Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Expert Review - (2022)Volume 10, Issue 1
Establishment of infant microbiome-first 100 days: The intestinal bacterial colonization begins when the fetus lies in the lower uterus and following birth the gut microbiota is transiently dominated by Enterobacteriaceae and Staphylococcus. The infant microbiota undergoes first transition during lactation, resulting in dominance of the gut microbiota by Bifidobacterium and some lactic acid bacteria. The second transition occurs during the weaning period with introduction of solid foods, leading to establishment of an adult-type of complex microbiota dominated by Bacteroidetes and Firmicutes. The dynamic gut microbial ecology is gradually altered and stabilized during early childhood.
Expansion of gut microbiome-the early childhood: The third transition occurs during the early childhood as various alterations influenced by dietary, host, and environmental factors. There occur interactions with the developing immune system in the gut and by about 3 years of age, a fully functional, adult-like gut microbiota is established. Compared with infants, the gut microbiome during childhood is more stable with less variability. Further, the gut microbiota is affected by geographical region and food culture. There are several factors, which through altering gut microbiota early in life influence the development of immune system and immune health and have an impact on the overall health during later life.
The normal and altered host-microbiota symbiosis: The host genetic and epigenetic factors such as gestational age, maternal nutrition, delivery mode, diet, pre- and probiotics, and antibiotics influence the gut microbial development during early childhood up to 3 years of life. The bacterial composition and diversity differ between breast-fed and formulafed infants, and solid food introduction is associated with alterations in the gut microbiota. Post-weaning, the diet is one of the major determinants of GI microbial colonization and diversity. The microbiota acquired in early life has long-term implications for host metabolism and immunological, gastrointestinal, dermatological, and neurological functions.
Fallouts of the altered co-existence and dysbiosis: The recent research suggests that the gut microbiome plays a significant role in age-associated physical frailty in older adults. Simultaneously, the advanced age associated deterioration in nutrient intake and absorption including dentition, changes in taste and smell, salivary function, digestion, and intestinal transit time are associated factors. The host factors like unhealthy diet and use of medications, especially antibiotics, have impact on the gut microbiota composition and function and contribute to gut dysbiosis, a state of disrupted gut microbial homeostasis, leading to increased IR and adiposity manifesting as metabolic syndrome, obesity, and type 2 diabetes.
Modulation of the gut microbiome: The health and healthy aging as a composite encompass not only on genetics, lifestyle choices and a positive attitude, but also the gut microbiome. The disturbed and disbalanced microbiome has been related to various harmful effects on health and accelerated aging. In fact, various studies reinforce that the view that unavoidable consequences of aging, in part, may represent the reversible effects of a sublime microbial dysbiosis. It may be possible to overcome dysbiosis and reset and restore normal microbiome through dietary and lifestyle modifications, probiotics and prebiotics, logical antibiotic therapy and bariatric surgery, and fecal microbial transplantation.
Gut microbiome; First 100 days; Early childhood microbiota; Adult microbiota; Symbiotic existence; Microbiota aberrations; Gut dysbiosis; Prebiotic; Probiotic; Synbiotics
Establishment of infant microbiome
Early life events and infant microbiome: The human intestinal bacterial colonization begins when the fetus lies in the lower uterus [1]. Though, the actual infant microbiota is established after birth and passes through various transition phases. The first transition occurring soon after birth, during lactation, results in dominance of the gut microbiota by Bifidobacterium and some lactic acid producing bacteria. The second transition occurs during the weaning period, with the introduction of solid foods and weaning from breast feeding, which leads to establishment of an adult-type of complex microbiota dominated by Bacteroidetes and Firmicutes. The third transition occurs much later during the adulthood as sublime or obvious dysbiosis, loss and altered composition of the gut microbiota.
The gut microbial ecology is dynamic during infancy and gradually gets stabilized during early childhood [2]. In the first 3 years of life, the gut microbiome changes through interactions with the developing immune system in the gut and have an impact on the development of GI related and the overall host immune system [3]. The process of establishing the gut microbiome is influenced by various environmental and host factors, and in turn is a potential determinant of health and longevity [4] (Figure 1).
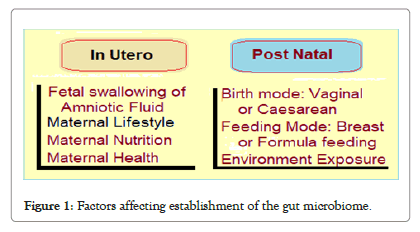
Figure 1: Factors affecting establishment of the gut microbiome.
Factors influencing infant microbiome: The early establishment of gut microbiota is initially affected by the maternal health and the delivery mode (caesarean delivery vs. vaginal delivery). The breast feeding vs. formula milk feeding, antibiotic usage and the introduction of solid foods and cessation of breast feeding are other important factors which have impact. Further, extent of exposure to the environment affects the infant microbiome.
The hygiene hypothesis correlates the exposure to the environment and lifestyle factors with development of immunity and related dysfunctions during childhood and later. The intestinal bacteria and their metabolites, including short chain fatty acids (SCFA) have been linked with proliferation and differentiation of T cells-regulatory T (Treg) and helper T (Th) cells, and Ig A or IgG secreting B cells [5].
Gut microbiotas in mother’s uterus: During pregnancy, the gut bacteria in the mother are transferred from oral cavity to uterus, followed by the bacterial efflux from the placenta to fetus. In the mice studies, the genetic and taxonomic composition of the placental microbiota has been found to closely resemble that of the oral cavity. The microorganisms are also present in umbilical cord, amniotic fluid, fetal membranes, and meconium. Further, the meconium microbiota resembles the microbiota of the amniotic fluid and placenta, thus confirming its origin from the uterus of the mother, which colonizes the fetal gut by bacteria through swallowing of the amniotic fluid. The placental microbiome has been found to be composed of commensal microbiota, Firmicutes, Tenericutes, Proteobacteria, Bacteroidetes and Fusobacteria phyla [6].
Evolution of microbiota during infancy: There are certain factors determining the evolution of the gut microbiota during the early course of human life.
Effect of mode of new-born delivery: The mode of delivery affects the development of gut microbiota in early life. In general, the gut microbiota of a new-born resembles the microbiota it encountered during birth [7]. Thus, in vaginally delivery, the infant’s gut microbiotas resemble the vaginal microbiotas, which are dominated by Lactobacillus, Prevotella and Sneathia [8]. Whereas, in case of delivery by caesarean section, the microbiotas of bear similarity to skin microbiota, which is dominated by Staphylococcus, Corynebacterium and Propionibacterium, and have poor representation of Bacteroides and a low bacterial diversity up to 3-4 months after birth.
Breast milk vs. formula milk feeding: The infant feeding methods, breast milk feeding or formula feeding, have an impact on the development of the gut microbiota. The human milk contains proteins, fats and carbohydrates, as well as immunoglobulins and endocannabinoids. The main components, human milk oligosaccharides (HMOs), such as galacto-oligosaccharide (GOS), are partially digested in the small intestine and reach the colon, where they are fermented, mainly by Bifidobacterium using enzyme, lacto-N-biosidase to produce short-chain fatty acids [9]. The HMOs, thus, have a clear probiotic effect by selectively stimulating the development of a Bifidobacterium-rich microbiota especially Bifidobacterium infantis.
Further, the breast milk contains as many as 600 bacterial species, such as Lactobacillus, Leuconostoc, Streptococcus, Enterococcus, Lactococcus and Weissella, as well as Bifidobacterium species. In breastfed infants the gut is mostly colonized by aerobic organisms, whereas microbiotas of formula-fed infants are enriched with anaerobic organisms such as Bacteroides and Clostridium [10]. In addition, breast milk contains immunoglobulins-IgA and IgG, antimicrobial compounds such as lysozyme and lactoferrin, immune regulatory cytokines such as TGF-β and interleukin 10 (IL- 10), and lymphocytes which influence the selection of bacteria that colonize the gut and modulate development of the immune system in infancy. The immune regulatory cytokines such as IL-10 and TGF-β in breast milk also facilitate tolerance of the host immune system to gut microbiota. Fatty acids (FAs) in breast milk are also associated with host health and development of the immune system.
Weaning and introduction of solid foods: Following weaning, solid foods intake promotes the growth of bacteria enriched in genes coding to allow the utilization of a larger variety of carbohydrates, vitamin synthesis, and xenobiotic degradation [11]. A large shift in the gut microbiome from Bifidobacteriumdominant to Bacteroidetes- and Firmicutes-dominant occurs during the weaning period with introduction of solid foods and indigestible carbohydrates [12]. Additionally, an increase in short chain fatty acid (SCFA)-producing species is observed in accordance with intake of starch and plant fibers and increased expressions of genes involved in amino acid metabolism and vitamin biosynthesis follow complementary feeding and weaning.
Gradually the diversity of gut microbiota is increased and there develops a stable adult-like microbiota. The infant gut rapidly acquires a functional gene pool-dominated by carbohydrate metabolism genes, which is broadly similar to that of an adult. There also occurs a functional shift in the infant microbiotas gene-pool during the first year of life, as the earlier microbiotas enriched in bacteria with genes that facilitate lactate utilization to solid foods enrich microbiotas with genes that can code for the utilization of a lager variety of carbohydrates, vitamin biosynthesis and xenobiotic degradation. Approximately by the 3 years of age, bacterial composition and diversity resembles that of adults and acquires a stable form.
Impact of use of antibiotics and drugs: Various factors have their impact on the gut microbiome during the first 100 days and early childhood. The exposure to exogenous factors like use of antibiotic and drugs adversely affect the development of the gut microbiota [13]. It shifts the composition of the gut microbiota, in infants, toward a higher abundance of Proteobacteria and lower abundance of Actinobacteria populations, decreasing the overall microbial diversity and selecting the drug-resistant bacteria to flourish [14]. Further, the antibiotic use in early life increases the risk of developing allergic diseases such as asthma, atopic disease, eczema and T1DM [15,16] (Figure 2).
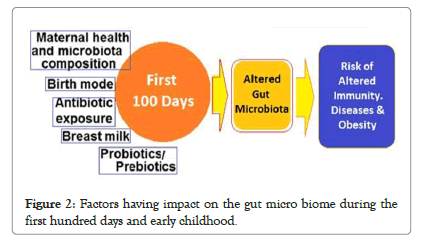
Figure 2:Factors having impact on the gut micro biome during the first hundred days and early childhood.
Microbiota during early childhood
Expansion and stabilization of microbiota: After birth, the gut microbiota of a new-born is transiently dominated by Enterobacteriaceae and Staphylococcus. Thereafter, an infant's gut microbiota is dominated by Bifidobacterium and some lactic acid bacteria. Bifidobacterium-dominated microbiota is maintained until the introduction of solid food. After weaning, the Bifidus flora is outnumbered by Bacteroide, Prevotella, Ruminococcus, Clostridium and Veillonella, which now colonize the infant's gut. Eventually, by about 3 years of age, a fully functional, adult-like gut microbiota is established.
Compared with infants, the gut microbiome during childhood, show less variability and is more stable. Still, the gut microbiota is affected by geographical region and food culture. The gut microbiotas of children in advanced countries are dominated by Bacteroides and Firmicutes, whereas those in developing countries are dominated by Prevotella. The gut microbiota-type is often associated with level of affluence/development, and urban and rural habitat. The gut microbiotas, thus, differ between advanced and developing countries and geographical areas. In the Eastern Asians, it is BB-type (Bacteroides-Bifidobacterium-dominated); P-type (Prevotella-dominated) in central and South-East Asia, and B-type (Bacteroides-dominated) in USA.
There are various other factors which through altering gut microbiota early in life have an influence on the development of immunity and immune health later in life. The early life physical and psychosocial environments and nutritional, hormonal and metabolic factors interact with genetics influencing the immune health during childhood and later in life. The proper evolution and establishment of the gut microbiota is of prime importance and the importance of the critical time window of the First 100 days, or the first three months of life has been highlighted. The aberrations during the first 100 days of life leading to microbial dysbiosis can potentially lead to illness, death, or long-term disability.
The Host-Microbiota symbiosis: Infants are born relatively sterile and acquire adult-like microbial colonies around 3 years of age, when the stable gut microbiotas acquire the well-balanced hostmicrobiota symbiotic states called ‘Enterotypes’ dominated by Bacteroides, Prevotella, and other Firmicutes. The host-microbiota symbiosis is an important feature of the infant gut microbiome development. It is perturbed by C-section, perinatal antibiotics and formula feeding, and the perturbed infant microbiomes have been linked to increased risk of metabolic and immune diseases, whereas its restoration may decrease the risk of associated diseases. Immune factors in infancy, such as the presence of maternal antibodies such as IgG and IgA maternal antibodies through the placenta and breast milk, and intestinal gut microbiota-primed B-cell trafficking, play important roles in the selection of intestinal bacteria habitats in GI tracts [17].
The host and microbiota cross-talks: Development of gut microbiotas between infancy and weaning is associated with immune system development [18]. It has been documented that the bacterial colonization is indispensable for the normal development of immunity. There occur bidirectional communications (host microbiota cross talks), in which the gut microbiome and metabolites contribute to development of host immune system, intestinal homeostasis and metabolism, and the host immune system influences the development of the gut microbiota [19,20]. The gut bacterial colonization impacts the differentiation of naïve T cells into Forkhead box P3 (Foxp3)+ Treg cells or various types of Th cells such as Th1, Th2, and Th17. Treg cells suppress the differentiation of naïve T cells into Th cells and have various antiinflammatory effects, including suppression of the inflammatory activities of mast cells, basophils and eosinophils, and suppression of IgE and induction of IgG. The gut bacteria such as Lactobacillus, Bifidobacterium, Bacteroides, Clostridium and Streptococcus, and their metabolites such as butyric acid and propionic acid, have been shown to induce Treg cells in mouse models.
On the other hand, each type of Th cell plays a distinct role in shaping and amplifying the immune response by producing cytokines that can suppress other types of Th cells. These cytokines improve the barrier function of the GI tract and protect against pathogen and fungi. Mutual regulation of Th1 and Th2 cells is a critical factor for immune homeostasis, and excessive Th1 or Th2 activation results in chronic inflammation and autoimmune or allergic disease.
Microbiota aberrations, altered immunity and other fallouts: The gut bacterial colonization during infancy is closely related to the development of the immune system and an aberrant development during infancy and early childhood has been shown to be associated with autoimmune or allergic disorders. Further, the establishment of food tolerance is a crucial event mediated by bacterial colonization for normal function of the digestive tract after weaning.
The gut microbiotas of infants with food allergies during the first two years of life have a relatively low abundance of lactic acid bacteria and Veillonella, and a low diversity involving Enterobacteriaceaedominant microbiotas, with colonization by Clostridium paraputrificum and tertium [21]. Further, the children who resolved milk allergies at later age (by 8 years) develop microbiotas with higher abundances of Firmicutes and Clostridiaceae and those with persistent allergies continue to show higher abundances of Bacteroides [22].
Microbiota-mediated hygiene hypothesis: The allergic disorders have risen significantly during the recent decades. This phenomenon has been explained by the ‘hygiene hypothesis’, which holds that less exposure to inanimate and animate ecological factors like microbes and parasites may lead to the over reactive immune system. There occurs an imbalance between type 1 and type 2 Th cells, due to modern hygiene biased environment impacting humoral immunity in human body. Further, the prevalence is higher in affluent and urban children than in economically poor and rural children. The childhood food allergies, asthma, atopic dermatitis, and allergic rhinitis have been associated with this phenomenon.
The deranged host-microbiota symbiosis: The bacterial species composing the gut microbiome, exist in the GIT in a symbiotic relationship sanctioned by the host immune system. The gut microbiota helps in the evolution of immunity and immune tolerance and acts as a barrier against pathogens and opportunistic commensals. It serves several functions such as metabolizing indigestible polysaccharides, detoxifying toxic dietary ingredients and metabolites.
The disturbed symbiotic relationship and the resultant dysbiosis has been linked with disorders and disease conditions like inflammatory bowel disease (IBD), irritable bowel syndrome, obesity, IR and MetS, allergic and autoimmune diseases, including asthma and atopic dermatitis, and degenerative disorders including neurodegenerative diseases. The link in relation to childhood atopy and asthma and gut microbiome has been endorsed by several studies including the CHILD study [23]. The bacteria Faecalibacterium, Lachnospira, Veillonella and Rothia (FLVR) have been found in low levels in the babies who later developed asthma [24].
Normal and altered host-microbiota symbiosis
The gut microbiota development: The gut microbial development during early childhood is a complex process that continues for up to 3 years of life. The host genetic and epigenetic factors such as gestational age, maternal nutrition, delivery mode, diet, preand probiotics, and antibiotics influence the process [25]. Diet is one of the major determinants of GI microbial diversity. The bacterial composition and diversity differ between breastfed (BF) and formula-fed (FF) infants, and solid food introduction has been associated with alterations in the gut microbiota. Breastfeeding may compensate for factors shown to negatively impact the infant's GI microbiota. Diet also interacts with other environmental factors and life events during infancy and early childhood to affect GI colonization.
The microbiota acquired in early life have long-term implications for host metabolism and gastrointestinal (GI), immune and neurological functions, and the reduced diversity and dysbiosis are linked to childhood and later life disorders, including necrotizing enterocolitis, eczema, asthma, inflammatory bowel diseases, irritable bowel syndrome, obesity, diabetes, and autism.
Breast feeding and human milk: The human milk (HM) plays a substantial role in establishing the gut microbiota. Complex oligosaccharides and bacteria present in HM may contribute. The microbes in HM are one of the contributors to the differences in the gut microbiota of BF vs. FF infants [26]. HM has been shown to be a source of potentially probiotic bacteria for the infant and contains bacterial genera also present in the infant gut microbiota, including Staphylococcus, Streptococcus, Lactobacillus, and Bifidobacterium.
Differences in the colonization patterns and microbial composition in BF vs. FF infants are brought about by complex sugars present in HM, known as human milk oligosaccharides (HMO). The HMOs comprise of glycans – L-fucose, D-glucose, D-galactose, N-acetylglucosamine and N-acetylneuraminic acid. Abundance of HMOs decreases throughout lactation and maternal genetics helps in modulating both the composition and orientation the gut microbiota. The HMOs are resistant to enzymatic hydrolysis and pass intact through the infant stomach and upper GI tract to the distal small intestine and colon, and exert prebiotic effects, inhibit the pathogenic bacteria-thus shape the composition of gut microbiota, and modulate the infant immune system. The bovine milk, the base of most infant formula, contains only trace amounts of less complex oligosaccharides.
Impact of prebiotic supplementation: There is a definite role of prebiotic supplementation on the colonization of the gut microbiota in the infants on FF. A prebiotic is nondigestible compound that, through its metabolization by microorganisms in the gut, modulates composition and activity of the gut microbiota, conferring beneficial physiological effects to the host’. Prebiotics are resistant to gastric acidity and enzymatic hydrolysis in the upper gastrointestinal tract and enter the colon intact, where they are metabolized by colonic microbiota [27].
The most studied prebiotic addition to infant formula is a 9:1 mixture of short-chain galacto-oligosaccharides (scGOS) and longchain fructo-oligosaccharides (lcFOS). The other prebiotics added to infant formula include, GOS, FOS, oligofructose and inulin, polydextrose (PDX), lactulose (LOS) and acidic oligosaccharides (AOS). Short chain prebiotics, such as FOS and GOS, are mainly fermented in the ascending colon, while longer-chain prebiotics, like PDX and inulin, are fermented along the entire colon. The prebiotics modulate the composition of the infant fecal microbiota by reducing luminal pH, result in more acidic stools with increased stool frequency and softness, and making the stool characteristics of FF infants resemble those of BF infants (Figure 3).
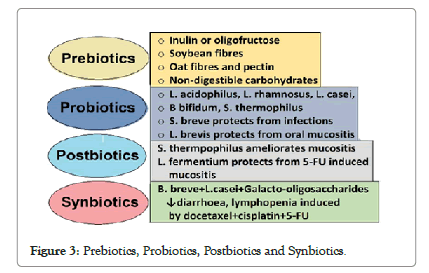
Figure 3: Prebiotics, Probiotics, Postbiotics and Synbiotics.
Development of GI tract and microbiome: The maturation of the human GI tract starts in utero and continues after birth. The gut physiology such as epithelial barrier mechanisms, accessory structures like endocrine and exocrine glands, and intestinal immune system develop fully after several months or years after birth. The regional-specific tissue features, such as gastric crypts and intestinal villi, differentiate in various sections of the gut and the structural and functional development of the GI tract is affected by the composition and the activity of microbiota. The infant’s gut microbial composition increases in number and diversity with age. Around 3 years of age, the infants’ gut microbiota attains the diversity and complexity resembling the mature adult anaerobic gut microbiota [28].
Compared to prebiotics, the probiotics are ‘live microorganisms which, when consumed in adequate amounts, confer a health benefit on the host.’ HM contains a variety of potential probiotic bacteria, such as Bifidobacterium, Lactobacillus and Streptococcus, which serve as a continuous inoculum to the BF infant GI tract, partly contributing to differences in the fecal microbial composition between BF and FF infants. Furthermore, administration of specific probiotic bacteria has been shown to improve infant health, including shortening the duration of rotavirus diarrhea, preventing of antibiotic-associated diarrhea, reducing the incidence of eczema in high-risk children, and decreasing the risk of necrotizing enterocolitis in very low birth weight infants.
Modulation of microbiota during late-childhood: The infant GI microbiota undergoes rapid and profound changes during the first year of life. During this period, the diet plays a predominant role over other environmental factors in shaping the microbial composition. The maternal nutrition during pregnancy and lactation potentially affects the composition and function of the microbiota of the baby.
Under normal circumstances, the gut microbiota has a symbiotic relationship with the host during which, among other things, contributes to the storage and harvesting of energy; development of the host immune system; maintenance of intestinal homeostasis; and nutrient processing. Interactions between gut microbes and the host also have a profound effect on an individual’s health later in life, while perturbation of the gut microbiota, dysbiosis, is associated with pathological conditions, such as inflammatory bowel disease (IBD), obesity, allergic, and autoimmune diseases.
The link with general health, obesity and T2DM: The human gut is inhabited by over 100 trillion microbes of about 1000 species. These microbes have co-evolved with humans over millennia and live together in symbiotic state. The most populous bacterial phyla are Bacteriodetes and Firmicutes, constituting more than 90% of the gut microbiota, and various species in lower abundance constitute the remainder. The gut microbial load is low in the stomach and increases exponentially from the duodenum, the jejunum, and the ileum to the colon, harboring about 109-1013 bacteria [29]. This dynamic population of millions of microbes is gut microbiome, having almost 10 bacterial cells for every one of human cells, which together form the meta-organism. The gut microbiota encodes over 150 times more genes than the human genome and influences the host physiology and homeostasis, in addition providing numerous metabolites for maintenance of intestinal and general health.
The lifestyle changes including dietary alterations accompanying aging have impact on gut microbiota. The alterations in gut microbiota, which modulate cardiometabolic and inflammatory processes, with aging mean loss of various physiological functions leading to accelerated aging-related health loss in older adults [30]. There occur changes in core microbiota taxa leading to low microbial diversity and gut dysbiosis. Though, the core microbiota may decline and be supplemented by other abundant species, the full core microbiota is rarely lost. These changes lead to increase in inflammatory markers and oxidative injury, intestinal inflammation, and insulin resistance (IR), precipitating T2DM and metabolic syndrome (Figure 4).
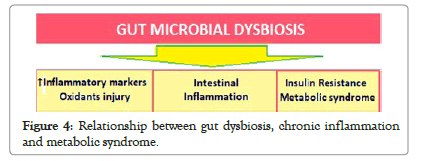
Figure 4: Relationship between gut dysbiosis, chronic inflammation and metabolic syndrome.
The groups of organisms that are mostly affected by aging, are the diversity-associated taxa, comprising of Prevotella and associated genera [31]. The extreme-aged older adults (centenarians) have a microbiota different from that in older adults [32]. Further, several specific co-abundant taxa seem to be associated with old age and malnutrition. Thus, the gut microbiota of older adults differs from that of younger adults. Furthermore, the degree of retention of the core microbiome is associated with age, general health and care, and dietary factors [33,34].
The Age-related Alterations: The age-associated loss of diversity in the core microbiota groups is associated with changes in innate immunity, sarcopenia bringing-about increased frailty, and reduced cognitive function with aging. There is no threshold of age at which the composition of the microbiota alters; rather, the changes occur gradually. With the age the composition of microbiota changes due to senescence of the gut and risk factors includes the altered physiological environment, with the lifestyle factors and diet playing a major role. There is often reduction in dietary amount of food and associated malnutrition [35,36]. There is decrease in variety of fibre-containing foods consumption leading to a decrease in microbiota diversity particularly with the co-abundant Clostridiales subpopulation [37] (Figure 5).
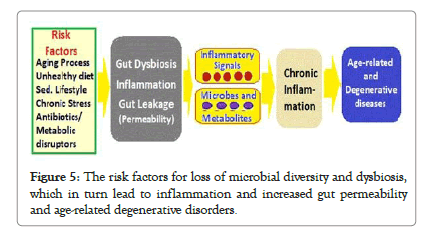
Figure 5: The risk factors for loss of microbial diversity and dysbiosis, which in turn lead to inflammation and increased gut permeability and age-related degenerative disorders.
The core microbiota decreases in abundance with aging, its loss playing a crucial role in the aging process [38]. The studies in fruit fly, Drosophila melanogaster, have documented the correlation between composition of food intake and diversity of the microbiota [39]. The microbiota composition alters, becoming less diverse and more instable over time in the older adults [40]. The loss of Clostridiales subpopulation is also significantly associated with increased frailty.
The extreme-aged older adults (centenarians), in general, have an overall decline in microbiome function parallel with various alterations in the loss of physiological functions [41]. With longevity, in centenarians, there occurs enrichment of subdominant taxa. The longevity adaptation seems to involve enrichment in health-associated gut bacteria [42]. Centenarians are a model for healthy aging because they have reached the extreme limit of life by escaping, surviving, or delaying chronic diseases. The gut microbiota in centenarians differs from that in older adults.
Fallouts of deranged symbiosis and dysbiosis
Alterations in microbiota with age: Once established early in life around 3 years of age, gradually after birth, the human gut microbiota is stably maintained depending on the host’s nutritional state and health status. For its communication with the host systems, microbiota-host crosstalk, involves various signaling networks and their mediators. In addition, the gut-brain axis connects gut microbiome with the central nervous system via neurons, hormones, or cytokines [43].
The cumulative abundance of core microbiota consisting of symbiotic bacterial taxa, mostly belonging to Ruminococcaceae, Lachnospiraceae, and Bacteroidaceae families, decreases with age, and there occurs an increasing abundance of subdominant species, as well as a rearrangement. These features are maintained during longevity and extreme longevity, when certain opportunistic and allochthonous bacteria such as Akkermansia, Bifidobacterium and Christensenellaceae turn into commensal and support health through becoming involved in establishing of a new symbiotic homeostasis with the aging host and contribute to aging-associated alterations in composition, diversity, and functional attributes of gut microbiota [44].
Gut microbiota and aging process: The changes in composition of gut microbiota and gut microbial diversity inversely correlates with biological age or functional age, rather than the chronological age. In general, there occurs compositional change in the functional core microbiome and the enrichment of non-core functions with advancing age [45-47]. The gut microbiota of the elderly becomes more diverse and variable with advancing age and the three main bacterial taxa in the core microbiota, become less abundant in older age groups, while certain health-associated species become more abundant in older age groups including centenarians and semi-supercentenarians. In general, the Ruminococcus and Coprobacillus (Firmicutes phylum), and Eggerthella (Actinobacteria genera) become abundant, but the overall gut microbiota richness decreases, while some microbial taxa associated with unhealthy aging emerge with increase in biological age.
The gut dysbiosis is the disruption of the commensal homeostasis between the host and gut microbiota due either altered host factors or microbial composition or both. The factors contributing to gut dysbiosis include unbalanced diet, environmental toxins, drugs, ROS, psychological stressors and various proinflammatory conditions. Gut dysbiosis due to antibiotics or high-fat or carbohydrate intake is associated with obesity and metabolic disorders. In turn, the gut dysbiosis has been associated with disorders like inflammatory bowel disease, obesity, diabetes, cardiovascular diseases, and neurodegenerative diseases. The altered innate immune response caused by dysbiosis, in the aging gut provokes chronic inflammation and oxidative injury leading to gut dysplasia and defective epithelial functioning, and accelerated aging and increased mortality.
Pathophysiology of inflam-aging: With advancing chronological age, the homeostatic relationship between the gut microbiota and the host deteriorates along with gut dysbiosis and the gut microbiota becomes more diverse with phylogenetic richness. The age-related decline in immune system functioning (immunosenescence) and a low-grade chronic inflammation (inflam-aging), may accompany aging. Because of its impact on human physiology, metabolism and immunity, the gut microbiome is a potential determinant of healthy aging. Indeed, the preservation of host-microbial homeostasis can counteract inflam-aging, intestinal permeability, and decline in muscle and bone mass and cognitive health [48].
The dysbiosis changes in the aging gut provoke oxidative stress and proinflammatory changes with altered innate immunity. In addition, the gut dysbiosis disturbs communication between the gut microbiota and the host through various biomolecules, CRindependent signaling pathways and epigenetic mechanisms, promoting proinflammatory immune responses and chronic degenerative reactions affecting the host health and longevity. There is evidence from the research that the chronic, age-related inflammation called inflam-aging is brought about by senescent cells, cell debris immunosenescence and microbial alterations. Thus, the age-associated changes in intestinal microbiota and their bidirectional relationship with the host, lead to chronic inflammation, and the dysbiotic gut microbiome appears to play a key role in age-related inflammation [49,50]. With the advanced age, the ability to resolve inflammation becomes impaired leading to sustained tissue infiltration by leukocytes and chronic release of pro-inflammatory cytokines and chemokines.
The concept of immuno-scence: The most important factor associated with age-related inflammation is decrease in the efficiency of the immune system, the immuno-senescence, characterized by decreased neutrophil function, naïve T cell number and cytotoxic capacity of natural killer cells, and lowered B-cell antibody production in response to antigen. The concept of immuno-senescence is thought to be the result of the chronic lifetime antigenic burden exhausting the finite capacity of the organism’s immune system, leading to long-term risks of chronic inflammation and disease. The gut microbiome appears to play an integral role in these age-related inflammatory changes. The advanced age is associated with changes in microbiota composition characterized by a loss of diversity in the core taxa associated with key geriatric syndromes including physical frailty and cognitive decline. Through the loss of gut microbial richness and altered microbiome composition, dysbiosis-sublime or obvious, has been documented to play a significant role in accelerated aging process and negative impact on longevity [51].
Impact of biomolecules from microbiota: The commensal microbes produce numerous biomolecules endogenously in the digestive tract, which may modulate health and aging process. These include various vitamins, fermentation products, gut-derived hormones, and bio-compounds relevant to the neurological health. Colanic acid, an exopolysaccharide, promotes mitochondrial fission and enhances the mitochondrial unfolded protein response under stressful conditions. SCFAs are fermentation products of dietary fibers by the anaerobic gut microbiota, which enter the circulation from the gut and have certain beneficial roles in energy metabolism. Acetate reduces serum cholesterol and triglyceride levels, propionate lowers glucose levels, and butyrate improves insulin sensitivity [52].
There occurs age-related decline in the concerned genes and in SCFAs production, which has been associated with frailty. SCFAs have both negative and positive effects on health, in a dose dependent manner. Some SCFAs are the main causal factor for the α-synuclein-related pathology caused by the gut microbiota. In the mouse model of Parkinson’s disease, α-synuclein aggregates activate immune cells, including phagocytic microglial cells in the CNS and treatment of germ-free mice overexpressing α-synuclein with a mixture of acetate, propionate and butyrate leads to neuroinflammation and motor deficits [53]. Another SCFA, butyrate inhibits histone deacetylases (HDACs) and has a profound effect on the host epigenome and aging process. The inhibition of HDACs by butyrate promotes histone lysine acetylation, leading to an open chromatin state and transcription activation. Butyrate increases lifespan in Drosophila. In aging mice, butyrate counters muscle atrophy and enhances memory functioning [54-56]. The activation of FOXO by HDACs can cause skeletal muscle atrophy in mice via autophagy, and inactivation of HDAC by butyrate can reverse the atrophy.
Impact of aging on gut microbiota: Though the human ageing process is associated with gradual declines in functions across almost all organs, the bacterial organisms in the gut do not age per se. However, various age-related functional alterations in the older adult are accompanied by changes in lifestyle patterns, diet and other gut-related physiological functions influencing the gut microbiota composition and stability. The high-fat diet can potentially alter gut bacteria and leads to dysbiosis, which leads to increased gut permeability and metabolic endotoxemia, contributing to lowgrade inflammation and insulin resistance and diabetes, adiposity, and other metabolic disorders (Figure 6).
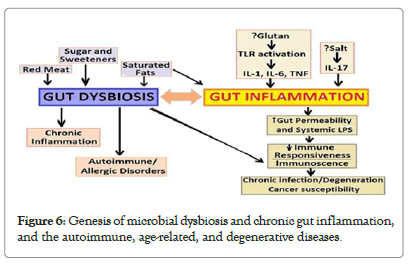
Figure 6: Genesis of microbial dysbiosis and chronic gut inflammation, and the autoimmune, age-related, and degenerative diseases.
Gut dysbiosis and neurodegeneration: The gut microbiota is also causally linked to the development of neuro-degenerative disorders [57]. The enteroendocrine cells in gut epithelium express α-synuclein, which is close to α-synuclein-containing enteric neurons, prompting the hypothesis that α-synuclein originates from the gut and reaches the central nervous system and may be a causative factor at least partially for the Parkinson’s disease [58].
The cerebral deposition of amyloid Aβ (Aβ) plaques is a critical risk factor for various types of dementia, including Alzheimer’s disease. The causative factor of the gut dysbiosis in Alzheimer’s disease has been proposed. Transgenic mice expressing Aβ precursor protein start to accumulate cerebral Aβ early on, and their gut microbiota composition differs greatly compared with that of the non-transgenic littermates. Transgenic mice rendered germ free show lower Aβ levels and significantly reduced cerebral Aβ deposition compared with conventionally reared transgenic mice. Furthermore, transfer of the gut microbiota from conventionally reared transgenic mice into germ-free transgenic mice was shown to induce the Aβ pathology. These observations indicate the role of microbiota through the gut-brain axis in development of neurodegenerative disorders [59,60].
Impacts on the host immunity and health
In general, the gut microbiome remains relatively resilient over time, however, erratic diet, illness, and antibiotic use can lead to dysbiotic alterations potentially affecting various physiological functions, including energy metabolism, immunological, endocrinal, and neurological functions.
Gut dysbiosis and physical frailty: The recent research suggests that the gut microbiome plays a significant role in age-associated physical frailty in older adults [61]. The age-related dietary changes also appear to contribute to the dysbiosis. In addition, advanced age is associated with deterioration in nutrient intake and absorption due to loss of dentition, changes in taste and smell, salivary dysfunction, indigestion, and altered intestinal transit time, contributing to dysbiosis. The host factors like unhealthy diet and use of medications, especially antibiotics, have impact on the gut microbiota composition and function and contribute to gut dysbiosis, a state of disrupted homeostasis of the gut microbiome, which leads to increased IR and may manifest as adiposity and T2DM. Further, adiposity and T2DM are associated with an altered gut microbiota, inflammation, and gut barrier disruption [62].
Gut dysbiosis and the immunity: The changes in gut microbial diversity and density leading to dysbiosis have been shown to have impact on immunity leading to chronic inflammation in the various organs. The failure to regulate the inflammatory responses is a contributor to the development of chronic inflammatory and degenerative disorders affecting the gut and other organs including the central nervous system and contributes to age-related cognitive decline. The brain amyloid content and circulating inflammatory analytes have been associated with the inflammatory bacteria taxa such as Escherichia and Shigella and inversely associated with the anti-inflammatory E. rectale taxon. A recent study has linked brain amyloidosis and chronic inflammation among cognitively impaired elders with the abundance of pro- and anti-inflammatory gut microbiota.
There appear to exist a gut microbiota signature that promotes intestinal inflammation and subsequent systemic low-grade inflammation, which in turn promotes insulin resistance and development of T2DM [63]. The animal studies have also endorsed that shifts in the composition of the gut microbiome influence metabolism and energy balance, and the chronic inflammation and increased gut permeability may play a significant role in development of adiposity and T2DM [64]. The immune system is brought up by commensal bacteria, especially bacteria in the gut. Homeostasis of the gut microbiota is important in modulation of the host immunity and control of inflammation [65].
Accompaniments of gut dysbiosis: The gut microbiota-derived metabolites, lipopolysaccharides (LPSs) and SCFAs have impact on IR and adipogenesis [66,67]. LPSs cause low-grade inflammation through the induction of inflammatory cytokines by immune cells and adipocytes. Whereas SCFAs, which are end products of the microbial fermentation of macronutrients such as acetate and butyrate, modulate gene expression and reduce chemokine and proinflammatory cytokine production by monocytes, acting on intestinal tissue immune cells locally as well as systemically. The high-calorie diets contribute to obesity and T2DM, and there appears to exist a link between diet, obesity, and the gut microbiota. The studies in mice have demonstrated that a high-fat diet (over 60% calories derived from fat) decreases the gut microbial diversity. In an important study, Chatelier, et al. documented that the diversity of human gut microbiome correlated with IR, fatty liver, and increased C- reactive protein and leptin concentrations and decreased serum adiponectin concentrations in both nonobese and obese Danish individuals [59,68].
The age-related changes in microbiome, the increased use of medications including antibiotics and consumption of highsaturated fat and high-sugar diet appears to contribute to the depletion of certain beneficial components of the microbiome [69]. This, in turn, leads to chronic activation of the immune system, altered immunity due to chronic activation of innate and adaptive immune systems. The increased intestinal permeability is associated with inflammatory bowel disease (IBD), and other disorders linked with the gut-brain axis.
Dysbiosis and intestinal epithelium: There occur changes in the intestinal epithelial barrier with age [70]. The gut barrier and nutrient transport functions decline with aging, and dysbiosis weakens the intestinal barrier function. In the Irish ELDERMET cohort study, there has been documented alterations in the core microbiota of those over 65 years of age, characterized by a greater proportion of Bacteroides spp. and distinct abundance patterns of Clostridium groups compared to younger individuals [71]. The older individuals also display a loss of diversity-associated taxa, including Prevotella and associated genera, contributing to instability in the microbiome composition. Biagi et al. [41] reported that a group of centenarians from Northern Italy displayed low species diversity compared to younger adults ~30 years of age. The specific changes occur within Firmicutes (one of the two dominant phyla in the gut) subgroups and enrichment of Proteobacteria an opportunistic, which can overtake commensal bacteria and induce pathology.
These microbiome changes are also accompanied by loss of genes for SCFAs production and decreased saccharolytic potential, while proteolytic functions become more abundant compared to the intestinal metagenome of younger adults [72]. Further, these changes in microbiota have been linked with increased plasma concentrations of inflammatory cytokines IL-6 and IL-8. These observations are supported by the studies in Drosophila, documenting that the age-related changes potentiate chronic inflammation and increased intestinal permeability [73,74]. The mice studies by Thevaranjan et al. also support this hypothesis [63,75]. In the study, the germ-free mice did not display an agerelated increase in systemic pro-inflammatory cytokines, but co-housing germ-free with old and conventionally raised mice increased circulating pro-inflammatory cytokines. Further, anti- TNF therapy reversed age-related microbial changes in the mice.
Fallouts of aberrant gut microbiome: The health in general and pathophysiological response to a disease state and aging is dependent not only on genetics, lifestyle choices and a positive attitude, but also on another fast emerging factor, the gut microbiome. The latter plays a significant larger role in staying healthy in all age groups [76]. Further, In one of the largest studies on human gut microbiota, has documented that the composition of the gut microbiome of healthy older adults was essentially similar to that of healthy younger people. So much so that the healthy older adults and elderly (up to 90 years of age) have the rich and thriving microbiome akin to younger people in 30s and 40s [77]. The gut microbiomes of the elderly have several characteristics, including instability among gut bacterial species counts and reduction in bacterial diversity [78]. The aberrations in microbiome appear to be related to various harmful alterations in health, accelerated aging and disease.
The gut microbiota is a potent biological modulator of several physio-pathological states. The renin-angiotensin system (RAS), including the local gastrointestinal RAS (GI RAS), has emerged as a potential mediator of microbiota-related effects [79]. In fact, there is a bidirectional interaction between the microbiome and RAS, and the gut bacteria and their metabolites may modulate GI and systemic RAS. As in COVID-19, SARS-CoV-2 infection causes a defect of the gut-blood barrier, increasing the penetration of microbes, bacterial lipopolysaccharide, or peptidoglycan into the circulation, possibly disrupting the immunological reaction to COVID-19 infection, and resulting in multisystem dysfunction or septic shock [80,81]. It has been suggested that in the pathogenesis of SARS-CoV-2, any disturbances in host-microbiota crosstalk could act as an initiating or reinforcing factor and the gut microbiome modulates colonic ACE2 and thereby influence COVID-19 infectivity [82]. Further, in the hospitalized patients with COVID-19 there was noted a disparity in intestinal microflora diversity, characterized by lower levels of probiotic bacteria, such as Lactobacillus and Bifidobacterium and a significantly higher abundance of opportunistic pathogens, such as Streptococcus, Veillonella, and Actinomyces [83,84].
Diet and lifestyle modifications and therapeutics
Modulation of gut microbiota and its outcomes: The recent research has reinforced the importance of restoring balanced microbial diversity and modulation of gut microbiota has various favorable outcomes for general health and longevity. The preservation and restoration of normal gut microbiome may be achievable through dietary and lifestyle modifications, probiotics and prebiotics, logical antibiotic therapy and bariatric surgery, and fecal microbial transplantation. Simultaneously, overcoming dysbiosis and resetting the aberrant gut microbiome to that of a healthier person have innumerable advantages in form of disease prevention and reducing the severity and improving outcomes (Figure 7).
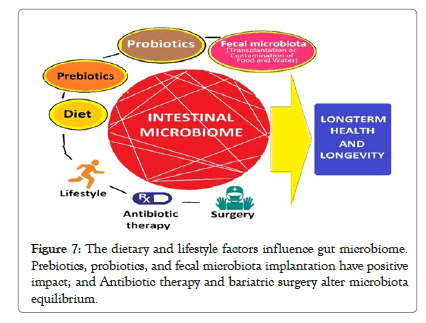
Figure 7: The dietary and lifestyle factors influence gut microbiome. Prebiotics, probiotics, and fecal microbiota implantation have positive impact; and Antibiotic therapy and bariatric surgery alter microbiota equilibrium.
Modulation through nutrients and nutraceuticals: The composition of gut microbiome naturally shifts over time and swings towards having more Bifidobacteria with aging. This natural phenomenon, however, is hampered by a diet high in processed food and low in prebiotic fibers, bouts of stress and inactivity, exposure to antibiotics and environmental pollutants. The Bifidobacteria plays a key role in many areas of well-being and important for maintaining strong bones and joints, reducing temporary bouts of inflammation , maintaining strong memory and good mental health, promoting healthy digestion and nutrient absorption, helping body balance energy levels, protecting intestinal wall and supporting the immune system. Further, having a balanced gut microbiome with Bifidobacteria species can control certain factors accelerating the shortening of telomeres and delay the aging process at cellular and tissue levels.
The dietary alterations can alter the degradative activity of the colonic microbiota in a physiologically relevant setting due to altered expression of bacterial genes. While most of the complex plant polysaccharides are not digested but fermented by a variety of anaerobic bacteria such as Bifidobacterium and Bacteroides species, resident in the colon using the undigested polysaccharides as energy source. These gut microorganisms harbour innumerable genes involved in catabolism of carbohydrates. In addition, a potential source of genetic diversity is horizontal transfer of genes from environmental microorganisms to gut bacteria, which is able to alter the ability of bacteria to harvest energy from indigestible polysaccharides in the food. On the downside, these bacterial strains can trigger induction of enzymes capable of degrading the intestinal mucin layer in case of sparse fibre intake, affecting intestinal health, and causing dysbiosis.
In a study, fibre-free diet in mice reduced the thickness of colonic mucus layer and increased susceptibility to disease caused by a mouse enteric pathogen. In another study, the microbial production of short chain fatty acids from dietary fibre influenced mouse lung disease and immune responses. Further, the microbial metabolism of other components of diet, such as L-carnitine in meat, has been linked to atherosclerosis. The nutrients, thus, influence the properties of gut microbiota and able to keep a flexible network of genes by the differential expression of bacterial enzymes.
Modulation through therapeutic interventions: As apparent, the human gut biosphere through the gut epithelium actively senses microbes and plays a role in maintaining host-microbial homeostasis at the mucosal interface. This relationship is altered in microbial dysbiosis. There are probable interactions between the infecting viruses and the gut microbiota, with the latter playing a variable but critical role in modulating the viral infectivity through diverse mechanisms to exert an inhibitory effect, and the crosstalk between the gut microbiome and host immune system is vital for preservation of health and protection from disease states including viral infections such as COVID-19. The probiotics and prebiotics appear to influence viral replication, transmission and persistence, and the outcome of various viral infections. On the other hand, the microbiota may facilitate genetic recombination of viruses and enhance their infectivity. There is substantial evidence from experimental studies to highlight the potential for microbe-based probiotic therapeutic approach to critical viral infections.
The immunomodulatory capacity of specific probiotics is strainspecific and results from a combination of signaling pathways activated as a result of a specific microbe-derived ligands interacting with the corresponding pattern recognition receptors and domains on host cells. The probiotics induce changes in dendritic cell phenotype and function, T-cells, natural killer cells and alveolar macrophages forming the basis of the protective effect of probiotics. Apart from the probiotic bacteria, their components are also able to induce potentially beneficial effects for host cells. The compounds, such as lactic acid, acetic acid and γ-aminobutyric acid produced by probiotic bacteria are capable of enhancing body immunity and controlling sepsis. The exopolysaccharides (EPSs) are biological high-molecular long-chain polysaccharides that are secreted by microorganisms. The EPS secreted by Lactobacillus acidophilus were found to inhibit TGE viral infection and improved levels of IFN-ɤ, IL-6, IL-8. Further, the probiotic therapy has been shown to facilitate CD4 recovery in HIV-1 infected patients.
The fecal microbial transplantation (FMT) is another possible therapeutic intervention to restore the normal composition and diversity of gut microbiota. As documented, the presence of various groups of commensal bacteria provides various health advantages by enhancing metabolism, potentiating the immune system and resistance to anaplastic process, and helping in endocrine signaling and brain function. Various bacterial taxa associated with these benefits include Bacteroides, Bifidobacterium, Clostridium clusters XIVa/IV and Lactobacillus. Considering rather in a straightforward, the FMT is a therapeutic modality to manipulate the human gut microbiota, by which a healthy donor microbiota is transferred into an existing disturbed microbial ecosystem. The FMT is a therapeutic intervention in form of administration of a form of fecal material from the donor into the intestinal tract of the recipient in order to directly modify the recipient’s microbial composition suitably to confer health benefits.
FMT results in normalization of microbial diversity and community structure in patients by multiple mechanisms including competition for nutrients among C. difficile and other microbiota, direct suppression by antimicrobial peptides, bile-acidmediated inhibition of spore germination and vegetative growth; and activation of immune-mediated colonization resistance. There is increasing acceptance for the therapeutic use of FMT, partially due to its perception as a ‘natural’ treatment and its relatively inexpensive implementation. Depending on the research in this field, FMT is likely to become a potential therapy for various infectious and no-infectious conditions including asthma in the future. The recent advancement in our understanding of gut microbiota has given a conceptual pathophysiological background to FMT and led to it being used in the treatment of several diseases associated with the disruption of gut microbiota, such as obesity, diabetes, IBD, metabolic syndrome, irritable bowel syndrome, anorexia nervosa, autoimmune diseases, multiple sclerosis, cancer, neuropsychiatric disorders, and cardiovascular diseases.
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed] [Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
[Crossref] [Google scholar] [Pubmed]
Citation: Nikhra V (2022) Establishment, Expansion and Modulation of Gut Microbiome: Birth to First 100 Days to Early Childhood and Later. J Prob Health. 10:252.
Received: 29-Dec-2021, Manuscript No. JPH-21-14175; Editor assigned: 05-Jan-2022, Pre QC No. JPH-21-14175 (PQ); Reviewed: 19-Jan-2022, QC No. JPH-21-14175; Revised: 24-Jan-2022, Manuscript No. JPH-21-14175 (R); Published: 31-Jan-2022 , DOI: 10.35248/2329-8901.20.8.252
Copyright: © 2022 Nikhra V. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.