Journal of Developing Drugs
Open Access
ISSN: 2329-6631
ISSN: 2329-6631
Research Article - (2014) Volume 3, Issue 2
Nitazoxanide (NTZ) and other thiazolides are effective against intracellular protozoa’s, anaerobic or micro aerophilic bacteria, viruses and tumour cells. Concerning their potential effects against Escherichia coli, the published results are scarce and conflicting. In order to investigate whether thiazolides are effective against aerobically growing E. coli, we examined mutants of the TolC efflux system for their sensitivity to nitro thiazolides, including NTZ, and bromothiazolides. We determined the susceptibilities of tolC mutants to various thiazolides and found that tolC mutants of E. coli were susceptible to both nitro thiazolides and bromothiazolides indicating a mechanism of action different from nitro reduction. Moreover, we showed that thiazolides induced a spy:lacZ transcriptional fusion indicating that thiazolides generate stress in the bacterial envelope. Moreover, wild type strains became susceptible to thiazolides if the tolC efflux system was inhibited. Taken together, our results show that thiazolides are effective against E. coli if their export from the cells is impaired.
<Keywords: Antibacterial chemotherapy; Chaperones; Drug efflux; Multidrug resistance
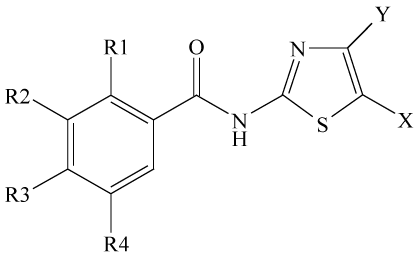
In the mid-1990s, the thiazolides nitazoxanide (NTZ; AliniaTM, Romark Laboratories, FL, USA; Table 1) was approved by the FDA for treatment of persistent diarrhoea caused by Crypto sporidium parvum and Giardia duodenalis in children and adults [1]. Meanwhile, a series of NTZ-derivatives had been synthesized and tested for activity against intracellular protozoans, bacteria, viruses and tumour cells. The respective in vitro, in vivo, and clinical studies have been reviewed elsewhere [2]. Susceptibility of G. duodenal is to thiazolides is dependent on the presence of a nitro group [3]. Therefore, enzyme systems reducing the nitro group to a toxic intermediate or radical have been extensively investigated as potential targets in this parasite [4,5].
 |
||
| Compound | X | R2 |
|---|---|---|
| NTZ (Nitro 1) | NO2 | H |
| Nitro2 | NO2 | CH3 |
| Bromo1 | Br | H |
| Bromo2 | Br | CH3 |
Table 1: Overview of thiazolide compounds mentioned in this article. R1 was OCOCH3, Y, R3 and R4 wereH for all compounds.
Soon after its launch, NTZ was investigated with respect to potential activities against anaerobic or micro aerophilic bacteria since resistance had appeared to the major current drug metronidazole [2]. It was found that some metronidazole-resistant strains isolated from patients were NTZ susceptible [6]. In clinical studies, NTZ was effective against Clostridium difficile colitis [7,8]. Moreover, NTZ derivatives have been identified that may have greater efficacy than NTZ against C. difficile and the microaerophilic Campylobacter jejuni and Helicobacter pylori [9,10]. In the anaerobic or micro aerophilic bacteria, reduction of the nitro group is regarded as the main mode of action [11,12]. Interestingly, in vitro studies indicate that NTZ and analogues may be active against aerobic bacteria such as Escherichia coli [13], Mycobacterium tuberculosis [14-16], and Staphylococcus sp. [10,17]. This is of particular interest since these pathogens often exhibit multidrug resistance against traditional antibiotics.
NTZ may also interact with membranes. In M. tuberculosis, NTZ disrupts the membrane potential and pH homeostasis in a similar way as the salicylanilide niclosamide [15]. In E. coli, the molecular mechanisms of action are unknown, and it is even unclear whether the inhibition of bio film production by the strains described in [13] can be generalized to an inhibition of other strains. In our experiments, E. coli BL 21 was not susceptible to NTZ, but became susceptible under semi-aerobic conditions only after expression of a G. duodenal is nitro reductase [5,18].
When investigating antibacterial effects of thiazolides and other compounds against E. coli, one must be aware that efflux pumps constitute a barrier that these compounds have to overcome. tolC is a channel-forming protein in the outer membrane that forms a complex with nine known inner-membrane efflux pumps and their cognate periplasmic membrane fusion proteins [19]. The tripartite complex forms a conduit for efflux of endogenous compounds and xenobiotics [20,21]. Deletion of tolC leads to increased susceptibility to a great variety of antibiotics, un couplers, detergents and other substances showing that it is the most important outer membrane protein involved in multidrug resistance [22]. Besides Acr B, eight other efflux pumps have been shown to be dependent on tolC for their efflux activities [23]. In the absence of tolC, none of these pumps are functional. E. coli tolC mutants have been used as model systems to investigate the mode of action of antibiotics such as rifamycin [24].
In order to investigate in more detail the antibacterial potential of thiazolides, we tested the susceptibility of E. coli strains lacking tolC to thiazolides with nitro or bromo group substitutions (Table 1). In addition, we have used strains carrying spy:: lacZ transcriptional fusions to test whether the thiazolides generate stress in the bacterial envelope. The expression of spy (encoding a periplasmic chaperone of proteins) is a good reporter of activation of the Bae and Cpx stressresponse systems which respond to misfolded envelope proteins [25].
E. coli strains
The E. coli strains used in this study are listed in (Table 2).
| Strain | Relevant genotype | Description | Reference |
|---|---|---|---|
| M5402 | Spy : lacZ | Wild type of M5403 | [23] |
| M5403 /p TolC | tolC /pTrc99AtolC+ | Complemented tolC-mutant | [23] |
| N7829 (GC4468) | Wild type of M5652 | [23] | |
| M5403 | tolC spy::lacZ | Defective for tolC-mediated efflux | [23] |
| M5403/pTrc99A | pTrc99AAmpr | Defective for tolC-mediated efflux | [23] |
| M5403/pTolC-turn1 mutant | Defective for tolC-mediated efflux | [29] | |
| M5652 | tolC | Defective for tolC-mediated efflux | this study |
Table 2: Bacterial strains used in this study.
Chemicals
If not otherwise stated, all biochemical reagents were from Sigma Life Science (St Louis, MO, USA). NTZ was synthesized at the Department of Chemistry and Biochemistry, University of Berne (kindly provided by Ch. Leumann). The other thiazolides were synthesized at the Department of Chemistry, University of Liverpool, which is gratefully acknowledged. The thiazolides, carbonyl cyanidem- chlorophenyl hydrazone (CCCP), and nalidixic acid were kept as 100 mm stock solutions in DMSO at -20°C.
Determination of drug susceptibility
Drug susceptibilities of the E. coli strains listed in (Table 2) were tested by a conventional disc diffusion agar procedure [5]. For this purpose, bacteria were grown to late logarithmic phase (A600 nm=1) in Luria-Bertani-medium (LB). Those strains harbouring a plasmid with an ampicillin resistance marker were grown in the same medium containing 100 μg mL-1 ampicillin. Then, 0.3 mL of bacterial suspensions was streaked on LB agar plates (9 cm diameter petri dishes containing 20 mL agar medium ± 100 μg mL-1 ampicillin). Whitman filter discs (5 mm diameter) were soaked with 7 μL of compound solutions or solvent controls as indicated. The discs were air-dried for 5 min, and placed on the plates. The plates were incubated at 37°C for 24 h in a humid chamber. Then, growth inhibition zone diameters were measured and the surface of the inhibition zones around the disc was indicated (in mm2) after subtraction of the surface of the disks (19.6 mm2).
Beta-galactosidase assay in E coli reporter strains
Overnight cultures of E. coli strains M5402 (WT) and M5403 (tolC) carrying spy::lacZ transcriptional fusions in LB medium were diluted 10-fold in LB and aerated for 1 h at 37°C to an A600 of 0.5. The cells (0.75 ml) were then diluted with equal volumes of pre warmed LB containing compounds or solvent controls as indicated and aerated for 120 min at 37°C. The cells were then assayed for beta-galactosidase specific activity (expressed in Miller units) as previously described [23].
Statistical methods
Statistical analysis of the results was performed with suitable tools from the open source software package R [26]. Differences exhibiting p values of <0.01 were considered significant.
Strains defective in the tolC-dependent efflux system and corresponding wild type strains (Table 2) were tested by disc diffusion assays with four thiazolides, two with a nitro group (including NTZ) and two with a bromo group. Moreover, nalidixic acid has been included as a positive control for a tolC-dependent antibiotic [22]. In a previous study, we had found that NTZ was inhibitory for E. coli growth but only when a) the Giardianitro reductase GINR1 was expressed in the cells and b) when they were grown in semi-aerobic but not aerobic conditions [5] suggesting that bromo-thiazolides would not be effective. In fact, all of the tolC mutants were susceptible to thiazolidesas well as to nalidixic acid, and the thiazolides with a bromo group gave even larger inhibitory zones than when a nitro group was present. The presence of a methyl group did not alter this picture (Figure 1). There was no detectable inhibition zone with DMSO as a solvent control.
Figure 1 : E. coli efflux pump mutants are susceptible to thiazolides.
Susceptibility of E. Coli efflux pump mutants and their corresponding wildtypes (listed in Table 2) to nalidixic acid (A) and to thiazolides (listed in Table 1) with a nitro group (B) or a bromo group (C). Susceptibilities were determined by disc diffusion assays as described in Materials & Methods. Discs were soaked with stock solutions yielding initial amounts of 700 n moles of thiazolides or 7 nmoles of nalidixic acid in the disks or with equal amounts of DMSO as a solvent control. Mean values ± SE of the surfaces of the inhibition zones are given for 3 replicates. Values marked by asterisks are significantly different from the control (paired t-test, two-sided, *, p<0.01).
To test whether thiazolides engender a stress response in E. coli, we assayed the M5402 (WT) and M5403 (tolC) strains for the expression of a spy: lacZ transcriptional fusion, an indicator of cell envelope stress. As previously shown, nalidixic acid induced spy expression in tolC, but not in the wild type [23]. All four thiazolides induced spy expression in the tolC mutant but not in the wild type indicating that nitro-reduction is not necessary for this effect (Figure 2).
Figure 2: Bromo- and nitrothiazolides induce the expression of a spy::lacZ fusion.
The wild type (M5402) and tolC mutant (M5403) strains were grown for 2.5 h in the presence of various thiazolides, nalidixic acid (Nal; all 1 μM) or DMSO as a solvent control. Beta-galactosidase activity was determined as described [18] and expressed as relative values to the corresponding solvent controls of each strain. Mean values (± SE) are given in triplicates. Values marked by asterisks are significantly different between the treatments and the solvent control (paired t-test, two-sided; *, P<0.01).
These findings prompted us to investigate whether E. coli wild type strains may be inhibited by thiazolides in the presence of a tolC inhibitor. To inhibit tolC, we chose CCCP in amounts that inhibit tolC efflux, but not growth [27,28], in our case 14 nmol. Previous tests had shown that initial amounts of 28 n mol CCCP and more gave clear inhibition zones around the disk (data not shown). As shown in Figure 3, NTZ and the bromothiazolide caused distinct inhibition zones with the strain N7829 reaching plateaus at 175 nmoles of compounds initially applied on the disk. Similar results were obtained with wild type M5602 (data not shown).
Figure 3: Thiazolides inhibit E. coli wild types if applied together with CCCP.
A nitro- (nitro1) and a bromothiazolide (bromo1) were applied in amounts as indicated with or without 14 nmoles of CCCP. Disc diffusion assays were performed as described. Mean values ± SE of the surfaces of the inhibition zones are given for 3 replicates. All values are significantly different from the respective controls without thiazolides (paired t-test, two-sided, *, p<0.01).
Our results show that tolC mutants of E. coli are susceptible to both nitro- and bromo-thiazolides. The tolC protein has to be functional since a mutation in turn 1 of the protein [29] is sufficient to induce thiazolide susceptibility. This is strong evidence that resistance to these drugs in E. coli is promoted by TolC-dependent efflux reducing the active concentration of the drug. Furthermore, the sensitivity seen in the tolC mutants is unlikely to be due to a mechanism involving partial reduction of the nitro group.
Another interesting clue to the mechanism of action of the thiazolides in E. coli is our finding that they increase expression of a spy::lacZ transcriptional fusion. This suggests that they increase mis folding of envelope proteins. Perhaps this is related to the disruption of the membrane potential and pH homeostasis brought about by NTZ in M. tuberculosis [15].
Taken together, thiazolides clearly exhibit antibacterial activities also against gram negative, aerobically growing bacteria such as E. coli. In wild type E. coli, this effect is, however, blocked by the presence of the tolC dependent efflux system. Inhibition of this efflux system, e. g. by sub lethal concentrations of CCCP, renders E. coli wild types susceptible to thiazolides. Thus, applied together with a more specific efflux pump inhibitor [30], thiazolides may provide an alternative to present chemotherapies, even in difficult cases such as S. aureus [31].
We wish to thank C. Huber (Institute of Parasitology, University of Berne, Berne, Switzerland) for technical support. This study was supported by a grant from the Swiss National Science Foundation (grant No. 31003A_138353).