2 ml of Manganese sulfate arrangement (MnSO4) was included utilizing pipette. The plug was supplanted and the segments blended. 2 ml of tetraoxosulphate (VI) corrosive was added to break up the hasten framed and the jug was shaken tenderly. At that point 100 ml of the subsequent arrangement was moved into a funnel shaped carafe and titrated against 0.025N Sodium Thiosulphate Solution utilizing 2 ml starch arrangement as a marker. A solid blue vanishing denoted the end purpose of the response. The aggregate sum of sodium thiosulphate (in ml) utilized then substituted into the capacity underneath to get the disintegrated oxygen.
DO=K.200 Volume of thiosuphate used/Volume of sample titrated
Where K=constant to correct for added reagents: 2 ml MnSO4, 2 ml alkaline iodide and 2 ml tetraoxosulphate VI acid.
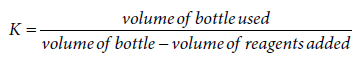
Total organic content : The total organic content of the sediment sample collected were stored in the freezer and later brought out to thaw before analysis. After thawing, the samples were air dried until a constant weight was obtained. The samples were put in crucibles and weighed up to 50g. Then the sample in each crucible was heated in a muffle furnace for 8 hours after which each crucible was brought out, left to cool and re-weighed. The loss in weight after heating in the muffle furnace was calculated as loss on ignition using the formula:
Loss on ignition=Loss of weight on ignition/Initial weight before ignition × 100
Acidity: The acidity of the water was determined by titration method 2310B (APHA 1998). 10ml of the sample was measured into a flask and 2 drops of phenolphthalein (indicator) was added. This was titrated to end points of pH acidity 3.7 (m-acidity) and pH 8.3 acidity using NaOH, 0.02M titrant against KH C8H4O4. The titre obtained is the standard and point for titration of total acidity which can be calculated as follows:
Acidity, as mg CaCO3/L=Titre (ml) × molarity × 50,000/Aliquot titrated (ml)
Turbidity (NTU): Turbidity was measured with the use of the turbidimeter, or nephelometer, which determines turbidity by the light scattered at an angle of 90° from the incident beam a 90E detection angle is considered to be the least sensitive to variabtions in particle size. Bephelometry has been adopted by Standard Methods as the preferred means for measuring turbidity because of the method’s sensitivity, precision, and applicability over wide range of particle size and concentration. The nephelometric method is calibrated using suspensions of formalin polymer. The preferred expression of turbidity is NTU (Figure 12).
Laboratory procedures for heavy metals
Analysis of heavy metals: The hurl metals researched in this work were dead set at the Chemistry Laboratory of the University of Lagos utilizing AA 689 pie unican 2003 nuclear ingestion spectrophotometer (AAS). The examination of substantial metals was completed in residue tests and water tests. The specimens were broke down for Silver (Ag), Copper (Cu), Cadmium (Cd), Chromium (Cr), Iron (Fe), Manganese (Mn), Nickel (Ni), Lead (Pb), Zinc (Zn). The specimens were first made to experience corrosive assimilation. An aliquot of the filtrate was taken and the absorbance was resolved at their attributes wavelength utilizing the AAS. More than one recreates into fire, heater and vapor were resolved for every example and the outcome reported as mean qualities. The mean fixation in parts every million (ppm) for the metals in the examples were then decided (Figure 13).
Concentration of samples (ppm)=absorbance concentration/ absorbance of standard
Note: the calibration standard of each metal was used to standardize the solution.
Digestion of sediment samples for heavy metal analysis: 20 g of the sediment sample was oven dried at 80°C for 3-6 hours. After drying, visible remains of organisms and debris were removed. The dried samples were crushed using a mortar and pestle and sieved through a 200 m sieve to normalize for particle size. From the dried sieved sediment sample, 5 g was placed in a beaker and digested according to the method adopted by Ageiman and Chau, (1976), Bryan and Langston (1992), see appendix. The AAS obeys the law of Beer Lambert which states that the absorbance is directly proportional to the concentration at a particular wavelength.
To prepare a standard solution, the molecular weight of the compound is divided by the molecular weight of the element. This is done by calculating the amount of heavy metals in the sample using the following formula:
X=CV/W (1)
Where X=amount of element in sample
C=Concentration read out from the AAS
W=Weight of sample in grams
V=Volume of solution
OR
X=Molecular weight of the compound/Molecular weight of element
For example: PbNO3/Pb=331.21/207.19=1.6 g
Therefore, 1.6 g in 1 litre of distilled water is called the stock standard or primary standard. To prepare 100 ppm from the stock standard i.e. 1000 ppm in 50 ml, we use the following formula:
M1V1=M2V2 (2)
Where M1=stock standard
V1=volume from stock standard
M2=Concentration you are preparing for
V2=Volume you are preparing for
Therefore V1=M2V2/M1
Where M2=100 ppm
M1=1000 ppm
V1=100 × 50/1000=5 ml
5 ml of stock standard into 100 ml of dilute water=100 ppm
From 100 ppm, you can prepare a lower concentration by using serial dilution formula.
For example:
To prepare 2 ppm from 100 ppm in 50 ml …………… 2 × 50/100=10/10=1 ml
Mathematical models
Statistical analysis: All statistical methods to be used would be adapted from Ogbeibu (2005). Data obtained during the experiment would be subjected to One-way analysis of variance (Anova). Comparisons among treatments means were carried out by using least square difference at a significance level of 0.05. All computations would be performed by the statistical package SPSS 17.0.
The variables
The variables used are either dependent or independent and they include some water quality parameters such as Air Temperature, Water Temperature, Water depth, Transparency, Conductivity, floating vegetation, phytoplankton and zooplankton, Dissolved Oxygen, BOD5, Alkalinity, Phosphate, Nitrate, Salinity, Sulphate, COD and Trace elements i.e. Cr, Pb, Cd, Cu, Fe.
The data obtained from the five stations over the six months period of sampling showed charges in the physic-chemical parameters and heavy metal contents of the different study sites, Bad odour in the area was also observed to be as a result of saw milling activities and transportation of logs of wood. Floating vegetation was observed to vary major with season as the major floating vegetation (water hyacinth) occurs in abundance during the rainy month than the dry months.
A summary of the results of physicochemical and heavy metals obtained is as presented below with the range of each stations across the months and the standard deviations.
Air and water temperature
Air and water temperature raised following almost a similar pattern the stations. Water temperature was observed to be higher than air temperature. The highest air temperature (30°) was recorded in April and May and the lowest (24°) was recorded in June, July and August. The highest of water temperature (34°) was recorded in June (station 4) and the lowest (25°) was recorded in station 2 of the month of August. Further analysis shows that both air and water temperature variations are not F values of Air and water temperature records 0.995 and 0.753 respectively [58].
Conductivity
Conductivity values vary less significantly with no regular pattern. Highest conductivity (1.050 NS/cm) and the lowest (1.000NS/cm) were recorded in April (Station 3 and 4 respectively). F value shows that there is no significant statistical difference in conductivity values (P>0.05) as the recorded value was 0.415.
Transparency
Fluctuations in the transparency level of the sampling site almost followed a decreasing pattern across the month. The highest transparency value (1.01 m) was recorded in the April while the lowest (0.08 m) was recorded in July. Transparency value varied significantly (P<0.05) as F value was recorded as 0.019.
Depth
Depth of the study areas varied significantly (P<0.05) with no regular pattern. The highest depth (2.15 m) was the lowest depth (0.64 m) was recorded in station.
pH
PH values varies with no regular pattern the highest pH values (7.9) was the lowest pH (6.78) was recorded in June (station 3) variation in pH value was not significant as (P>0.05) the F- value was recorded as 0.62.
Total solid
There was fluctuation in the total solid levels across station and months. Total solid level varied significantly (P<0.05). The lowest value of 2106 was recorded at station 1 (August). F-value for suspended solid was recorded as 0.001.
Chloride
Fluctuation in chloride levels varies with no regular pattern. The highest value for chloride was recorded in June (station 5) The F value for chloride (0.010) shows significant difference in fluctuation shows significant (P<0.05).
Salinity
Salinity fluntuation almost follows a decreasing regular pattern across the month. The highest salinity (22.8) was recorded in March (station 1 which is closest to the source of pollution) and the lowest salinity (5.7) was recorded in the month of June at station 5 (control). F value for salinity (0.091) showed that there is no statistical difference in salinity variation (P>0.05).
Alkalinity
Alkalinity values varied with no regular pattern, the highest alkalinity value was recorded in March at station 5 (105.2) while the lowest value (36.5) was recorded in July (station 2). Alkalinity variation was less significant (P>0.05) as the F. value was recorded as 0.516.
Dissolved oxygen (DO)
Dissolved oxygen varied in an increasing pattern across the months. (March-August). The highest oxygen level (7.42) was recorded in July (stations 5) and the lowest (of 3.45) was recorded in march (stations 3). F-value of dissolved oxygen shows that there is no significant difference (F=0.786 P>0.05).
Biological oxygen demand (BOD)
BOD varied with no regular pattern. Highest value of BOD (348) was recorded in March (station 5), the lowest (54.2) was recorded in August (station 4). F-value of BOD (0.913) showed that there was no statistical difference in BOD (P>0.05).
Nitrate (NO3)
NO3 values varied with no regular pattern. The highest nitrate value (2.201) was recorded in April (station 1) while the lowest value (0.133) was recorded in July (station 4). F. values of Nitrate (0.801) shows that Nitrate fluctuation is less significant (P>0.05).
Sulphate (SO4)
Sulphate levels varied with a decreasing pattern across the months. Highest sulphate level was recorded in April at lowest value (0.68) was recorded in July at station 4. F- value of sulphate (0.978) shows that there was no significant difference (P>0.05) in the values of sulphate. There was no regular pattern of variation also in sediment samples.
Phosphate (PO4)
Phosphate levels varied with no regular pattern (Figure 2). The highest value was recorded in July at station 1 (with value of 0.86) while the lowest value of 0 was recorded at the same month (July) at station 4. F-value was recorded as o.432 and this shows that it is less significant (P>0.05). Variation in sediment samples has no regular pattern also.
Heavy Metals
Cupper (Cu)
The level of cupper varied with no regular pattern for water and sediment. Highest value of cupper (0.042) was recorded in the month of March (station 5) while the lowest value (0.0) was obtained in July (station 3). F-value of cupper (0.336) showed that values of cupper is less significant (P>0.05).
Chromium (Cr)
The values of Cr wasn observed to vary across the months with no regular pattern in water and sediment. The highest value (0.012) was recorded in June (station 4) while the lowest values f value for water sample (0.001) was significant (P<0.05).
Lead (Pb)
Values for lead were very low and have no regular pattern of variation in water and sediment. Highest Pb value was recorded as 0.005 in the month of May (station 4) and lowest values (zero) were recorded in several stations across the month. F-values for water sample (0.163) was less significant (P>0.05).
Nickel (Ni)
Values of Nickel showed a decredasing regular pattern across the months (march-August) in water samples. The highest values was recorded in which (station 1) F-value (0.00) shows statistical significance in Nickel concentrations (P<0.005). there was no regular pattern for sediment Nickel.
Zinc (Zn)
There was fluctuation in the level of Zn in water and sediment across the sampling months (Figure 2). The highest value (0.211) was recorded in march (station 2) and lowest value (0.002) was recorded in July (station 5) for waater samples. F-value (0.004) shows statistical significance in water (P<0.05).
Iron (Fe)
There was fluctuation in iron levels. The highest level (0.487) was recorded in March. The lowest (0.052) was recorded in August (station 2) F-value (0.006) shows significant levels of iron in water (P<0.05).
The creeks and lagoons of south western Nigeria, apart from their more ecological and economics significance, serves as sink for disposal of an increasing array of waste types such as wood waste sewages etc [11].
In the case of okobsba (study sites) the major suspected source of pllation is the sawmill, located at the bank of the Lagos lagon at okobaba. The present study (carried out in okobaba area) is discussed in two sections as it relates to the result obtained as follows.
1. Physical and chemical conditions of water and sediment samples obtained from the sample locations.
2. Soluble nutrients and heavy metals of water and sediment samples.
The result obtained from this study showed that there was no significant difference in both air and water temperatures, (P>0.05), this means that the fluctuations in temperature was reduced throughout the period of the sampling (March–August).
Although the fluctuation in both air and water varied with no regular pattern. However pattern of fluctuation in water temperature still follows almost the same pattern with that of air temperature (Welcome, 1979). The range of air and ruster temperatures observed in this study was observed to be similar to those reported for water bodies in south western Nigeria [59,60].
According to Edokpayi and Nkwoji temperature was not considered an important ecological factor in the tropics.
Transparency level followed a decreasing pattern across the stations, ther was a significant difference (P<0.05) in the transparency of the watter at different location and at different month of the year. Being a lotic water body, there is the movement of excessive nutrient and the concentration of pollutant is reduced due to water current.
Increase in transparency also increase primary productivity by making available, volume of water in which phytoplankton communities are photosynthetically efficient (Francisco and Martine1993). Record showed that transparency is lower in station 1 (close to the sawmill) and becomes higher as it more to station 5 (control) Depth also varied significantly (P<0.05).
Conductivity of water is given as the index of total ionic content which therefore indicates freshness of the water body.
Conductivity at sampling station shows no regular pattern across the mouth and values were not statistically significant (P>0.05). Usually water with conductivity of value of below 100 mS/cm are fresh water while those above 40,000 NScm-1 indicate marine waters. Those between these two limits are brackish. The values recorded (range of 1000 to a brackish one and followed that of natural African lagoon).
pH can drastically affect the structure and function of the ecosystem both directly and indirectly, it could lead to increase concentration of heavy metals in water through increase concentration of heavy metals in water through increased leading from sediment [60].
The pH of water was slightly alkalinic almost throughout the study period (6.8-7.9). This pH range was similar to those reported earlier for Lagos lagoon. Chukwu and Nwankwo [16] recorded a very similar pH range (7.2-8.2) at an adjacent water body of the Lagos lagoon. However the pH of sediment samples was slightly acidic as most of the values fall below 7.0.
Total suspended solid is the amount of particulate matter that is in the water column. High levels of suspended solid would result in a reduction in light penetration which in turn will reduce primary productivity and decrease dissolve oxygen in the water body [61]. Suspended solid recorded for this study was high. The organic matter distribution in the Lagos lagoon has been attributed to anthropogenic inputs [62]. There was significant difference in the suspended solid values at study station (P<0.5). Further test (LSD) showed that are close to the Okobaba saw mill (station 1 and 2) (Figures 14 and 15).
The dissolved oxygen (DO) and bio-chemical oxygen demand (BOD) always have an inverse relationship existing between them. It is the dissolved oxygen in water. That is being depleted or utilized by the micro-organisms and hence the elevated value biochemical oxygen demand values. It means that for a high Do there is a less BOD and vice versa. This is the case in this study. Both dissolved oxygen and the biochemical oxygen demand (Do and BOD) showed no significant difference of the study station.
The low Do observed in the study stations (especially in station 1 and 2) could be attributed to high organic content, decayed plant and animal materials and domestic affluent. This requires large quantities of dissolved oxygen for decomposition. Biochemical oxygen demand also provides a measure of the effect of pollution on a receiving water body. The high BOD in the study showed that the water body had received an enormous level of organic pollutant into it.
Salinity range (5.7-22.8) observed in the study areas was brackish. This fell within the range reported by other reports within the environment [63,64].
Nitrate index is an index derived from organic residue from plant, animals, sewage and fertilizers (Lund 1972, Boyd 1982). Nitrate levels across stations falls within the range of 0.133 mg/l-2.2 mg/l.
Nitrate and other dissolved nutrient recorded for this study was observed not to be significantly different (P>0.05) values for soluble nutrient were also low and varied between months.
Heavy metals such as Corium, Nickel Zinc and Iron showed significant difference in level of variation (P<0.05) while Copper and Lead are less significant in sampling station water concentration of heavy metal was also reported to be higher in sediment samples than in water samples of the same location. However, both samples (water and sediment) still falls below WHO standards for permissible heavy metal concentrations.
Correlation (Table 5) for water parameters showed that there are strong positive correlations between chloride and total solid, nitrate and air temperature, sulphate and air temperature, biochemical oxygen demand and iron, biochemical oxygen demand and cupper. There are correlations between salinity and biochemical oxygen demand, alkalinity and air temperature, water temperature and transparency.
There are strong inverse correlations between dissolved oxygen and temperature, alkalinity and dissolved oxygen, dissolved oxygen and biochemical oxygen demand, total solids and iron. There are also correlations between total solid and transparency and between dissolved oxygen and salinity [65-68].
Extremely weak correlation was observed between biochemical oxygen demand and conductivity, copper and phosphate, copper and depth, biochemical oxygen demand and depth. All other correlation in the matrix is weak.
Table 6 also showed correlation matrix between parameters of sediment samples. Total organic carbon and total organic matter had a strong positive correlation existing between them. Others that had strong positive correlations include phosphate and pH, nickel and copper. Positive correlations also exist between zinc and nickel, phosphate and cupper, copper and zinc [69-74].
| Correlations | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | MOISTURE CONTENT | TOTAL ORGCARBON | TOTAL ORGMATTER | NO2 | PO4 | Cu | Cr | Pb | Ni | Zn | ||
| pH | 1 | |||||||||||
| MOISTURE CONTENT | .403* | 1 | ||||||||||
| TOTAL ORGCARBON | -.252 | .395* | 1 | |||||||||
| TOTAL ORGMATTER | -.271 | .383* | .997** | 1 | ||||||||
| NO2 | .031 | -.082 | .084 | .084 | 1 | |||||||
| PO4 | .724** | .550** | .000 | -.027 | .067 | 1 | ||||||
| Cu | .490** | .490** | -.104 | -.127 | .143 | .523** | 1 | |||||
| Cr | .038 | -.322 | -.097 | -.105 | .107 | .140 | .283 | 1 | ||||
| Pb | -.026 | .463* | .491** | .507** | .151 | .256 | .242 | -.207 | 1 | |||
| Ni | .145 | -.528** | -.229 | -.235 | -.081 | -.034 | .017 | .655** | -.428* | 1 | ||
| Zn | .007 | -.027 | .349 | .345 | .240 | -.071 | .267 | .513** | .106 | .543** | 1 | |
*Correlation is significant at the 0.05 level (2-tailed).
**Correlation is significant at the 0.01 level (2-tailed).
Table 6: Correlation matrics for physico-chemical parameters, heavy metals and dissolved nutrients of okobaba waters sediment (March-August, 2012).
Inverse correlations exist between nickel and moisture content, nickel and lead. Very weak correlations exist between lead and pH, nitrate and pH, nitrate and copper. There is no correlation between phosphate and total organic carbon (Figure 17).
The results obtained from this report has revealed that physical is the deposition of sawdust and other wood wastes from the Okobaba sawmill. This has reduced the quality of water and made it unfit for human use. The reduction in level of dissolved oxygen (DO) and increased Biochemical oxygen demand (BOD) has also made it difficult for life forms to be found abundant in the water body (Figures 18 and 19).
Reduction in DO, increased BOD and rise in level of heavy metal such as Zn, Pb, Zn, Fe and Cr were generally attributed to the wastes generated from the Okobaba sawmill. It is also noted that these anthropogenic inputs had resulted in the modification of habitat when comparing between the stations (Compare Station 1and 5). It is observed that the lagoon is no longer what it use to be [57,75-81] and obviously, this pollution would continue to occur if nothing is done to reduce or even stop the main sources of pollution of this water body (Figure 20).
Further analysis could still be carried out to show the effect of the pollution on the organisms present in the area.
In other to control the pollution, the following point could be adopted.
1. Revocation of Okobaba sawmill to area that could contain them without causing problems to the environment.
2. Transportation of logs of wood should not be by water but by any other environmentally friendly means.
3. Regular biological assessment must be carried out on the environment and the result obtained should be used to remedy the pollution level of the water.