Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2014) Volume 5, Issue 1
Using the recently proposed equations for calculating thermal expansion coefficient (α) and isothermal compressibility (βT) from density (ρ) and ultrasonic velocity (u) values, six binary organic liquid mixtures have been considered. These are: n-heptane+toluene (I); n-heptane+n-hexane (II); toluene+n-hexane (III); cyclohexane+nheptane (IV); cyclohexane+n-hexane (V), and n-decane+n-hexane (VI) at 298.15 K. Literature data for ρ and u of these mixtures are employed to compute α, βT, as well as γ, Pint and Γ. Calculated values of βT are compared with the earlier ones, and the agreement is found to be good.
Keywords: Isothermal compressibility; Thermodynamic properties; Ultrasonic velocity; Organic liquid mixtures
Estimation of thermodynamic properties through empirical modeling have immense applications in industry, pollution control, oil recovery and separation processes. The measurements of ultrasonic velocity and density data of liquid and liquid mixtures provide very simple method for estimating a number of useful and important thermodynamic properties e.g. adiabatic compressibility, intermolecular free length, free volume, acoustic impedence, relative association, relaxation time etc. Such studies have been carried out by several workers [1-13]. On the other hand, various statistical mechanical theories [14-17] (Flory theory, hole theory, hard sphere equations of state etc.) have been employed to compute theoretically the ultrasonic velocity, density, excess volume, excess adiabatic compressibility, internal pressure, solubility parameter, non-linearity parameter, guineisen parameter etc. of liquid mixtures. However, thermal expansivity and isothermal compressibility and their related properties (heat capacities ratio, internal pressure etc.) have not been calculated from ultrasonic velocity (u) and density (ρ) values. Recently [16,18,19], two empirical relations based on dimensional analysis were obtained showing the direct relations between thermal expansion coefficients (α) and isothermal compressibility (βT) with ultrasonic velocity (u) and density (ρ). In the present communication we have applied these relations for the estimation of thermal expansivity (α), isothermal compressibility (βT), internal pressure (Pint), heat capacity ratio (γ) and pseudo guineisen parameter (Г) of binary organic liquid mixtures. The experimental data of ρ and u for these binary mixtures have been taken from literature (20).
α and βT of a liquid system is related to ρ and u by the following expressions:
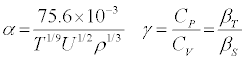 (1)
(1)
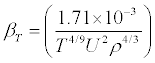 (2)
(2)
where all the symbols have their usual meanings. The internal pressure, Pint, of liquid is given by
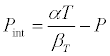 (3)
(3)
where P is the external pressure when P = 0, he above equations becomes
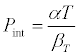 (4)
(4)
Substituting the values of α, βT from eqs (1) and (2) into above equation, we get
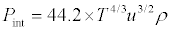 (5)
(5)
The heat capacity ratio 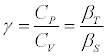 (6)
(6)
Where Cp and Cv are respectively the heat capacities at constant pressure and at constant volume, βs is the isentropic compressibility, defined by
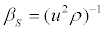 (7)
(7)
Combining eqs (2), (6) and (7), we gets
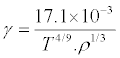 (8)
(8)
The value of pseudo-guineisen parameter, Г is given by
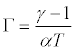 (9)
(9)
Obtaining the values α, from eq. (1) and γ from eq. (8), we have computed the value of Γ from eq. (9).
The method utilized in the present paper is easy to implement on variety of simple and complex fluid systems comparatively recently proposed methods based on various version of SAFT (Statistical associating fluid theory for variable range interactions (SAFT-VR) of the generic Mie form, SAFT+cubic and Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT). For the calculations of α, βT and other thermodynamic properties from equestions (1) to (9) we, have considered the following binary organic liquid mixtures:
(I) n-heptane(x1)+toluene(x2)
(II) n-heptane(x1)+n-hexane(x2)
(III) n-hexane(x2)+toluene(x1)
(IV) n-heptane(x2)+cyclohexane (x1)
(V) n-hexane(x2)+cyclohexane(x1)
(VI) n-hexane(x2)+n-decane(x1)Ultrasonic and density measurement of these systems were carried out by Pandey et al. [16-19] at 298.15 K. These data have been taken for the present calculation Table 1. Calculated values of α, βT, Pint,
| 298.15 K [Experimental] | |||||
| Component | V | α х 103 (deg-1) | βT х 1012 (cm2 dyne-1) | ||
| Toluene | 106.87 | 1.086 | 142.40 | ||
| n-Heptane | 147.47 | 1.258 | 112.40 | ||
| Cyclohexane | 108.76 | 1.215 | 112.77 | ||
| n-Hexane | 131.56 | 1.381 | 166.90 | ||
| n-Decane | 195.94 | 1.050 | 116.30 | ||
| γ and Г of all the pure components are recorded in Table 1 | |||||
| 298.15 K [Calculated] | |||||
| Component | α х 103 (deg-1) | βT х 1012 (cm2 dyne-1) | Pint х 10-9 (dyne cm-2) | γ | Г |
| Toluene | 1.167 | 9729.2 | 3.322 | 1.427 | 1.226 |
| Cyclohexane | 1.235 | 122.0 | 3.021 | 1.480 | 1.302 |
| n-Hexane | 1.409 | 206.5 | 2.034 | 1.565 | 1.344 |
| n-Heptane | 1.357 | 178.0 | 2.273 | 1.546 | 1.348 |
| n-Decane | 1.331 | 164.5 | 2.411 | 1.512 | 1.289 |
Table 1: Experimental and calculated values of α, βT, Pint, γ and Г for pure components at 298.15 K
Similarly computed values of these properties for the systems (I) to (VI) are presented respectively in Tables 2-7. For each binary mixture, the percentage and average percentage deviation for the βT values are given. Since the experimental values of βT are known, only deviation in βT values was calculated. Calculations of βT for the afro said organic liquid mixture [20, 21] and also other mixtures have been carried out on the basis of various hard sphere models and Flory’s statistical theory.
| (x1) | α х 103 (deg-1) | βT х 1012 (cm2 dyne-1) | Pint х 10-9 (dyne cm-2) | γ | Г | %D βT |
|---|---|---|---|---|---|---|
| 0.2979 | 1.190 | 105.2 | 3.371 | 1.424 | 1.195 | 6.32 |
| 0.3162 | 1.194 | 106.4 | 3.343 | 1.425 | 1.193 | 6.31 |
| 0.3325 | 1.196 | 107.3 | 3.322 | 1.427 | 1.197 | 6.41 |
| 0.3519 | 1.200 | 108.5 | 3.295 | 1.429 | 1.199 | 6.54 |
| 0.3709 | 1.203 | 109.7 | 3.267 | 1.431 | 1.201 | 6.68 |
| 0.3902 | 1.206 | 111.0 | 3.240 | 1.432 | 1.201 | 6.86 |
| 0.4029 | 1.199 | 111.9 | 3.220 | 1.434 | 1.214 | 6.90 |
| 0.4282 | 1.212 | 113.2 | 3.192 | 1.436 | 1.206 | 7.03 |
| 0.4484 | 1.206 | 114.6 | 3.163 | 1.438 | 1.218 | 7.18 |
| 0.4684 | 1.210 | 116.0 | 3.133 | 1.440 | 1.219 | 7.32 |
| 0.4874 | 1.222 | 117.0 | 3.135 | 1.442 | 1.213 | 7.42 |
| 0.5023 | 1.231 | 120.2 | 3.051 | 1.450 | 1.226 | 7.73 |
| 0.5227 | 1.225 | 121.8 | 3.020 | 1.452 | 1.237 | 6.47 |
| 0.5442 | 1.238 | 123.1 | 2.997 | 1.457 | 1.238 | 8.00 |
| 0.5446 | 1.242 | 124.7 | 2.968 | 1.456 | 1.231 | 8.15 |
Average percent deviation =7.04
List of abbreviations used in Table
х: Mole fraction
ρ: Density
u: Ultrasonic velocity
α: Thermal expansion coefficient
βT: Isothermal compressibility
Pint: Internal pressure
γ: heat capacity ratio
Г: Pseudo gruneisen parameter
Table 2: Calculated values of α, βT, Pint, γ and Г of binary system— n-heptane(x1) + toluene at 298.15 K.
| (x1) | α х 103 (deg-1) | βT х 1012 (cm2 dyne-1) | Pint х 10-9 (dyne cm-2) | γ | Г | %D βT |
|---|---|---|---|---|---|---|
| 0.3388 | 1.394 | 198.1 | 2.098 | 1.559 | 1.344 | 13.31 |
| 0.3598 | 1.393 | 197.1 | 2.106 | 1.558 | 1.343 | 13.26 |
| 0.3782 | 1.393 | 197.4 | 2.104 | 1.560 | 1.348 | 13.27 |
| 0.4001 | 1.390 | 195.9 | 2.116 | 1.557 | 1.344 | 12.35 |
| 0.4193 | 1.390 | 195.7 | 2.117 | 1.558 | 1.346 | 13.18 |
| 0.4394 | 1.389 | 194.9 | 2.124 | 1.557 | 1.344 | 13.13 |
| 0.4604 | 1.388 | 194.3 | 2.129 | 1.556 | 1.343 | 13.10 |
| 0.4782 | 1.386 | 193.5 | 2.135 | 1.556 | 1.345 | 13.05 |
| 0.4986 | 1.385 | 192.7 | 2.141 | 1.555 | 1.344 | 13.02 |
| 0.5191 | 1.383 | 191.6 | 2.151 | 1.554 | 1.343 | 12.95 |
| 0.5395 | 1.381 | 190.8 | 2.157 | 1.553 | 1.304 | 12.90 |
| 0.5594 | 1.378 | 189.1 | 2.173 | 1.550 | 1.338 | 12.81 |
| 0.5795 | 1.378 | 189.2 | 2.171 | 1.551 | 1.341 | 12.82 |
| 0.6025 | 1.377 | 188.5 | 2.178 | 1.550 | 1.339 | 12.77 |
| 0.6185 | 1.376 | 188.1 | 2.181 | 1.550 | 1.340 | 12.75 |
Average percent deviation =12.97
List of abbreviations used in Tables:
х: Mole fraction
ρ: Density
u: Ultrasonic velocity
α: Thermal expansion coefficient
βT: Isothermal compressibility
Pint: Internal pressure
γ: heat capacity ratio
Г: Pseudo gruneisen parameter
Table 3: Calculated values of α, βT, Pint, γ and Г of binary system— n-heptane(x1) + n-hexane at 298.15 K.
| (x1) | α х 103 (deg-1) | βT х 1012 (cm2 dyne-1) | Pint х 10-9 (dyne cm-2) | γ | Г | %D βT |
|---|---|---|---|---|---|---|
| 0.4074 | 1.278 | 139.9 | 2.723 | 1.467 | 1.225 | 9.46 |
| 0.4299 | 1.274 | 137.9 | 2.752 | 1.465 | 1.224 | 9.30 |
| 0.4486 | 1.268 | 135.7 | 2.787 | 1.459 | 1.214 | 9.11 |
| 0.4704 | 1.264 | 133.9 | 2.814 | 1.460 | 1.220 | 8.97 |
| 0.4919 | 1.259 | 131.9 | 2.846 | 1.457 | 1.217 | 8.80 |
| 0.5114 | 1.254 | 129.8 | 2.881 | 1.454 | 1.214 | 8.60 |
| 0.5290 | 1.251 | 128.2 | 2.907 | 1.452 | 1.211 | 8.47 |
| 0.5491 | 1.243 | 125.2 | 2.960 | 1.446 | 1.203 | 6.78 |
| 0.5584 | 1.232 | 124.9 | 2.965 | 1.447 | 1.207 | 7.98 |
| 0.5880 | 1.242 | 123.0 | 3.000 | 1.444 | 1.202 | 6.53 |
| 0.6088 | 1.238 | 120.8 | 3.039 | 1.442 | 1.203 | 6.18 |
| 0.6275 | 1.232 | 119.2 | 3.070 | 1.440 | 1.197 | 6.85 |
| 0.6453 | 1.211 | 116.2 | 3.129 | 1.434 | 1.196 | 4.97 |
| 0.6637 | 1.217 | 115.1 | 3.152 | 1.433 | 1.193 | 6.30 |
| 0.6829 | 1.204 | 114.0 | 3.175 | 1.436 | 1.203 | 7.12 |
Average percent deviation =7.69
List of abbreviations used in Tables:
х: Mole fraction
ρ: Density
u: Ultrasonic velocity
α: Thermal expansion coefficient
βT: Isothermal compressibility
Pint: Internal pressure
γ: heat capacity ratio
Г: Pseudo gruneisen parameter
Table 4: Calculated values of α, βT, Pint, γ and Г of binary system— toluene(x1) + n-hexane at 298.15 K.
| (x1) | α х 103 (deg-1) | βT х 1012 (cm2 dyne-) | Pint х 10-9 (dyne cm-2) | γ | Г | %D βT |
|---|---|---|---|---|---|---|
| 0.2227 | 1.318 | 158.0 | 2.485 | 1.517 | 1.315 | 10.83 |
| 0.2689 | 1.312 | 155.5 | 2.515 | 1.522 | 1.334 | 10.65 |
| 0.3158 | 1.623 | 153.4 | 2.542 | 1.513 | 1.060 | 10.50 |
| 0.3588 | 1.308 | 153.6 | 2.539 | 1.516 | 1.323 | 10.51 |
| 0.4016 | 1.305 | 150.7 | 2.575 | 1.513 | 1.318 | 10.30 |
| 0.4433 | 1.296 | 147.8 | 2.614 | 1.508 | 1.314 | 10.08 |
| 0.4861 | 1.291 | 145.6 | 2.643 | 1.507 | 1.317 | 9.94 |
| 0.5270 | 1.287 | 143.9 | 2.666 | 1.505 | 1.316 | 9.78 |
| 0.5642 | 1.284 | 142.3 | 2.688 | 1.503 | 1.313 | 9.65 |
| 0.6019 | 1.279 | 140.4 | 2.716 | 1.501 | 1.313 | 9.50 |
| 0.6414 | 1.275 | 138.7 | 2.741 | 1.500 | 1.315 | 9.36 |
| 0.6795 | 1.271 | 136.9 | 2.767 | 1.498 | 1.314 | 9.22 |
| 0.7164 | 1.268 | 135.3 | 2.792 | 1.497 | 1.314 | 9.08 |
| 0.7521 | 1.263 | 133.5 | 2.820 | 1.494 | 1.317 | 8.93 |
| 0.7877 | 1.260 | 132.0 | 2.845 | 1.491 | 1.307 | 8.80 |
Average percent deviation = 9.80
List of abbreviations used in Tables:
х: Mole fraction
ρ: Density
u: Ultrasonic velocity
α: Thermal expansion coefficient
βT: Isothermal compressibility
Pint: Internal pressure
γ: heat capacity ratio
Г: Pseudo gruneisen parameter
Table 5: Calculated values of α, βT, Pint, γ and Г of binary system- cyclohexane(x1) + n-heptane at 298.15 K.
| (x1) | α х 103 (deg-1) | βT х 1012 (cm2 dyne-1) | Pint х 10-9 (dyne cm-2) | γ | Г | %D βT |
|---|---|---|---|---|---|---|
| 0.2189 | 1.368 | 183.7 | 2.220 | 1.543 | 1.331 | 12.51 |
| 0.2541 | 1.354 | 179.4 | 2.260 | 1.541 | 1.340 | 12.23 |
| 0.2981 | 1.347 | 172.5 | 2.364 | 1.536 | 1.334 | 11.80 |
| 0.3150 | 1.346 | 171.9 | 2.333 | 1.535 | 1.333 | 11.77 |
| 0.3647 | 1.338 | 167.9 | 2.375 | 1.531 | 1.331 | 11.50 |
| 0.3897 | 1.331 | 164.7 | 2.410 | 1.529 | 1.333 | 11.28 |
| 0.4258 | 1.325 | 161.7 | 2.442 | 1.525 | 1.328 | 11.09 |
| 0.4777 | 1.317 | 157.7 | 2.489 | 1.521 | 1.326 | 10.81 |
| 0.5029 | 1.311 | 155.0 | 2.522 | 1.519 | 1.327 | 10.65 |
| 0.5675 | 1.301 | 150.2 | 2.581 | 1.514 | 1.325 | 10.26 |
| 0.5799 | 1.299 | 149.1 | 2.596 | 1.512 | 1.321 | 10.18 |
| 0.6012 | 1.294 | 146.9 | 2.625 | 1.511 | 1.324 | 10.01 |
| 0.6695 | 1.283 | 142.1 | 2.692 | 1.505 | 1.320 | 9.51 |
| 0.7187 | 1.274 | 138.1 | 2.749 | 1.501 | 1.318 | 9.32 |
| 0.7888 | 1.264 | 133.6 | 2.819 | 1.495 | 1.313 | 8.94 |
Average percent deviation = 10.79
List of abbreviations used in Tables:
х: Mole fraction
ρ: Density
u: Ultrasonic velocity
α: Thermal expansion coefficient
βT: Isothermal compressibility
Pint: Internal pressure
γ: heat capacity ratio
Г: Pseudo gruneisen parameter
Table 6: Calculated values of α, βT, Pint, γ and Г of binary system-cyclohexane(x1) + n-hexane at 298.15 K.
| (x1) | α х 103 (deg-1) |
βT х 1012 (cm2 dyne-1) |
Pint х10-9 (dyne cm-2) |
γ | Г | %D βT |
|---|---|---|---|---|---|---|
| 0.0187 | 1.401 | 201.8 | 2.068 | 1.556 | 1.331 | 13.52 |
| 0.0588 | 1.396 | 198.8 | 2.092 | 1.553 | 1.328 | 13.35 |
| 0.1008 | 1.388 | 194.7 | 2.126 | 1.550 | 1.329 | 13.13 |
| 0.1436 | 1.381 | 190.6 | 2.169 | 1.547 | 1.328 | 12.90 |
| 0.1896 | 1.377 | 188.6 | 2.176 | 1.547 | 1.332 | 12.78 |
| 0.2348 | 1.367 | 183.2 | 2.225 | 1.541 | 1.327 | 12.46 |
| 0.2886 | 1.359 | 178.6 | 2.267 | 1.539 | 1.330 | 12.18 |
| 0.3350 | 1.351 | 174.7 | 2.305 | 1.536 | 1.330 | 11.99 |
| 0.3872 | 1.345 | 171.4 | 2.338 | 1.534 | 1.325 | 11.73 |
| 0.4449 | 1.338 | 167.8 | 2.376 | 1.531 | 1.331 | 11.50 |
| 0.5009 | 1.333 | 164.3 | 2.414 | 1.529 | 1.333 | 11.26 |
| 0.5627 | 1.321 | 159.8 | 2.465 | 1.525 | 1.332 | 10.95 |
| 0.6248 | 1.313 | 155.7 | 2.513 | 1.523 | 1.335 | 10.66 |
| 0.6944 | 1.306 | 152.6 | 2.551 | 1.522 | 1.340 | 10.43 |
| 0.7655 | 1.298 | 149.7 | 2.600 | 1.520 | 1.343 | 9.65 |
Average percent deviation = 11.89
List of abbreviations used in Tables:
х: Mole fraction
ρ: Density
u: Ultrasonic velocity
α: Thermal expansion coefficient
βT: Isothermal compressibility
Pint: Internal pressure
γ: heat capacity ratio
Г: Pseudo gruneisen parameter
Table 7: Calculated values of α, βT, Pint, γ and Г of binary system- n-decane(x1) + n-hexane at 298.15 K.
Unfortunately, in both the papers there is controversy about the experimental values of βT which have been used to compare the theoretical results. In one case authors [21] considered the βT values obtained from Flory theory as the experimental ones which is not justified. In the second case, authors [20] employed purely empirical relation (combination of Auerbach and Mc Gowan) for the experimental βT values which is absurd. A perusal of Tables 2-7 shows that the average percentage deviations of the calculated βT values for mixtures (I) to (VI) are respectively 7.04 and 12.97, 7.69, 9.80, 10.79 and 11.89. Keeping in view the uncertainties in the experimental βT values employed, our agreement is quite good, and far better than the earlier results.
The values of internal pressures are increasing with increasing mole fraction for the systems II, III, IV, V and VI. This decrease in Pint values may be attributed to the existence of columbic forces in the mixture [22]. In case of system I, the behavior is opposite due to lack of columbic forces. βT values are also increasing for all the systems except system- I. In system-I, intermolecular forces are strong due to the presence of π electrons. Similar observation was observed in case of thermal expansion coefficients [23,24] For system –I, α values are increasing with the mole fraction of component I which may be interpreted in terms of closure approach of unlike molecules [25]. γ values are found to follow the general trend.
The support and encouragement of Prof. J.D. Pandey, Deptt. of Chemistry, University of Allahabad is highly appreciated.