Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
+44 1300 500008
ISSN: 2167-0412
+44 1300 500008
Research Article - (2016) Volume 5, Issue 4
Objective: A combination of rutin and ascorbic acid were exploited in several pharmaceutical activities and so simple and high sensitive liquid chromatographic method for the simultaneous determination of the cited ingredients in methanolic extracts of different Libyan herbal plants was proposed.
Methods: The chromatographic analysis was performed using 250 × 4.6 mm Brownlee BIO C18 column, 5 μm particle size, with UV detection at 254 nm and isocratic elution of methanol–phosphate buffer pH 3.2, which is initially in 45:55 (v/v) for 0.5 min (equilibrium state), then varied to 60:40 (v/v) isocratic for 8 min and then back to 45:55 for 2 min, at a flow rate 1 ml/min and at ambient temperature.
Results: The percentages of rutin and ascorbic acid found were 0.7, 0.1 in Salvia fruticosa, 1.72, 2.31 in Thymus vulgaris, 0.44, 0.35 in Rosemary officinalis, 2.87, 3.56 in Matricaria chamomilla L., 2.10, 0.62 in Artemisia absinthium respectively.
Conclusion: The proposed method was suitable for the identification and quantification of the binary combination of rutin and ascorbic acid in Libyan herbal plants.
Keywords: Rutin; Ascorbic acid; HPLC; Libyan herbs
Rutin (3’,4’,5,7-tetrahydroxyflavone-3β -D -rutinoside), a kind of the most abundant bioactive flavonoid called as Vitamin P, was shown to act as a scavenger of various oxidizing species, i.e., superoxide anions, hydroxyl radicals, and peroxyl radicals. As a result of these biological effects, its several pharmacological activities were widely exploited, including antibacterial, anti inflammatory, antitumor, antiallergic, antiviral and antiprotozoal. Therefore, rutin was widely present in multivitamin preparations and more than 70 herbal remedies [1]. Ascorbic acid, also known as Vitamin C, was a vitamin soluble in water, which interferes with oxidative–reductive and other metabolic processes in an organism, was important for the activity of enzymes, keeping the balance between some enzymatic groups and has great importance for physiological permeability of capillaries. Some works were claimed better results from the use of rutin in combination with ascorbic acid than from rutin alone in pharmaceutical preparations [2]. Both vitamins have antioxidant properties. Rutin and ascorbic acid can often be found together in fruits, vegetables, teas and herb plants. Hence, it is beneficially to examine highest possible percentages of both vitamins in number of herbal plants cultivated in Libya using simple, sensitive and efficient chromatographic method as demonstrated below.
Various methods for the determination of rutin in drugs and extractants have been reviewed [3]. The simultaneous determination of rutin and ascorbic acid in pharmaceutical products has been achieved by UV spectrophotometry [4,5], voltammetry [6-8], and HPLC [9,10]. However, high performance liquid chromatography [11,12] and capillary electrophoresis [13,14], were the commonly used methods only for separating and determining rutin in plants. The amount of rutin analyzed by HPLC were found 1.18% in Sage, 0.16% in rosemary, 2.49% in Thymus vulgaris L. [15], 0.059-0.093% in Salvia tomentosa [16], 1.18% in Sage, 2.49% Thymus vulgaris L., 1.73% in Thymus serpyllum L, 2.26% in Thymus sibthorii [17] and 0.0979% in water extracts of A. millefolium leaves [18]. The main aim of the present work was to estimate simultaneously both rutin and ascorbic acid and to compare the results published with that obtained in the medicinal herbs, especially cultivated in Libya.
Instrumental
Apparatus and chromatographic separation is preformed on modular HPLC system, Perkin Elmer HPLC Series PE-200 (USA), a Brownlee BIO C18 reversed-phase analytical column, 5 μm particle size, with dimension 250 × 4.6 mm operated at ambient temperature, equipped with a P200 pump, solvent degasser DGU-3A, an automatic sampler AS200, Rheodyne injector with 200 μL loop, UV/VIS detector Series 200 with controlled wavelengths at 254 nm and communication Network chromatography Interface NCI 900.
Chromatographic conditions
The components were separated isocratically with a mobile phase consisting of methanol-buffer solution. The buffer solution is prepared from potassium dihydrogen phosphate KH2PO4 0.01 M solutions adjusted to pH 3.2 with 85% ortho-phosphoric acid. The isocratic elution begun with linear equilibrium elution initial at 45% methanol over the first 0.5 minutes, followed by elution with 60% methanol over the next 8 minutes and then elution with 45% methanol for 2 minutes (Table 1). The flow rate was 1 ml/min and data were collected at 254 nm. The injection volume was 10 μl.
| Step | Time | Solvent A buffer solution |
Solvent B methanol |
| 1 | 0.5 | 55 | 45 |
| 2 | 8.00 | 40 | 60 |
| 3 | 2.00 | 55 | 45 |
Table 1: Detailed program analysis for standard rutin and ascorbic acid.
Five samples of the Libyan herb species, viz Sage, Thymus, Rosemary, Chamomile, Artemisia, were collected from the local places of El-Beida city, were dried and ground to a fine powder immediately before an experiment.
Rutin Standard rutin was purchased as rutin trihydrate (molar mass=664.6 g/mole) from MP Biomedicals, LLC-France, and ascorbic acid was purchased from BDH Laboratory Supplies Poole, BH15 1TD England. Methanol was obtained from Sigma-Aldrich Chemie (GmbH, Switzerland) and orthophosphoric acid from Sigma-Aldrich Chemie (GmbH, Seelz), potassium dihydrogen phosphate was purchased from Riedel-De Haen AG. Seelze-Hannover.
Preparation of standard stock solution
Solutions with concentrations of 1.2 mg/ml rutin and 3.2 mg/ml ascorbic acid in absolute ethanol were prepared.
Linearity and range
The linearity of the method was determined at concentrations ranging from 60-240 and 160-800 μg/ml for rutin and ascorbic acid, respectively. The calibration curves were constructed by plotting peak area versus concentrations and all measurements were triplicate.
Samples preparation
0.5 g dried plant powder was extracted with 100 ml absolute ethanol in the hot plate apparatus for 2 hours. The ethanolic solution was concentrated by rotary vapor under reduced pressure at 50°C and the remaining residue was dissolved in ethanol, and completed to 5 ml in volumetric flask which was then introduced into HPLC.
Optimization of chromatographic condition
In order to develop and validate an efficient method for the analysis of rutin and ascorbic acid in crude raw material, different detection wavelengths (uv-range), and different compositions of the mobile phase were explored. According to the preliminary studies, the detection wavelength of 254 nm and the mobile phase of methanol/ buffer phosphate solution of pH 3.2 (40:60) (v/v) in an isocratic flow were selected. Before and after this step, the ratio of 55:45 (v/v) mobile phase was necessary to achieve fast and maximum separation and selectivity within 10 min. Beyond this time, other minor components were detected over 30 min. So, it was preferably to extend the time of elution up to 30 min to wash the column by 55:45 mobile phase before replicate the procedure. Figure 1 shows a chromatogram with well separated peaks for rutin at 5.4 min and ascorbic acid at 2.85 min.
Validation parameters
The developed method was validated according to the guidelines of the International Conference on Harmonization (ICH) for validation of analytical procedures [19] and USP [20] for its linearity, LOD, LOQ, precision, accuracy, and specificity.
Linearity
Calibration curves were constructed as a function of the concentrations of standard analytes (X) versus their peak areas (Y). Calibration curves were linear over a large concentration range of 60- 240 μg/mL for rutin and 160-800 μg/mL for ascorbic acid. Calibration curves as shown in Figure 1 exhibited good linear regressions, Y=192.07X-5138.63, r=0.998 for rutin and Y=260.2X-13362, r=0.996 for ascorbic acid (Table 2).
| Component | regression parameters, µg/ml λ, 254 nm | ||||
|---|---|---|---|---|---|
| Slope S | Intercept | r | LOD | LOQ | |
| Rutin | 192.0 | -5138 | 0.998 | 17.91 | 59.68 |
| Ascorbic acid | 260.2 | -13362 | 0.996 | 59.37 | 197.91 |
Table 2: Regression parameters for the determination of rutin and ascorbic acid by the proposed methods.
Accuracy and precision
The accuracy of the proposed method was determined by a recovery study, carried out by adding standard solutions to the herbal extracts. The overall recovery percentages were in the range of 97.54 - 103.21 for rutin and 94.30 - 98.23 for ascorbic acid indicating a satisfactory accuracy. The relative standard deviation RSD (or coefficient of variation) for triplicate injections of the standard solutions and measurement of peak areas was found to be less than 1.5%, indicating the high repeatability of the developed method. Therefore, this HPLC method can be regarded as selective, accurate and precise.
Limits of Detection (LOD) and Limits of Quantitation (LOQ)
Based on the values of standard deviation of the response (Sy/x), calculated as the standard deviation of Y-intercepts of regression lines, and slope (S) obtained from the linear regression equation described above, the LOD and LOQ values were calculated, according to the ICH guidelines, as follows:
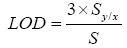
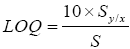
LOD of an individual analytical procedure is the lowest amount of an analyte in a sample that can be detected but not necessarily quantified. LOD was found to be 18 and 59 μg/mL for rutin and ascorbic acid respectively (Table 2). LOQ of an individual analytical procedure is the lowest amount of analyte in a sample that can be determined with suitable precision and accuracy. LOQ was found to be 60 and 198 μg/ mL for rutin and ascorbic acid respectively.
Analytical application of herb plants
Assay results for the determination of rutin and ascorbic acid by applying the chromatographic conditions to the Libyan herbal extracts are given in Table 3. Figure 2 shows a typical separation of rutin and ascorbic acid from other ingredients in a plant. The highest content was found in Chamomile with 2.82%, 3.52% of rutin and ascorbic acid, respectively. The recoveries of rutin found in the Libyan varieties were fluctuate but close to the range mentioned in the literature which may be attributed to various agricultural environments.
| Name | Rutin% | Ascorbic acid% | ||
|---|---|---|---|---|
| Mean | RSD | Mean | RSD | |
| Rosemary | 0.435 | 0.8135 | 0.345 | 0.0936 |
| Thymus | 1.714 | 0.6641 | 2.311 | 1.1971 |
| Chamomile | 2.817 | 0.1571 | 3.520 | 1.3353 |
| Artemisia | 2.099 | 1.2740 | 0.645 | 0.0783 |
| Salvia | 0.765 | 0.2116 | 0.174 | 0.7866 |
Table 3: Recoveries % of rutin and ascorbic acid in herbal extract.
Average of triplicate measurements for every plant
The developed method was suitable for the identification and quantification of the binary combination of rutin and ascorbic acid in Libyan herbal plants. A highest percentage was found 2.8%, 3.5% of rutin and ascorbic in Chamomile acid and the lowest was found in Rosemary with 0.43 and 0.34%.