Forest Research: Open Access
Open Access
ISSN: 2168-9776
+44 1300 500008
ISSN: 2168-9776
+44 1300 500008
Research Article - (2015) Volume 4, Issue 3
With the increasing concentration of carbon dioxide in the Earth’s atmosphere as the result of deforestation, there is a pressing need to estimate biomass and carbon pools in tropical forests. This is, particularly, essential in Africa where reliable biomass data is lacking. The present study was aimed at classifying land use land cover, estimating above ground biomass using remote sensing data and allometric equations, and determining the importance value of species in Lake Hawassa Watershed. Pantropic allometric equations were used that relate tree variables obtained by non-destructive measurements to the oven dry biomass. Local species specific biomass equations were also used to compare the results. The results indicated that the natural forest had lower mean above ground biomass (200.9 Mg/ha) than the plantation forest (223.6 Mg/ha). The pantropic allometric equations overestimated the above ground biomass by about 13.0% and 20.5% for natural and plantation forests, respectively, compared to the local equations. This variation is likely to be the main source of uncertainty for biomass computed using generalized equations. The species sampled ranged from 1 to 22 per plot and the overall mean stand density was 785 stems/ha. Cupressus lucitanica (60.09%), Grevillea robusta (28.65%), and Eucalyptus citriodora (20.87%) were the species with the highest importance value. The majority of tree species belonged to the diameter at breast height class of 5–25 cm accounting for 79.1% and 73.3% in plantation and natural forests, respectively. The total above ground biomass of the forest in the study area in 2011 was estimated at 1.72 Megatons. Although using generalized allometric equations demonstrated variations in above ground biomass estimates compared to the local species specific equations, results from this research effort can be used in absence of area specific models.
<Keywords: Above ground biomass; Allometric equations; Forest inventory; Importance value index; Remote sensing
Ethiopia is a country rich in biodiversity with a wide range of ecological physiographic heterogeneity, having arid lowlands in the east to rainforest in the west and high-altitude afro-alpine vegetation in the central highlands [1]. Ethiopia’s flora is estimated to range from 6500 to 7000 species of higher plants of which 12% are endemic [2]. This diversity, including that of Lake Hawassa Watershed is, however, threatened by environmental degradation and deforestation as a result of population growth [3]. The population density of the Lake Hawassa Watershed was about 588 persons/ km2 [4].
Human driven influence on woodlands and forests is high and complex. According to Thomas and Bekele [5], 75% of urban and 82% of rural Ethiopia’s energy consumption depends on traditional fuel (charcoal and fuel wood) extracted from forests. Consequently, the current size of forest in Ethiopia has become small (less than 3%, though there is no consensus on the figure), which once covered about 40% of the country [6].
Carbon is stored by trees and the removal of these trees or deforestation adds CO2 to the atmosphere when the carbon contained in the forest biomass is burnt or decomposed [7]. The forest not only provides the local people with many resources that are essential for their livelihood, but also contributes to the environmental stability by preventing soil degradation. Besides, forests, as both carbon sources and sinks, can play a major role in combating global climate change.
African landmass is primarily tropical with a wide variety of vegetation communities [8,9]. An earlier work in the tropics by Chave et al. [10] has shown that one ha of tropical forest may shelter as many as 300 different tree species. Because of this diversity, it is practically difficult to develop allometric equations for all species present in the ecosystem. The literature review also didn’t find allometric equations developed to estimate above ground biomass (AGB) of the tree species inventoried.
Though destructive sampling methods were initially used to estimate living tree biomass [7], several studies [11-13] have used allometric equations as an alternative method for biomass estimation. In the current study, AGB was also estimated using generalized allometric equations developed for similar biophysical environment.
Generally, in Ethiopia, there is limited number of reports on biomass studies, and the existing studies have focused on small diameter ranges and on few species from Eucalyptus and Acacia genera. In their earlier study, Fantu et al. [14] developed allometric equations for the three Eucalyptus species using destructive sampling method while Zewdie et al. [15] applied the tree variables, diameter at stump height (DSH) and height to develop allometric equations for Eucalyptus globulus coppice - shoot age ranging from one to nine years. In the former study, the Eucalyptus trees harvested were part of a plantation forest in Degaga and Kofele districts in the northern part of our study site, while in the latter the samples were harvested in a plantation forest located around Addis Ababa, at an altitude of 2300-3200 m above sea level. Due to the age limits of sampled trees (between 9 and 14 years old) and variations in biophysical environment, the developed equations were not found suitable to apply for our study site. In their reports, Woldemariam et al. [16] summarized the results of above ground live biomass and carbon stock of the Harana tropical rain forest in the Bale zone, not to be much far away from our study site, using generalized allometric equations. Eshete and Stahl [17] studied the amount of biomass accumulated in the five Acacia species that belong to the natural setting. The land-use types of Harana were Afromontane forest managed for Coffee production and unmanaged natural forest for which the AGB were estimated at 341.2 and 418.2 Mg/ha, respectively. Whereas the study site of the Acacia species is situated within the Ethiopian Rift Valley, far north (≈80 km) from our study site and it was highly disturbed woodland due to its proximity to large cities and the main road. The biophysical environment of the two sites was different from the Lake Hawassa Watershed. Besides, variation existed in management practices between the two sites and our study area. In a similar scenario, Negash et al. [18] developed allometric equations in the Rift Valley Escarpment, found adjacent to our study site for estimating the AGB of Coffea arabica, which is native to Ethiopia, while Abate et al. [19] reported the AGB for Munessa-Shashemene forest. These areas have similar biophysical environment with our study site. However, Coffea arabica in the current study area was sampled from a natural forest different from the other study area where the Coffee plants were sampled in an agroforestry system. Consequently, owing to the difference in management practices and degree of disturbances, it was not possible to use the equations developed for Coffea arabica to estimate AGB of the same species in our site. The Munessa-Shashemene forest site has not only similar biophysical environment with our study area which is critical for the model transfer [20], but also part of this site falls in the district where our study site is located. Therefore, the allometric equations developed for Croton macrostachyus (in natural forest) and Cupressus lucitanica (in plantation forest) were used to validate the AGB estimated in the current study.
The review indicated that no study has fully addressed the AGB and above ground carbon (AGC) distribution of all species in our study site. Thus, given the large potential storage of carbon in tropical forest, it is worthwhile to direct our effort to estimate AGB/carbon stock. This was performed utilizing the technique of remote sensing combined with field measurements [21-23] which have become common in forest investigation providing realistic and cost effective way of estimating AGB and carbon stock.
The specific objectives of the present study were to (i) analyze satellite image data and delineate forest cover for the base year (2011), (ii) explore and identify the existing tropical forest allometric equations that best estimate the tree based AGB and extrapolate it to the entire study area, (iii) estimate the potential AGB/carbon accumulated in the Lake Hawassa Watershed, and (iv) evaluate the diversity and dominance of species in the ecosystem. Certainly, the analysis performed and the estimated AGB are useful to understand and manage forest resources in the area.
Study area description
The study area is located in the Lake Hawassa Watershed ecosystem (latitude 6°49′ to 7°14′ N and longitude 38°16′ to 38°44′ E) covering an area of 143973.4 ha (Figure 1). Out of this, 8130.5 ha were covered with forest in 2011. It extends in both Southern Nations Nationalities and People’s Regional State (SNNPRS) and Oromiya Regional State, Ethiopia. The selected site is a mosaic of parts from eight districts from which much of the area (77.6%) falls in the SNNPRS.
The study area accommodates both plantation and natural forests. Wondo Genet area, in particular, is a home for various tree species and harbors a number of wild animals. The dominant tree species in the plantation forest were Cupressus lucitanica, Grevillea robusta, Eucalyptus citriodora, Juniperus procera, and Pinus patula. Tree species dominant in the natural forest were Celtis Africana, Podocarpus gracilior, Croton macrostachyus, Albizia gummifera, Teclea nobilis, and Cordia Africana.
This area was selected for the study because of its ecological and economic importance. It is one of the few remaining patches of forest in the region near two big cities (Hawassa and Shashemene), and it contains a number of tree species including the major lumber plantations such as Cupressus lucitanica, Podocarpus gracilior, Aningeria adolfi-friederici, and Pinus patula.
The Wondo Genet area is endowed with rich water resources, fertile soil, and wildlife. Topographically the study area comprises hills, rugged surfaces, depressions, and flat plains with an altitude ranging between 1571 and 2962 m above sea level. The Watershed is classified as tropical sub-humid type (Moist Weyna Dega) agro climatic zone [24,25], receiving considerable amount of precipitation averaging 1134 mm on an annual basis and temperatures between 12.5°C and 27.2°C [26]. The rainfall pattern of the forest site (Wondo Genet) is bimodal with the main rainy season between July and September, and short rainy season from February to April [27]. The raw rainfall and temperature data used (1984-2003) were from Wondo Genet and Hawassa meteorological stations, respectively. The temperature data from Hawassa station was used due to the absence of recorded data at Wondo Genet station.
The forest areas considered for AGB estimation were predominantly open to dense canopy (30-80%), most of which are found at the south and south-eastern foothills of Abaro Mountain. In areas with higher precipitation, trees were taller, forests were more dense and with fairly-closed canopy. Such areas occur in the Abaro and Wondo Genet sample plots. The natural forest in some hillsides and their foot slopes had dense undergrowth while understory vegetation in some plantation forest and easily accessible sites were little due to a freely roaming animal population. There were signs of illegal cutting of trees and ring-barking causing more tree mortality. In areas which were once harvested, there were regenerating sites with juvenile trees and coppices often in plantation forest.
Most of the forest areas were surrounded by agricultural lands and built-up areas. As a result, the forests were under intense pressure from anthropogenic activities to open-up new farmland, settlements, illegal tree felling for firewood, and construction materials, causing a serious ecosystem degradation and disturbance of the wild life.
Classification of satellite image to delineate forest cover
Remote sensing has long been identified as an effective and efficient tool in forestry studies [28,29] and several researches [21-23] have been conducted to estimate forest biomass and carbon stocks using remote sensing data combined with field measurements. Land cover interpretation and vegetation status of the study area was analyzed using a Landsat TM image from January 10, 2011. The image was downloaded from Global Visualization Viewer at http://glovis.usgs.gov website. This image was segmented into a series of non-overlapping and homogeneous landscape units and land cover classification was performed using a hybrid method with the goal to improve its accuracy. The classified segments were then mosaicked and partitioned into eight parts representing components of districts (Figure 2) that constitute the study area. The digital analysis and delineation of forest class was performed using ERDAS Imagine 2011 and maps were prepared in ArcGIS 10.1. A more detailed description of the image processing and the classification techniques can be found in Wondrade et al. [30].
Forest inventory
The tallying of woody plants in sample plots included the measurement of stem diameter at breast height (DBH), diameter at stump height (DSH), and total height (H). Since it was impractical to measure the height and diameter of all trees in the entire area, H and DBH of trees in the randomly selected plots were measured to represent all trees in the entire forest.
The field survey was conducted during the dry season of January and February, 2012. Sampling of trees in the two forest types was undertaken by a random method within the forest strata in an attempt to sample a broad range of representative trees in the watershed. But in the field, difficulty to access some sample plots on steep slopes limited the strict adherence to the sampling plan.
Four plots were dropped after taking the measurements for being on the boundary and measurements in eight other plots located in the south eastern part of the study area were constrained by natural barriers and security problems during the field work. A total of 48 rectangular plots of 1225 m2 each were set-out, of which 10 plots were in natural forest and 38 in plantation forest. The size of the rectangular plots was 35 m x 35 m. The sample plot size (0.1225 ha) was determined based on the requirement described by Reid and Stephen [31], who recommended to use sample plot size of 0.2-0.02 ha for a forest with stocking rate (trees/ha) ranging from 100-1000. In another study by Chave et al. [32], it was suggested that a total sampling size of 5 ha allows a land scape scale estimation of the AGB with an error of ± 10%.
Hand held Global Positioning System (GPS) device, measuring tape, colored measuring rope, compass, and pegs were used to set-up sample plots. In each plot, DBH and H of all individual trees with DBH ≥ 5 cm were marked, measured, and identified. Trees with DBH<5 cm were not measured since they normally contribute a small amount of biomass [29]. DBH was measured from a conventional height of 1.3 m [33-35] from the ground level using tree caliper and diameter tape. Individual tree height in each plot was systematically measured using a clinometer and a graduated pole for low trees. When used correctly, the Suunto Clinometer has an accuracy of ± 0.5 m for a 20 m tall tree, i.e., about 2.5% [36].
Tree species identification
All trees within the sample plots were recorded and identified. Both vernacular and scientific names of tree species were first identified by the support of local people and forest technicians immediately in the forest. For those tree species that could not be identified outright in the field, sample leaves, (seeds, fruits, and flowers when available) were coded and collected in a botanical press for later verification. Species identification of such plants was performed using books published by Bekele-Tessema [37] and Kelecha [38], and Arboretum at Wondo Genet College of Forestry. Despite considerable efforts to identify tree species, we were not able to identify botanical names of two plants identified with local names as Dinbicho in Sidamigna and Muka kara in Oromgna languages, respectively.
Allometric equations
The study area falls under the tropical moist zone where species’ richness is prevalent. In such highly diverse tropical forest, it is difficult to develop species-specific regression models, as used in the temperate zone [39]. This is because generation of local species-specific equations by destructive method is prohibitively costly, time taking, and may not be feasible in conservation areas. On the other hand, the most common method of estimating biomass from forest is through allometric equations [7,13]. However, very few allometric equations exist for Sub-Saharan Africa [12]. Therefore, the possibility of using existing mixed species allometric equations established for forests in other continents that best describe the relationship of biomass and tree variables was explored. After literature review, several equations were collated, but used the pantropic allometric equations developed by Brown [40] and Chave et al. [10] for plantation and natural forest, respectively. These models are also widely used and recommended for estimating carbon stocks in tropical forests [24,41].
Brown’s equation was based on volume/ha data and the biomass density were calculated by taking into account the biomass of the other above ground living tree components. It has the following functional form:
AGB density (t ha−1) = VOB × WD × BEF (1)
Where, VOB=inventoried volume over bark of free bole (first main branch), WD=volume-weighted average wood density (ton of oven-dry biomass per m3 green volume), BEF=biomass expansion factor (ratio of above ground oven-dry biomass of trees to oven-dry biomass of inventoried volume), and t ha-1= ton per hectare.
Volume weighted average wood density was calculated as:
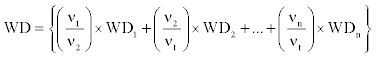 (2)
(2)
Where, V1, V2,………Vn= estimated volume of species 1,2,….,n; Vt = total volume
WD1, WD2, …., WDn = wood density of species 1, 2,…., n
Available in previous studies [42,43] and 0.580 g/cm3, the arithmetic mean for tropical Africa [32,44], when wood density of species or species itself is unknown.
The volume of trees in each sample plot was calculated using equations developed and used by several authors [40,45,46] which has the following general form:
VOB = BA × H × FF (3)
Where,
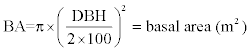
H = the measured tree height (m),
DBH = the measured diameter at breast height (cm) and
FF = tree form factor
Tree form factors vary not only between species, but also with age, site, composition, and crown size [47]. They take a value of 0.33 for cone shaped trees and 1 when no tapering. In our volume calculation, 0.42 was used, because diameters were actually measured at 1.3 m above the ground and most trees carry a bit more volume than the cone form would suggest.
Research outputs based on data across the tropics has shown that tropical broadleaved forest biomass expansion factors (BEF) are significantly related to stem wood biomass (SWB) and BEFs can be calculated according to the following model [48,49]:
BEF = Exp{3.213 – 0.506 × ln(SWB)}
For SWB < 190 t/ha (4)
= 1.74 for SWB ≥ 190 t/ha (5)
Where SWB=biomass of inventoried volume (t/ha), calculated as the product of VOB/ha (m3/ha) and wood density (t/m3), ln=natural logarithm, and Exp{}=e raised to the power of {}. No model for calculating BEF for coniferous forests was available to do the type of analysis performed for the broadleaved forests. Therefore, an estimated mean value of 1.3 presented in ref. [40] was used.
The best pan-tropic model for moist forest based on DBH and wood density [10,33] was applied to the current data to estimate AGB of natural forest.
TAGB = WD × Exp{-1.499 + 2.148 ln(DBH))2 - 0.0281 (ln(DBH))3}(6)
WD is species specific wood density in g/cm3 and available from the same source given in equation (2) and mean wood density of 0.570 g/cm3 for unknown species and for those species with an unknown wood density.
The equation of Chave et al. [10] was developed based on a much wider sampling size that involved ca. 2410 trees with DBH ≥ 5 cm directly harvested in 27 study sites across the tropics. Although this model includes the diameter range from 5-156 cm, it excludes Africa in its sample data. However, some other studies [12,50], indicated that Chave’s model is less biased and has immediate applicability to Africa.
Estimation of total above ground biomass and carbon stock.
The available above ground tree biomass was estimated by applying allometric equations to the inventoried individual tree and extrapolated to the entire area. The allometric equations convert forest structural variables (DBH, H, and WD) into biomass and carbon [13,33]. For plantation forest, the volume of individual trees in each sampling plot, wood density, and biomass expansion factors were used to derive AGB density. In Natural forest, AGB of each tree was estimated using DBH and wood density. The AGB content of the individual trees in a sample plot were then summed to get the total AGB density in that sample plot. The mean AGB density was computed for each forest type and these were then averaged to obtain the total mean AGB density for the entire sample plots. This total mean expressed in t/ha was later multiplied by the total forest cover (ha) in each district to obtain the AGB in tons, the summation of which gives the AGB of the forests in the entire study area.
The forest carbon stocks are widely estimated from the allometric equations for biomass. Generally, the carbon concentration of different parts of a tree is assumed to be 50% of its dry biomass [8,51,52]. Therefore, the estimated AGB in each district was converted to AGC stock using the conversion factor of 0.5 and the carbon stock values were expressed in tons and megatons.
Importance value index of tree species
To investigate the species composition in the Lake Hawassa Watershed ecosystem, the importance value indices were calculated for tree species. Tree species were coded using three letters from the genus, while the last two letters identify the species as used in the woody biomass inventory manual of Ethiopia [53]. The importance value indices (IVI) indicating the importance of species in an ecosystem were calculated using the following relationships [54,55].
IVI = Relative density + Relative dominance + Relative frequency (7)
Where
Relative Density = (Density /Total Density of all species) × 100
Density = Number of individuals / Sum of all plot areas,
Relative dominance = (Density/Total dominance of all species) × 100
Dominance = Basal area of each species/ Sum of all plot area,
Relative frequency = (Density/Total frequency of all species) × 100
Frequency = No. of plots which have at least 1 individual/ Total no. of plots sampled
Classification and accuracy
The classification of LULC from image data was aimed at extracting forest cover paying equal attention also to other classes. The magnitude of LULC classes and an overall accuracy are given in Table 1. In this process, plantation and natural forests with tree canopies of 30% and above were classified as forest leaving out other tree formations that were mixed with shrubs and grasses.
| LULC class | Water | Built-up | Cropland | Woody vegetation | Forest | Grass land | Swamp | Bare land | Scrub |
| Area (ha) | 9596.8 | 2465.6 | 81102.6 | 19557.3 | 8130.5 | 6608.3 | 6429.7 | 3998.0 | 6084.5 |
| An overall accuracy=85% and the total area=143973.4 ha. | |||||||||
Table 1: Spatial coverage of land cover classes for the base year 2011.
Forest structure
A total of 4617 trees belonging to 35 families, 51 genera, and 58 species were recorded in the stratified sample plots. Species’ composition calculated using IVI (Table 2), indicated that Cupressus lucitanica was the most important species accounting for 60.09% followed by Grevillea robusta (28.65%) and Eucalyptus citriodora (20.87%). The abundance of Cupressus lucitanica (62%) was also reported [19] for the contiguous Munessa-Shashemene forest.
| Species code | Scientific name | Vernacular name | RDen (%) | RDom (%) | RFre (%) | (IVI 300) |
|---|---|---|---|---|---|---|
| CUPLU | Cupressus lucitanica | Ye-ferenj Tsid (Am.) | 20.51 | 31.02 | 8.56 | 60.09 |
| GRERO | Grevillea robusta | Grevillea (En.) | 12.63 | 12.42 | 3.60 | 28.65 |
| EUCCI | Eucaluptus citriodora | Shito-barzaf (Am.) | 10.57 | 8.50 | 1.80 | 20.87 |
| JUNPR | Juniperus procera | Tsid (Am.) | 8.75 | 6.49 | 2.25 | 17.49 |
| EUCCA | Eucaluptus camaldulensis | Key-barzaf (Am.) | 7.62 | 3.39 | 1.80 | 12.81 |
| EUCGL | Eucaluptus globulus | Nech-barzaf (Am.) | 6.52 | 3.83 | 1.35 | 11.70 |
| CELAF | Celtis africana | Kawoot (Am.) | 2.84 | 4.48 | 4.05 | 11.37 |
| PODGR | Podocarpus gracilior | Zigba (Am.) | 5.63 | 1.91 | 3.15 | 10.69 |
| CROMA | Croton macrostachyus | Bisana (Am.) | 0.95 | 1.28 | 6.76 | 8.99 |
| PINPA | Pinus patula | Patula (Am.) | 1.80 | 4.71 | 1.35 | 7.86 |
| ALBGU | Albizia gummifera | Sassa (Am.) | 0.69 | 1.25 | 4.50 | 6.45 |
| TECNO | Teclea nobilis | Lela (Or./Sd.) | 1.95 | 1.06 | 3.15 | 6.16 |
| CORAF | Cordia africana | Wanza (Am.) | 1.49 | 2.31 | 1.80 | 5.61 |
| CAUEQ | Casuarina equisetifolia | Shewshewe (Am.) | 1.21 | 2.35 | 0.90 | 4.47 |
| ACOSC | Acokanthera schimperi | Keraru (Or./Sd.) | 2.06 | 0.53 | 1.35 | 3.94 |
| CAOMA | Cassipourea malosana | Tilo (Or.) | 1.04 | 0.67 | 1.80 | 3.51 |
| ANIAD | Aningeria adolfi-friederici | Kerero (Am.) | 0.32 | 0.92 | 2.25 | 3.50 |
| MACKI | Macaranga kilimandscharica | Shakere (Wl.) | 0.50 | 0.21 | 2.70 | 3.41 |
| XMUKX | No scientific name found | Muka kara (Or.) | 0.54 | 0.11 | 2.70 | 3.35 |
| ACASY | Acacia seyal | Wach'u (Am./Or.) | 0.48 | 1.01 | 1.80 | 3.29 |
| CHIMI | Chionanthus mildbraedii | Sigheda-dhaltu (Or.) | 1.06 | 0.37 | 1.80 | 3.23 |
| POLFU | Polyscias ferruginea | Yezinjoro wenber (Am.) | 0.22 | 0.65 | 2.25 | 3.12 |
| MILFE | Millettia ferruginea | Birbra (Am.) | 0.43 | 0.70 | 1.80 | 2.94 |
| JACAC | Jacaranda acutifolia | Jacaranda (Am.) | 1.62 | 0.85 | 0.45 | 2.93 |
| FARAN | Fagaropsis angolensis | Sisa (Or.) | 0.28 | 0.31 | 2.25 | 2.85 |
| PYGAF | Pygeum africanum | Tikur-inchet (Am.) | 0.97 | 0.94 | 0.90 | 2.82 |
| EKECA | Ekebergia capensis | Oloncho (Wl.) | 0.26 | 0.27 | 2.25 | 2.78 |
| EUCGR | Eucaluptus grandis | Key barzaf (Am.) | 0.63 | 1.68 | 0.45 | 2.76 |
| PITVI | Pittosporum viridiflorum | Ara (Or.) | 0.35 | 0.11 | 2.25 | 2.71 |
| DIPDA | Diphasia dainellii | Hadesa (Or.) | 0.37 | 0.05 | 2.25 | 2.67 |
| CALCI | Calistemon citrinus | Bottle-brush (En.) | 0.78 | 0.73 | 0.90 | 2.41 |
| BESAB | Bersama abyssinica | Teberako (Sd.) | 0.37 | 0.07 | 1.80 | 2.24 |
| SPANI | Spathodea nilotica | Ye-chaka nebelbal (Am.) | 0.26 | 1.50 | 0.45 | 2.21 |
| MASLA | Measa lanceolata | Gobacho (Sd.) | 0.24 | 0.06 | 1.80 | 2.10 |
| VERAU | Vernonia auriculifera | Reji (Or./Sd.) | 0.87 | 0.09 | 0.90 | 1.85 |
| OLEHO | Olea hochstetteri | Damot-weyra (Am.) | 0.24 | 0.19 | 1.35 | 1.78 |
| DRAST | Dracaena steudneri | Tonkicho (Sd.) | 0.22 | 0.17 | 1.35 | 1.73 |
| PAVAB | Pavetta abyssinica | Muka buna (Or.) | 0.30 | 0.04 | 1.35 | 1.70 |
| PSIGU | Psidium guajava | Zeytun (Am.) | 0.19 | 0.08 | 1.35 | 1.62 |
| MAYOV | Maytenus ovatus | Kombolcha (Or.) | 0.19 | 0.02 | 1.35 | 1.57 |
| MIMKU | Mimusops kummel | Kolati (Or.); Ishe (Am.) | 0.06 | 0.02 | 1.35 | 1.44 |
| ACAAL | Acacia albida | Grar (Am.) | 0.28 | 0.66 | 0.45 | 1.39 |
| ACAAB | Acacia abyssinica | Bazra-grar (Am.) | 0.28 | 0.54 | 0.45 | 1.28 |
| FICSU | Ficus sur | Shola (Am.) | 0.24 | 0.58 | 0.45 | 1.27 |
| ERIJA | Eriobotrya japonica | Woshmela (Am.) | 0.13 | 0.08 | 0.90 | 1.11 |
| SYZGU | Syzygium guineense | Dokma (Am.) | 0.06 | 0.13 | 0.90 | 1.10 |
| PERAM | Persea americana | Avocado (Am.) | 0.04 | 0.14 | 0.90 | 1.09 |
| BUDPO | Buddleja polystachya | Bulchano (Sd.) | 0.09 | 0.04 | 0.90 | 1.02 |
| FICVA | Ficus vasta | Warka (Am.) | 0.06 | 0.05 | 0.90 | 1.02 |
| FLAIN | Flacourtia indica | Huda (Or.) | 0.04 | 0.04 | 0.90 | 0.98 |
| NUXCO | Nuxia congesta | Bitana (Or.) | 0.04 | 0.01 | 0.90 | 0.95 |
| XDINX | No scientific name found | Dinbicho (Sd.) | 0.04 | 0.00 | 0.90 | 0.95 |
| COFAA | Coffea arabica | Buna (Am.) | 0.04 | 0.00 | 0.90 | 0.95 |
| VERAM | Vernonia amygdalina | Grawa (Am.) | 0.35 | 0.07 | 0.45 | 0.86 |
| DELRE | Delonix regia | Yediredawa-zaf (Am.) | 0.09 | 0.27 | 0.45 | 0.81 |
| EHRCY | Ehretia cymosa | Uraga (Or.) | 0.06 | 0.05 | 0.45 | 0.56 |
| RHUNA | Rhus natalensis | Tatesa (Or.) | 0.06 | 0.01 | 0.45 | 0.52 |
| PSYOR | Psychotria orophila | Digita (Am.) | 0.04 | 0.00 | 0.45 | 0.50 |
| Total | 100.00 | 100.00 | 100.00 | 300.00 |
Table 2: Importance value indices of tree species in the study area.
Note: Relative density (RDen), Relative dominance (RDom), Relative frequency (RFre). Vernacular names of identified tree species in some of the local languages and in English: Amargna (Am.), Oromgna (Or.), Sidamigna (Sd.), Wolaytgna (Wl.), English (En.). The first three letters of the vernacular names were taken for trees with unknown genus and species to begin and end with a letter ‘X’.
The stem density of plantation forest (800/ha) was higher than the density of natural forest (730/ha), while the overall stand density was 785 stems/ha. Basal area and volume of plantation forest were 25.0 m2/ha and 251.9 m3/ha, respectively. The tree species with the highest basal area and volume were Cupressus lucitanica, Grevillea robusta and Eucalyptus citriodora. These species accounted for 43.7% of the total tree plants. Eucalyptus grandis, having the highest mean height, stood 9th and 12th in terms of volume and basal area, respectively. However, Spathodea nilotica, a species with the highest mean DBH, was 13th both in volume and basal area.
The spatial distribution and species’ composition of the sampled trees revealed that species had variations in their abundance, frequency, and density across the study site. The pattern of forest structure also indicated that about one-fifth of the species had individual trees above the medium stage (20 cm ≤ DBH < 50 cm). Moreover, the mean height of about one-third of the tree species sampled (20 species) were less than 10 m indicating the infrequency of matured trees. The uneven population structure and insignificant number of matured trees can mainly be explained as an indication of human intervention in the forest and selective removal of such trees for timber production.
The majority of tree species both in plantation and natural forest belonged to the DBH class of 5-25 cm accounting for 79.1% and 73.3%, respectively. The number of species in the sampled forests ranged from 1 to 22 per plot, the highest being in the natural forest. Cupressaceae was the most dominant tree family as given in Figure 3b, while Flacourtiaceae and Lauraceae were the least abundant.
Figure 3: Distribution of sampled trees by DBH class (a) and by Family (b). Tree families with the highest occurrence both in natural and plantation forests: CUPR: Cupressaceae; MYRT: Myrtaceae; PROT: Proteaceae; PODO: Podocarpaceae; ULMA: Ulmaceae; RUTA: Rutaceae; APOC: Apocynaceae; BIGNO: Bignoniaceae; PINA: Pinaceae; MIMO: Mimosoideae; BORA: Boraginaceae; EUPH: Euphorbiaceae and others.
The high prevalence of trees with small DBH class in both forest types (Figure 3a) was an indication of disturbance where mature stems are affected by anthropogenic activities and tree mortality as observed during the field inventory. Such disturbance was reported [56] in the Harenna forest, Bale zone in the eastern part of our study area with a relatively similar altitude of 1500-2700 meters above sea level. The change in land use has also been one of the factors that have contributed to the deforestation as reported by Abate et al. [19] for one of the adjoining forest blocks in the area.
Previous studies [55,56] suggested that the patterns of plant population structure represented by an uninterrupted reversed J distribution indicate the presence of individual trees at all sizes implying a healthy and stable regeneration process. However, the observed DBH and Height distributions in the current study area were different from reversed J distribution which can also be attributed to the intense previous and current human interventions.
AGB and carbon estimation
The 48 plots contained trees with mean DBH of 17.4 cm (range 5-75.4), mean height 18.2 m (range 2-57 m), and WD of 0.570 g/cm3 (range 0.400-0.784). The mean AGB density per plot was estimated at 200.9 Mg/ha (range 87.7-308.6) for natural forest, whereas the estimated mean AGB density for plantation forest was 223.6 Mg/ha (range 51.9-401.7). The mean AGC density per plot was estimated at 100.5 Mg/ha and 111.8 Mg/ha for natural and plantation forests, respectively. It is evident from the figures that the plot level and mean values of AGB density reflected variability within and among the forest types. The amount of biomass and carbon stored in forest varies based on several factors including forest types, age, management practices, and level of human and natural disturbances [15,57,58].
The result revealed that large fractions of AGB and Carbon were stored in small (5-20 cm) and medium (20-50 cm) DBH classes where the number of mature trees were limited (Figure 4).
The sample plot with the highest AGB in the natural forest had 538.8 stems/ha compared to the plot with the lowest AGB having a stand density of 579.6 stems/ha indicating the presence of more biomass in less number of trees with larger size. Therefore, the major variation in the AGB is explained by the large DBH sizes and age differences. Four trees with DBH between 60.5 and 75.4 cm, in the plot with the highest AGB, contributed about 59.1% of the total biomass.
The results of AGB in the plantation forest revealed that the stand density of trees was not the main contributor for the increased AGB. This was exemplified by the plot with the highest and lowest AGB. The stand density of trees in the plot with the lowest AGB was 1 314 stems/ha, while the plot with the highest AGB had 278 stems/ha. The average DBH and height of trees sampled in the plot with the highest AGB were large enough (34.1 cm and 38.5 m, respectively) compared to the average DBH and height of trees (9.8 cm and 17.8 m, respectively) in the plot with the lowest AGB. This shows that the main factors for the variation of the AGB in plantation forest were the differences in DBH and height of individual trees in the sample plots. Thus, the overall mean AGB of the two forest types (212.3 Mg/ha) was used to estimate the biomass of the entire study area (Table 3). The mean AGC in the natural and plantation forests were estimated to be 100.5 and 111.8 Mg/ha, respectively.
| Regional State | District | Geographic area (ha) | Forest cover (ha) | Total AGB3(ton) |
|---|---|---|---|---|
| SNNP SNNP SNNP SNNP |
Hawassa Zuria | 84512.2 | 6550.6 | 1390683.9 |
| Boricha | 14286.3 | 72.7 | 15438.5 | |
| Shebedino | 12116.3 | 236.7 | 50251.4 | |
| Arbegona | 306.1 | 14.8 | 3133.5 | |
| Oromiya Oromiya Oromiya Oromiya |
Siraro | 11518.0 | 0.0 | 0.0 |
| Shashemene | 17124.2 | 521.4 | 110686.9 | |
| Kofele | 4038.2 | 733.7 | 155760.3 | |
| Kokosa | 72.1 | 0.7 | 152.9 | |
| Total | . | 143973.4 | 8130.5 | 1726107.3 |
| Mt=Megaton | 1.726 Mt | |||
Table 3: The estimated mean AGB of forest in the Lake Hawassa Watershed. Total AGB3 is the product of forest cover and the mean AGB of all plots (212.3 t/ha).
The absence of forest cover in Siraro district indicated the presence of heavy anthropogenic activities and active LULC changes on the border between the two regional states. This finding is consistent with the work of Dessie and Kleman [57], who reported the complete replacement of forest cover at the border by agricultural land in the same area.
Comparison of AGB and carbon with the available estimates
The total mean AGB density estimated for this study area was considerably lower than the range of forest AGB reported (245-513 Mg/ha) for the same life zone in countries such as Cameroon, Malaysia, and French Guiana [59]. Though all the sites fall in tropical moist forests, the large variation in AGB is likely due to differences in the type of forest, size of sampled plants and wood density of the inventoried forests. However, the current mean AGB density for plantation forest (223.6 Mg/ha) was within the range of mean AGB density estimated for Cupressus lucitanica (217 Mg/ha) and Eucalyptus globulus (255 Mg/ha) [19] in plantation forest adjacent to our study site.
The comparison of AGB from the pantropic allometric equations was made against local species specific equations with DBH ranging from 10 to 30 cm and 19 to 47 cm for Croton macrostachyus and Cupressus lucitanica, respectively (Table 4). In both cases, the pan tropic equations overestimated the AGB compared to the local equations for the specified DBH classes. The equations (ii), (iii) and (iv) overestimated the AGB on average by about 13.0, 19.5, and 42.0%, respectively, for the natural forest, whereas equations (vi), (iii), and (iv) overestimated by about 20.5, 59.0, and 82.8%, respectively, for plantation forest. It is evident from the figures that equation (ii) corresponding to natural forest and equation (vi) which was used for the plantation forest were found the best models giving results close to the local species specific equations. This was one of the reasons why we selected the two equations to estimate AGB and AGC in the current study area.
| a. AGB (kg) of trees from Croton macrostachyus (Natural forest) | ||||
| DBH (cm) | Equation (i) | Equation (ii) | Equation (iii) | Equation (iv) |
| 15 | 96.3 | 102.5 | 111.9 | 130.0 |
| 18 | 164.1 | 166.0 | 177.4 | 212.7 |
| 21.5 | 243.2 | 265.1 | 278.2 | 336.3 |
| 28 | 390.1 | 528.0 | 542.7 | 643.4 |
| b. AGB (kg) of trees from Cupressus lucitanica (Plantation forest) | ||||
| DBH (cm) | Equation (v) | Equation (vi) | Equation (iii) | Equation (iv) |
| 25 | 302.2 | 359.3 | 407.4 | 489.2 |
| 30 | 438.7 | 517.4 | 646.1 | 758.2 |
| 35 | 575.1 | 654.0 | 954.4 | 1086.8 |
| 40 | 711.6 | 936.3 | 1337.9 | 1475.1 |
Table 4: Comparison of AGB estimated using local and pan tropic allometric equations.
(i) - (vi) represent allometric equations used to compare AGB of trees with the specified DBH range
(i) AGB=22.601 × DBH-242.74; Abate et al. [19]
AGB= WD × Exp {-1.499+2.148 × ln(DBH)+0.207 × (ln(DBH))2-0.0281 × (ln(DBH))3}; Chave et al. [10]
(iii) AGB=Exp (−2.134+2.530 × ln(DBH)); Brown [40]
(iv) AGB= (38.4908-11.7883 × DBH+1.1926DBH2); Brown et al. [59]
(v) AGB=27.293 × DBH-380.14; Abate et al. [19]
(vi) AGB density: VOB × WD × BEF, converted to biomass; Brown [40]
Note: (i) and (v) are local species specific equations.
The higher percent variation observed in the plantation forest can be attributed to (1) inaccuracies in height measurement of trees caused by dense canopy, (2) the fact that the original data base used for developing the volume weighted equations was based on closed forests different from the present study site which contains open to closed canopy, and (3) probably the current plantation forest was not purely composed of broadleaf trees, rather, in some plots, with a mix of limited number of conifer trees. On the other hand, most conifer trees such as Pinus patula, Cupressus lucitanica, and Juniperus procera were with similar branching and leaf structure within species. Consequently, the variation in BEF of conifer trees will be less than that of broadleaf trees and can influence the result of AGB and carbon stock if BEF of broadleaf is applied. It was also observed that, tree branches near settlements and along routes was trimmed for fencing, fuel wood, and roofing materials which were believed to have contributed to the low AGB estimates using the local equations. This was exemplified by the proportion of biomass of tree components of the species used for comparison where by ≥ 90% of the AGB was allocated to stem wood and the remaining tree components (branches and foliage) accounted only for ≤ 10% [19]. In the absence of precise data, we believe that this will give first estimates of the AGB and AGC for the inventoried forests. Due to lack of other site specific equations, it was not possible to compare AGB for other species.
Chave’s equation was developed with very high coefficient of determination (R2 ≥ 99%) for many different forest types across various tropical countries and in the current study; it also demonstrated a comparable result (13.0%) with the area specific equation.
Generally, the reasons why we selected the two equations include: (1) the use of more variables, rather than only DBH, that can lead to an important improvement of biomass estimation [7], (2) equations published by Chave et al. [10] were less biased and more precise than other equations according to a research finding [12] in the tropical forest of Africa, (3) their mathematical simplicity, (4) similarity in biophysical environment where the equations were developed, and (5) the comparative closeness of the AGB with that estimated by the local species specific equations for the available range of DBH. Thus, the equations used can be applied in other tropical moist forests as long as the study area has similar biophysical environment and for the range of tree variables for which the equations were developed.
Uncertainty and errors
Though the allometric equations of Brown and Chave were the best equations found in literature for moist tropical forest worldwide [12,60], they were not without limitations. In estimating AGB of plantation forest, the volume of trees was computed using H, DBH, and form factor as variables. The uncertainty in measuring such tree variables might have contributed to the overestimation of AGB/carbon. Here, it has to be noted that uncertainty in measuring the height of small trees was less since such tree heights were measured directly using graduated poles. The absence of very large diameter trees (100 cm or more) and small number of harvested samples (<10) used to develop the site specific equation that were considered for comparison could also be one source of uncertainty in AGB estimation.
Special plants having the stature of trees, but having different branching and leaf structure such as Dracaena steudneri, may contribute to the AGB and carbon stock of the tropical forest. However, applying the same allometric equations to estimate AGB and carbon accumulation of such plants may be another explanation for the variation of the results.
The use of coarse resolution remotely sensed data where a pixel of forest may also contain a mixture of information may affect accurate estimation of AGB and carbon storage when extrapolated to the entire study area. This was exemplified by the accuracies achieved during image classification. The overall accuracy of the classified image from 2011 was 85%, while the producer’s and user’s accuracies of forest classification were 87.1% and 88.5%, respectively.
The growing population of the current study area is largely reliant on fuel wood for its energy consumption and livelihood benefits leading to immense depletion of the available forest resources. This is also expected to continue in the future unless the available forest cover is regularly inventoried and sustained to serve the coming generation. Moreover, the preservation of forest resources may contribute to the mitigation of global climate change which has elevated the need for assessment of biomass.
This paper endeavored to quantify the AGB and carbon using the generalized allometric equations developed for tropical moist forests. The abundance of tree species in tropical forest coupled with practical and cost constraints have prompted the use of existing pantropic allometric equations. The best tree allometric equations found in the literature were the equations of Brown [40] and Chave et al. [10]. The application of the selected models is important for the assessment of forest biomass and created a base line for tracking changes in the carbon storage considering the current study as one temporal instant.
The generalized equations, when compared to the available site specific equations, have generally shown the tendency to overestimate the AGB in the order of 20.5% and 13% in plantation and natural forests, respectively. This could be due to the uncertainties in model transfer and measurement of tree variables. It should be noted that using one species specific allometric equation for each forest type for validation may not be enough. In order to minimize the uncertainty in estimating AGB and validate the results achieved using the generalized equations, further research should consider developing local species specific allometric equations in the future.
Given the lack of data on biomass, the current study provided valuable estimates of AGB and AGC storage and fills the data gap in an area under-represented by existing literature.
This study was supported by Norwegian Agency for Development Cooperation (NORAD) as part of capacity building at Hawassa University, Ethiopia. We gratefully acknowledge the financial support provided by NORAD and Hawassa University to undertake this research. The authors are thankful to the forest technicians both at Hawassa College of Agriculture and Wondo Genet College of Forestry, for providing assistance in tree species identification and measurement. We also would like to express our sincere appreciation to all the crew members whose active involvement made the forest inventory possible.