Virology & Mycology
Open Access
ISSN: 2161-0517
ISSN: 2161-0517
Research Article - (2024)Volume 13, Issue 1
The prognosis of patients with Liver Failure (LF) depends significantly on the etiologies and clinical indicators. The retrospective cohort study included 637 LF patients between 2018 and 2020, including the subclasses of Acute Liver Failure (ALF), Subacute Liver Failure (SLF), Acute-on-Chronic Liver Failure (ACLF), Subacute-on-Chronic Liver Failure (SALF), and Chronic Liver Failure (CLF). Multivariate logistic regression analysis was used to screen clinical indicators of death patients. We analyzed the Receiver Operating Characteristic curves (ROCs) and cut-off values to assess prognosis criteria. HBV infection was present in 64.52% of LF patients. SALF (41.36%) is the main subclass of the Hepatitis B Virus-related LF (HBV-LF) group, while chronic liver failure (32.30%) is the main subclass of the non-HBV related LF group in Southeast China. Between 2018 and 2020, the incidence of HBV-LF decreased significantly, ranging from 72.36% to 59.74%, and the spontaneous survival rates of HBV-LF patients were substantially lower than those of the non-HBV-LF group (36.43%~44.93% vs. 58.97%~63.64%). Infection and cirrhosis were the primary causes of both groups. The age and total bilirubin value of the HBV-LF dead patients were significantly higher, and the number of days of hospitalization was significantly shorter than those of the survivors. The ages of the dead patients of the non-HBV-LF group were significantly higher than those of the survivors. The Prothrombin Time-International Normalized Ratio (PT-INR) of 2.05, 1.92, or 2.11, and Antithrombin III (AT III) of 24.50%, which were proposed as prognostic criteria for the HBV-SALF, non-HBVsubacute liver failure, non-HBV acute on chronic liver failure, and HBV acute liver failure subclasses, respectively. The incidence of HBV-LF is decreasing yearly. AT III, as a new prognostic criterion, has an excellent discriminative ability on the outcomes of the HBV-ALF subclass.
HBV; Liver failure; Prognostic; Antithrombin III; Acute on chronic liver failure
LF: Liver Failure; HBV: Hepatitis B Virus; HEV: Hepatitis E Virus; ALF: Acute Liver Failure; SLF: Subacute Liver Failure; ACLF: Chronic Acute Liver Failure; SCLF: Chronic Subacute Liver Failure; CLF: Chronic Liver Failure; M/F: Male/Female; HBV-LF: HBV-Related Liver Failure; SS: Spontaneous Survival; PT-INR: Prothrombin Time-International Normalized Ratio; TBil: Total Bilirubin; AT III: Antithrombin III; ROC: Receiver Operating Characteristic curves
Liver Failure (LF) is a clinical symptom of severe or chronic hepatic injury caused by various factors [1]. However, there is no consensus on the definition, classification, and prognostic criteria of LF subclasses in different countries [2,3]. For example, the classification system for Acute Liver Failure (ALF) includes the O'Grady system, the Bernuau system, and the Japanese system; all initially define liver failure as a severe liver injury, which may be reversible, and in the absence of pre-existing liver disease, hepatic encephalopathy may occur within eight weeks after the initial symptoms. Furthermore, a previous study reported that the prevalence and 30 days mortality rates in patients with Acute-on-Chronic Liver Failure (ACLF) determined by different criteria might be inconsistent worldwide. Given that LF is a complex pathophysiological process caused by multiple etiologies, it is difficult for treatment guidelines to include or address all of LF’s problems. Validation and comparative studies have shown that the European Consortium for the Study of Chronic Liver Failure (EASL-CLIF) criteria have better sensitivity for diagnostic and prognostic capacity, while the North American Consortium for the Study of End-Stage Liver Disease (NACSELD) is highly accurate in predicting mortality [4,5]. In addition, ACLF was first proposed by Japanese scholars in 1995, and through expert consultations between the EASL-CLIF and the Asia-Pacific Association for the Study of the Liver (APASL), a consensus for ACLF was finally confirmed in 2018 [6]. Based on our observations, the research team proposed a diagnostic criterion for the Chinese ACLF, almost identical to the APASL criteria [7,8].
The incidence of LF also varies with geography and time [9]. The etiology shows large geographical differences among countries, such as in the United Kingdom and the United States, mainly due to acetaminophen induced ALF. At the same time, Hepatitis B Virus (HBV) and Hepatitis E Virus (HEV) infections are the leading causes in Japan and India, respectively. The incidence of HBV varies widely in the Asia-Pacific region, and China has the highest rate of HBV infection [10]. The Chinese criteria divide the LF into ALF, Subacute Liver Failure (SLF), ACLF, Subacuteon- Chronic Liver Failure (SALF), and Chronic Liver Failure (CLF) subclasses which are also feasible for the exploration of effective treatment strategies. Xie, et al., analyzed the etiological classification in southwest China from 2000 to 2012 according to the Chinese criteria. Over the past two decades, with the use of birth-dose HBV vaccination and antiviral drugs in urban and rural communities in China, the incidences of HBV related cirrhosis, cancer, and LF may change [11,12]. The incidence of LF caused by HBV in Southwest China was 91.6%, but the survival rate increased yearly. You, et al., showed that the incidences of LF caused by HBV in northern China ranged from 86.5% to 69.2% between 2002 and 2011.
In recent years, there have been numerous reports on the etiologies of ALF and ACLF worldwide [13-15]. However, there are no data about the incidences, etiologies, and prognoses of various types of LF in Southeast China. Since 2018, the East coastal infectious diseases alliance of China has conducted annual surveillance of patients with LF according to the new diagnostic criteria. In recent decades, bilirubin has been a prognostic biomarker for short term death from ACLF, particularly for emerging interventions such as extracorporeal liver assist devices and early phase improvements in pharmacological therapies [16]. On the one hand, clinical and biochemical markers of liver dysfunction, such as the Child- Pugh classification, the End-Stage Liver Disease Model (MELD), and the King's standard (for ALF), analyze biochemical markers of liver synthesis function, such as Total Bilirubin (TBil), biliary globin and albumin, Prothrombin Time (PT), and the International Normalized Ratio (INR) to assess disease severity [17-19]. This study aimed to elucidate the clinical pathogenesis of patients with HBV-LF and non-HBV-LF in a retrospective cohort study and to develop new diagnostic criteria or prognostic criteria for the sub classical classification of LF diagnoses, such as age, the number of days of hospitalization, PT-INR, ALT, TBil, and Anti-thromboplastin III (AT III) levels. The cut-off value of these indicators helps determine the prognosis of LF subclass patients helps to promote etiological treatment and provides a theoretical basis for diagnostic criteria.
Participants and site descriptions
Retrospective cohort studies of LF were conducted through an annual survey of three liver centers in the Southeast coastal region of China, including three general tertiary hospitals in Wuxi, Shanghai, and Fujian. The medial records and demographic information of 689 LF patients from January 2018 to December 2020 were collected through a conventional medical record system. The inclusion criteria were as follows:
• Patients whose LF diagnosis met the criteria of the "guidelines
for the diagnosis and treatment of liver failure”.
• Patients’ ages >1 year.
• Patients who were hospitalized.
• Patients who were hospitalized for one day or more were
selected for efficacy and case mortality rate evaluations.
Death and survival: patients who died or survived during hospitalization, two weeks or six months post discharge. The survival rate of discharged LF patients was investigated by telephone follow-up at two weeks and six months post discharge.
The exclusion criteria were:
• Patients whose admission or discharge was not within this
time range.
• Patients with a lack of critical clinical, laboratory diagnostic
information in their medical records, and loss to-follow-up.
This study was conducted following the declaration of
Helsinki approved by the Fifth People's Hospital of Wuxi
clinical research ethics committee.
Demographic information and follow-up
Demographic data, such as the HBV-LF and non-HBV-LF etiological indicators, including sex, age, comorbidities, Prothrombin Time-International Normalized Ratio (PT-INR), TBil, AT III values, the number of days of hospitalization, the hepatic encephalopathy incidence, the number of patients who died during treatment or two weeks post discharge, and the number of patients who survived six months post discharge, were obtained from the database of the hospitals' clinical medical record systems and clinical laboratory centers. In addition, data on Spontaneous Survival (SS) after two weeks or six months were obtained from patients or legal surrogates through telephone follow-up.
Statistical analysis
GraphPad Prism 8.0.1 statistical software was used for data analysis. The population variables' frequency, distributions, and clinical outcomes were described proportionally, and the differences in the biochemical markers between the two groups were analyzed using the Mean ± Standard Deviation (X ± SD). Differences in the biochemical markers between the two groups were analyzed using Student's t-test, the Wilcoxon signed-rank test, and the Mann-Whitney test. Count data were analyzed using chi-square tests or rates of occurrence (in percentages). The factors affecting death from LF were analyzed by the multiple logistic regression calculation methods by SPSS statistics 22.0 software. The prognostic value of each clinical indicator for LF was analyzed by the Receiver Operating Characteristic (ROC) curve and Area Under the Curve (AUC) analysis. In all calculations, p-values less than 0.05 were considered statistically significant.
Proportions of LF patients in Southeast China
Between 2018 and 2020, we collected 689 LF patients’ information in 3 designated centers in southeast China. We removed LF patients who met the exclusion criteria, including 2 LF patients whose hospitalized days out of the study time frame, 3 LF patients due to incomplete clinical data, 47 LF patients due to loss of follow-up at 2 weeks and 6 months post discharge from hospital (n=52), and the remaining 637 LF patients were eligible (Figure 1A). After demographic and clinical analysis, HBVrelated LF (HBV-LF) was the dominant cause in each center (Figure 1B and 1C). Among the 637 patients, the number of patients in the HBV-LF group was significantly higher than in the non-HBV-LF group (~1.82-fold, 64.52% (411/637) vs. 35.48% (226/637)). According to the subclass classification, the three main subclasses of HBV-LF group were SALF (41.36%), ACLF (30.90%), and CLF (20.68%). The three main subclasses in the non-HBV-LF group were CLF (32.30%), SLF (24.78%), and SALF (22.57%) (Figure 1D). Therefore, HBV infection was the dominant cause of LF in Southeast China. The dominant subclasses of the two groups were HBV related SALF (HBVSALF) and non-HBV-related CLF (non-HBV-CLF) subclasses.
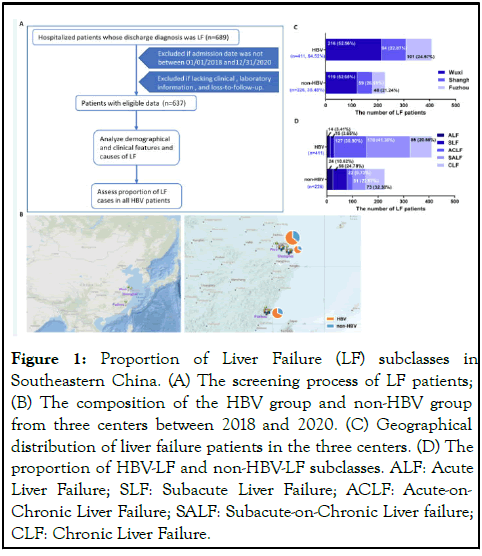
Figure 1: Proportion of Liver Failure (LF) subclasses in Southeastern China. (A) The screening process of LF patients; (B) The composition of the HBV group and non-HBV group from three centers between 2018 and 2020. (C) Geographical distribution of liver failure patients in the three centers. (D) The proportion of HBV-LF and non-HBV-LF subclasses. ALF: Acute Liver Failure; SLF: Subacute Liver Failure; ACLF: Acute-on- Chronic Liver Failure; SALF: Subacute-on-Chronic Liver failure; CLF: Chronic Liver Failure.
Incidences and Spontaneous Survival (SS rates of LF
The incidence and Spontaneous Survival (SS) rates and the proportion of ALF, SALF, and ACLF diagnoses changed year by year between 2000 and 2012 in southwest China 1. In our retrospective cohort study, the incidence of HBV-LF ranged from 72.36% to 59.74% between 2018 and 2020. Compared with 2018, the incidences of HBV-LF decreased significantly between 2019 (P<0.001) and 2020 (P<0.01). After obtaining the survival information of discharged patients (2 weeks), the SS rate in the HBV-LF group was up to 44.93% in 2019 and 63.64% in the non-HBV LF group in 2018. Compared to the non-HBV-LF group, the HBV-LF group showed significantly lower SS rates between 2018 and 2020, and the number of ACLF and SALF patients in the HBV-LF group was 2.50~8.50-fold higher. The number of CLF patients that occurred in both groups was similar. In general, the results suggest that the incidence of HBV-LF is decreasing yearly, and the incidence of ACLF and SALF is dominant (Table 1).
| Group | HBV | Non-HBV | |||||
|---|---|---|---|---|---|---|---|
| Year | 2018 | 2019 | 2020 | 2018 | 2019 | 2020 | |
| All patients, n (%) | Incidence a | 144 (72.36) | 138 (59.74) ¶ | 129 (62.32) § | 55 (27.64) | 93 (40.26) | 78 (37.68) |
| SS b | 63 (43.75) § | 62 (44.93) ¶ | 47 (36.43) ¶ | 35 (63.64) | 58 (62.37) | 46 (58.97) | |
| Subclass, n (%) c | ALF | 7 (4.90) | 3 (2.17) | 4 (3.10) | 6 (10.71) | 10 (10.75) | 8 (10.26) |
| SLF | 4 (2.80) | 8 (5.80) | 3 (2.32) | 9 (16.07) | 25 (26.88) | 22 (28.21) | |
| ACLF | 27 (18.88) | 50 (36.23) ¶ | 51 (39.53) ¶ | 5 (8.93) | 10 (10.75) | 6 (7.69) | |
| SALF | 77 (53.85) | 48 (34.78) ¶ | 45 (34.88) ¶ | 14 (25.00) | 19 (20.43) | 18 (23.08) | |
| CLF | 29 (20.28) | 29 (21.01) | 26 (20.16) | 21 (37.50) | 29 (31.18) | 24 (30.77) | |
| HBV/Non-HBV | ALF | 1.67 | 0.3 | 0.5 | N/A | N/A | N/A |
| SLF | 0.44 | 0.32 | 0.14 | N/A | N/A | N/A | |
| ACLF | 5.4 | 5 | 8.5 | N/A | N/A | N/A | |
| SALF | 5.5 | 2.53 | 2.5 | N/A | N/A | N/A | |
| CLF | 1.38 | 1 | 1.08 | N/A | N/A | N/A | |
Notes: SS: Spontaneous Survival; N/A: Not Available; ALF: Acute Liver Failure; SLF: Subacute Liver Failure; ACLF: Acute-on-Chronic Liver Failure; SALF: Subacute-on-Chronic Liver Failure; CLF: Chronic Liver Failure. a: Means a statistical comparison of the corresponding composition with 2018; b: Means a statistical comparison of the corresponding composition between the HBV-LF group and non-HBV-LF group; c: Means a statistical comparison of the subclass composition in each group by comparing with the ALF subclass; Statistical significant differences were analyzed using the Chi-square test. §, P<0.05; ¶, P<0.01.
Table 1: Incidences and spontaneous survival rates of LF patients.
Etiological component of the HBV-LF and non- HBV-LF
According to diagnostic guidelines, whether infectious or immune related diseases occurred first or LF appeared first. In addition, guidelines and related studies have confirmed that ACLF (or SALF) is a complex syndrome that presents with acute decompensation, organ failure (s), and high short term mortality and is often the focus of recent etiological studies [19,20]. LF surveillance data from the past three years showed that the leading four etiological components of HBV-LF were infection (59.61%), cirrhosis (37.71%), liver cancer (9.00%), and alcohol use (5.6%), while the leading four causes of non-HBV-LF were infection (34.96%), cirrhosis (28.32%), medication (16.81%) and alcohol abuse (9.73%) (Figure 2A). In both groups of LF, infection and cirrhosis in the HBV group were the main etiological component of the subclasses, particularly in ACLF, SALF, and CLF subclasses. Both groups had Primary Biliary Cirrhosis (PBC), a history of schistosomiasis, diabetes, liver cancer, alcoholic fatty liver or cirrhosis, Wilson's disease, HEV, and HCV. The ACLF and SALF subclasses had eight etiological components in the HBV group, and in the non-HBV group, the ACLF and CLF subclasses had 12 and 10 etiological components, respectively. However, the ALF subclass had the minor etiological components in both groups (Figure 2B).
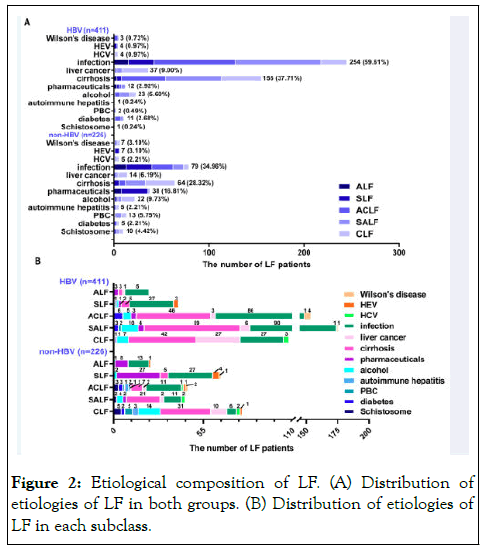
Figure 2: Etiological composition of LF. (A) Distribution of etiologies of LF in both groups. (B) Distribution of etiologies of LF in each subclass.
Demographic characteristics of HBV-LF and non- HBV-LF patients
After a demographic, clinical, therapeutic, and prognostic analysis of all LF patients, the demographic indicators showed that the overall sex ratio (M/F) in the HBV group was 4.55, and the highest sex ratio was in the CLF subclass (8.44). The sex compositions in the non-HBV group were significantly lower than that of the HBV group, and its highest sex ratio was also in the CLF subclass (1.61). Compared with ALF patients in the corresponding group, HBV patients with SLF and CLF were significantly older; non-HBV patients with ACLF and CLF were significantly older; Compared with ALF patients in the corresponding group, the clinical indicators showed that the number of days in hospitalization (SLF and CLF), ALT (ACLF, SALF, and CLF), and PT-INR (CLF) values in the HBV group were significantly lower; The TBil values were significantly higher in the HBV patients with SLF, ACLF, SALF, and CLF, while the AT III value was significantly lower in the HBV patients with SLF; The ALT (SLF and CLF) and PT-INR (SALF and CLF) values in the non-HBV group were significantly lower; The AT III value was significantly lower in the non-HBV patients with ACLF; The number of days in hospitalization (SLF, SALF, and CLF) and TBil value (SLF) in the non-HBV group were significantly higher. Compared with ALF patients in the corresponding group, the therapeutic and prognostic indicators showed that the incidences of hepatic encephalopathy were significantly higher in the HBV patients with ACLF (25.93%), SALF (35.19%), and CLF (31.48%); The rates of artificial liver support treatment were significantly higher in the HBV patients with ACLF (24.76%) and SALF (60.00%); the short term (during hospitalization and within two weeks post discharge) mortality rates were significantly higher in the HBV patients with ACLF (22.94%), SALF (45.29%), and CLF (26.47%). In the non-HBV group, the rate of artificial liver support treatment was significantly higher in the SLF patients (40.63%). In comparison, the short term mortality rate was significantly higher in the CLF patients (31.58%) compared with the ALF patients. Overall, consistent with the dominant subclasses, the highest mortality rates in the two groups were also in the HBV-SALF and non-HBV-CLF subclasses (Table 2) [21].
| Group | Class | ALF | SLF | ACLF | SALF | CLF | Total |
|---|---|---|---|---|---|---|---|
| (a) HBV | Male, n (%) | 11 (78.57) | 13 (86.67) | 104 (81.89) | 133 (78.24) | 76 (89.41) | 337 (82.00) |
| Female, n (%) | 3 (21.43) | 2 (13.33) | 23 (18.11) | 37 (21.76) | 9 (10.59) | 74 (18.00) | |
| M/F ratio a | 3.67§ | 6.50§ | 4.52* | 3.59¶ | 8.44* | 4.55* | |
| Age (years) b ` | 48.00 ± 4.03 | 52.93 ± 2.52¶ | 49.84 ± 1.17 | 49.82 ± 0.97 | 53.94 ± 1.14¶ | 50.11 ± 1.03 | |
| Hospitalization (d) b | 23.18 ± 5.21 | 17.73 ± 3.63§ | 22.12 ± 1.61 | 26.07 ± 1.59§ | 19.62 ± 1.68§ | 24.83 ± 1.32 | |
| ALT (IU/L) | 1533.00 ± 333.5 | 1012.00 ± 521.10 | 786.60 ± 746.70¶ | 836.20 ± 697.10¶ | 198.70 ± 1335.00* | 766.30 ± 767.00 | |
| PT-INR | 2.50 ± 0.34 | 2.32 ± 0.18 | 2.46 ± 0.05 | 2.17 ± 0.33 | 2.06 ± 0.44 * | 2.26 ± 0.25 | |
| TBil (μmol/L) b | 198.46 ± 24.65 | 342.41 ± 24.62& | 308.03 ± 18.05& | 298.69 ± 17.21¶ | 207.20 ± 18.18¶ | 298.04 ± 11.42 | |
| AT III (%) b | 30.33 ± 2.96 | 39.00 ± 5.96§ | 30.96 ± 2.31 | 29.72±1.55§ | 32.72 ± 2.90 | 31.70 ± 1.84 | |
| Encephalopathy, n (%) | 3 (5.56) | 1 (1.85) | 14 (25.93) ¶ | 19 (35.19) & | 17 (31.48) & | 54 (13.14) | |
| Artificial liver treatment, n (%) | 3 (2.86) | 6 (5.71) | 26 (24.76) & | 63 (60.00) & | 7 (6.67) | 105 (25.55) | |
| Mortality c, n (%) | 3 (1.76) | 6 (3.53) | 39 (22.94) & | 77 (45.29) & | 45 (26.47) & | 170 (41.36) | |
| (b) Non-HBV | Male, n (%) | 13 (54.17) | 29 (51.79) | 9 (40.91) | 29 (56.86) | 45 (61.64) | 125 (51.31) |
| Female, n (%) | 11 (45.83) | 27 (48.21) | 13 (59.09) | 22 (43.14) | 28 (38.36) | 101 (44.69) | |
| M/F ratio | 1.18 | 1.07 | 0.69 | 1.32 | 1.61 | 1.24 | |
| Age (years) b | 51.92 ± 3.66 | 54.69 ± 1.53 | 58.96 ± 2.86§ | 53.15 ± 2.47 | 57.45 ± 1.56 § | 56.18 ± 1.01 | |
| Hospitalization (d) b | 14.26 ± 2.33 | 22.91 ± 1.99§ | 14.26 ± 3.31 | 28.00 ± 2.88¶ | 25.68 ± 2.24¶ | 23.07 ± 1.16 | |
| ALT (IU/L) | 601.80 ± 50.29 | 551.50 ± 186.50§ | 646.7 ± 44.97 | 450.50 ± 151.20 | 85.19 ± 516.60 | 440.00 ± 161.70 | |
| PT-INR | 2.46 ± 0.56 | 2.27 ± 0.20 | 2.31 ± 0.15 & | 1.977 ± 0.49 | 1.94 ± 0.52 | 2.15 ± 0.31 | |
| TBil (μmol/L) b | 276.43 ± 29.49 | 353.68 ± 22.21 § | 274.06 ± 42.79 | 289.01 ± 35.61 | 223.70 ± 20.30 | 281.14 ± 12.39 | |
| AT III (%) b | 35.86 ± 4.64 | 33.80 ± 2.82 | 26.20 ± 5.95 § | 31.33 ± 3.13 | 35.98 ± 2.31 | 34.76 ± 1.47 | |
| Encephalopathy, n (%) | 5 (15.15) | 11 (33.33) | 1 (3.03) | 8 (24.24) | 8 (24.24) | 33 (14.60) | |
| Artificial liver treatment, n (%) | 8 (12.50) | 26 (40.63) & | 7 (10.94) | 15 (23.44) | 8 (12.50) | 64 (28.32) | |
| Mortality c, n (%) | 14 (14.74) | 22 (23.16) | 11 (11.58) | 18 (18.95) | 30 (31.58) ¶ | 95 (42.04) |
Notes: The normal reference value of Anti-thromboplastin III (AT III) is 60%-120%; the normal reference value of Total Bilirubin (TBil) is 5-21 μmol/L; the normal reference value of Alanine Aminotransferase (ALT) is 10-40 IU/L, and the normal reference value of the Prothrombin Time-International Normalized Ratio (PT-INR) is 5-21. The data in the table are the primary results of clinical testing at the time of admission; M: Male; F: Female; ALF: Acute Liver Failure; SLF: Subacute Liver Failure; ACLF: Acute-on-Chronic Liver Failure; SALF: Subacute-on-Chronic Liver Failure; CLF: Chronic Liver Failure.
a: Statistical differences in sex composition compared with the non-HBV group.
b: Mean ± SD.
c: Death during hospitalization or within 2 weeks after discharge.
Statistical analyses of significance were compared to ALF subclass in the corresponding group. Statistical significant differences were analyzed using the unpaired t-test (Mann-Whitney test) and Chi-square test; §, P<0.05; ¶, P<0.01; &, P<0.001; *, P<0.0001.
Table 2: Demographic and clinical indicators of the LF patients.
Prognosis analysis in HBV-LF and non-HBV-LF patients
LF is an acute deterioration of diseases such as cirrhosis, with multiple organ failure and high short-term mortality, and even some patients may undergo liver transplantation, which needs a longer period to know the outcome. Therefore, a telephone follow-up of survival status was carried out six months post discharge. The initial ALT, PT-INR, TBil, and AT III values were used as a reference for the clinical prognosis of the two LF groups. Regardless of the survival or death of patients, the sex ratio (M/F) in the non-HBV-LF group showed more men than women, but the sex ratio was higher in the HBV-LF group (Figure 3A). The age of HBV-LF patients who died were significantly higher than those of the survivors; The number of days of hospitalization in the dead HBV-LF patients was significantly lower than that of the survivors, and the initial TBil values of HBV-LF patients who died was significantly higher than those of the survivors. In addition, the age and PT-INR values of non-HBV-LF patients who died were significantly higher than those of the survivors; while AT III levels were significantly lower (Figure 3B). The HBV-LF group had a relatively high mortality rate of hepatic encephalopathy (82.98%) and a high rate of treatment with artificial liver support systems (67.62%).
The cirrhosis mortality rate in the HBV-LF group (58.23%) was higher than that of the non-HBV-LF group (50.00%). The mortality rate of liver cancer patients in the HBV-LF group (94.87%) was higher than that in patients in the non-HBV-LF group (87.50%) (Figure 3C). Thus, patients who died in the HBV-LF group were older, spent fewer days in the hospital, and had higher TBil levels, which may be used to evaluate their risk of death. However, patients who died in the non- HBV-LF group displayed relatively older and higher PT-INR values, and lower AT III levels, which may serve as prognostic factors in the evaluation [22-25].
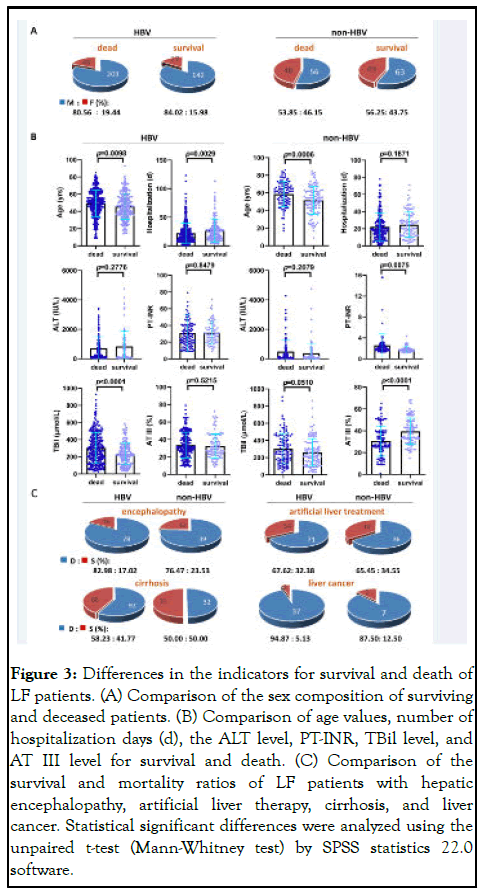
Figure 3: Differences in the indicators for survival and death of LF patients. (A) Comparison of the sex composition of surviving and deceased patients. (B) Comparison of age values, number of hospitalization days (d), the ALT level, PT-INR, TBil level, and AT III level for survival and death. (C) Comparison of the survival and mortality ratios of LF patients with hepatic encephalopathy, artificial liver therapy, cirrhosis, and liver cancer. Statistical significant differences were analyzed using the unpaired t-test (Mann-Whitney test) by SPSS statistics 22.0 software.
Logistic regression analysis of risk factors in HBV-LF and non-HBV-LF patients
To further analyze the significance of clinical indicators on the preliminary judgment of the prognosis, logistic regression analysis showed that the PT-INR of the HBV-SALF subclass (OR=7.999, 95% confidence interval (95% CI): 2.112-30.291, P=0.002) was an independent risk factor affecting the patient’s prognosis; The AT III level of the HBV-ALF subclass (OR=0.724, 95% CI: 0.526-0.995, P=0.047) was an independent risk factor affecting the patients’ prognosis; And the PT-INR of the non-HBV-SLF (OR=6.931, 95% CI: 2.315-20.750, P=0.001) or non-HBV-ACLF (OR=37.415, 95% CI: 1.811-773.011, P=0.019) subclass was an independent risk factor affecting the patient’s prognosis (Supplementary Table 1). Therefore, PT-INR or AT III as an essential indicator may become the prognostic criteria for HBV-SALF, HBV-ALF, non- HBV-SLF, and non-HBV-ACLF subclasses.
Predictive value of the ALT, PT-INR, TBil, and AT III for prognosis in HBV-LF and non-HBV-LF patients
To analyze the value of the above prognostic factors, we needed to calculate the ROC of the critical indicator. The AUC of the ROC for the PT-INR in the HBV-SALF subclass was 0.726 (95% CI: 0.612-0.840, P=0.001, sensitivity: 57.14%, specificity: 84.62%, cut-off: 2.05). Furthermore, the AUC of the ROC curve for AT III levels in the HBV-ALF subclass was 0.814 (95% CI: 0.596-1.000, P=0.032, sensitivity: 71.43%, specificity: 90.00%, cut-off: 24.50%). However, the AUC values for the PTINR in the non-HBV-LF-SLF and non-HBV-ACLF subclass were 0.786 (95% CI: 0.591-0.886, P=0.007, sensitivity: 65.00%, specificity: 79.17%, cut-off: 1.92) and 0.609 (95% CI: 0.596-0.943, P=0.004, sensitivity: 68.10%, specificity: 100.00%, cut-off: 2.11), respectively. Therefore, when the critical value of PT-INR is 2.05 or AT III is 24.50%, this indicator has a predictive value for the prognosis of HBV-SALF or HBV-ALF subclasses, respectively. When the cut-off value of PT-INR is 1.92 or 2.11, this indicator has a predictive value for the prognosis of non-HBV-LF-SLF or non-HBV-ACLF subclass, respectively. Other indicators and ROC values in these subclasses did not show significant predictive values (Supplementary Table 2) (Figure 4).
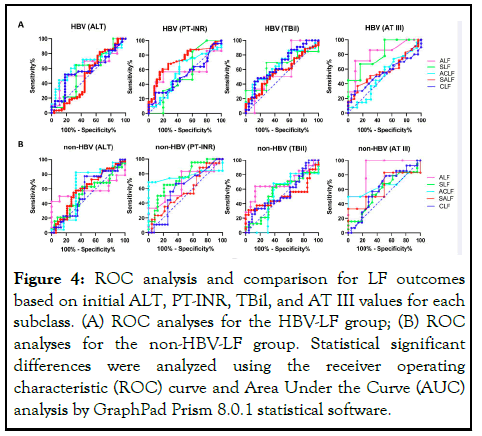
Figure 4: ROC analysis and comparison for LF outcomes based on initial ALT, PT-INR, TBil, and AT III values for each subclass. (A) ROC analyses for the HBV-LF group; (B) ROC analyses for the non-HBV-LF group. Statistical significant differences were analyzed using the receiver operating characteristic (ROC) curve and Area Under the Curve (AUC) analysis by GraphPad Prism 8.0.1 statistical software.
To our knowledge, this study is the first systematic investigation of the etiological composition or prognostic criteria for each LF subclass. In this multicenter retrospective cohort study, the department of infectious diseases of the Fifth People's Hospital of Wuxi (Wuxi infectious disease hospital) is the designated LF treatment center of Wuxi city. It is the central unit of "one city and one center for non-biological artificial liver treatment". The number of patients is relatively small at two participating tertiary general hospitals in Southeastern China, where LF patients are also being treated in other public hospitals. In recent decades, there have been limited reports on the proportions of HBV-LF in Southeast China, so it is impossible to speculate on the morbidity or mortality trends of HBV-LF. In some studies conducted in Southwestern and Northern China, the incidences of HBV-LF were 91.6% (years: 2000-2012) and 69.2% (years: 2002-2011), respectively, whereas, in our study, it was 64.52% (years: 2018-2020). According to statistical analysis in Southwest China, 87.3% of ACLF patients were mainly caused by HBV infection, which is the leading cause of SALF and ACLF. Current guidelines and recommendations suggest that the ACLF data include the ACLF and SALF subclasses, and we used consistent classification criteria for calculation and comparison. In contrast, our ACLF incidence rate in this study was reduced to 80.27% (297/370, recalculated based on Figure 1C), and our data were lower than those in Southwest China a decade ago.
The morbidity and mortality rates of LF are affected by regional economic development and medical services. Southeast China is a region with a developed economy and medical technology, so further assessment of the morbidity and mortality rate of HBVLF in Southeastern China and an understanding of the economic burden of health care are needed. Similar to previous data in Northern and Southwestern China, the short term mortality rate in HBV-LF patients is significantly higher than in non-HBV-LF patients. However, it has been shown that our overall SS rate is higher than before 2012. That is, timely intervention is the key to preventing deaths and obtaining a successful treatment. Therefore, if the subclasses of LF can be predicted and diagnosed earlier, the timely use of artificial liver support will help to reduce the incidence of hepatic encephalopathy and death. LF can be induced by liver diseases of various etiologies, resulting in impaired or decompensated liver function and alterations in its composition, detoxification, drainage, biotransformation function, and other abnormalities. LF patients have different causes or trigger factors, including hepatotropic viruses, drugs, alcohol, genetic disorders, and cirrhosis. In the past 30 years, with the explosive growth of China's economy and the improvement of social openness, alcohol consumption has significantly improved, and alcoholic cirrhosis has shown an upward trend growth. The outcome of alcohol-related cirrhosis may be different from HBV related cirrhosis. Alcoholic cirrhosis is more likely to lead to hepatic encephalopathy and LF. Patients with HBV-related cirrhosis are at increased risk of liver cancer and hypersplenism. Similarly, the highest proportion of the sex ratio (M/F) of LF patients in our study was 76:9 (8.44), occurring in the HBV-CLF subclass, which predominantly had underlying diseases cirrhosis and liver cancer. However, the incidence of non-HBV-LF was similar to other countries, caused by pharmaceuticals, Chinese herbal medicines, anti-tuberculosis drugs, infections, and alcoholism.
Our study also has several important findings. First, age, the number of hospitalization days, PT-INR, and AT III values are likely to be used as prognostic criteria for the outcomes of LF subclass patients. In addition, drug or alcohol induced non- HBV-LF patients who received early non-biological artificial liver support therapy recovered more quickly than the HBV-LF group. However, our previous study showed that the success rate of nonbiological artificial liver therapy in LF individuals reached only 55.56%, and cirrhosis and liver cancer remain the leading causes of LF death. Furthermore, our study showed that SALF was the main subclass of the HBV-LF group in Wuxi, with the incidence of HBV-LF decreasing from 8.36% in 2018 to 6.24% in 2020 (Supplementary Table 3).
Accumulating studies have demonstrated the value of clinical predictive markers or mortality models for the outcomes of ACLF patients, including the TBil, MELD score, PT-INR, and Neutrophil-Lymphocyte Ratio (NLR) values. The MELD score is accepted worldwide as an effective and reliable indicator of prognosis for LF patients and is used to assess the entire course of treatment. However, there was a lack of detailed predictive evaluation for each LF subclass. Furthermore, because the definition of ACLF varies in Eastern and Western countries, the triggering event and prognosis may also be different. Hence, this study aims to calculate meaningful diagnostic criteria by analyzing a single cause. In addition, based on previous studies, the Neutrophil-Lymphocyte Ratio (NLR) has been identified as a potential marker of HBV-LF survival and prognosis. Here, we calculated the ALT, PT-INR, TBil, and AT III values and found that the PT-INR value (≥ 2.05, AUC=0.726) in the HBV-SALF subclass showed a higher ROC curve that could be used as a predictive indicator of outcomes for patients with HBV-SALF (sensitivity of 57.14%, specificity of 84.62%). In addition, the PT-INR value can also be used as a predictive indicator of outcomes for patients with non-HBV-SLF (≥ 1.92) or non-HBVACLF (≥ 2.11). However, the AT III level (≤ 24.50%) can be used as a predictive outcome indicator for patients with HBV-ALF. Therefore, the above initial indicator values can be used as a new reference for the prognosis of outcome for each LF subclass patient. In summary, the AT III value as a prognostic criterion for the LF subclass is also the first time to propose compared with the previous studies.
This study has provided evidence that the initial PT-INR or AT III value may be a potential prognostic indicator for different LF subclass patients. The limitation of our retrospective study is that after dividing these patients into subclasses, the number of people is significantly decreased. In our study, the AUC of the AT III value in the HBV-ALF subclass was higher than 0.8, this indicator can be considered excellent. However, the AUC of the PT-INR value in HBV-SALF, non-HBV-SLF, or non-HBVACLF subclass was higher than 0.6, so the prognostic criteria of PT-INR had relatively limited clinical significance in the LF subclass patients. At the same time, AT III as the prognostic criterion has not been explored in the prediction of the LF subclass patients. Therefore, this indicator is worth further application in the LF patients’ clinical tests. Furthermore, these indicators, combined with the LF patients' age, days of hospitalization, or TBil value, may become a more objective criterion for assessing their prognosis.
We would like to thank the Wuxi municipal statistical information center for providing technical support for clinical medical data search.
This work was funded by the national natural science foundation of China (81701550, 81670528), the top talent support program for young and middle aged people of the Wuxi health committee (BJ2020094), the innovation team for infectious diseases of the Wuxi health committee (CXTD2021009), and the key laboratory of medicine of Wuxi Municipal health commission (ZDXK2021006).
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
YY and RM contributed to the study concept, design, and analysis. CL, JH, NK, LG, DC, DZ, RM, and YQ collected the information and statistical analysis. YY drafted the manuscript, and DZ, RM, and YQ checked, edited, and finalized the manuscript. All authors read and approved the final manuscript.
The study protocol was approved by the ethics committee of the fifth people's hospital of Wuxi (no. 2021-007-1) and performed in accordance with the latest version of the declaration of Helsinki and guidelines. Using the medical record data obtained from previous clinical diagnosis and treatment for research, the patient information was kept strictly confidential, and the informed consent waiver was determined by the ethics committee of the fifth people's hospital of Wuxi.
Not applicable.
There was no financial support for the research or conflicts of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Yan Y, Lyu C, Han J, Kang N, Davgadorj C, Ge L, et al. (2023) Etiology and Prognostic Criteria of Liver Failure in Southeast China: A Multicenter Retrospective Cohort Study between 2018 and 2020. Virol Myco. 13:281.
Received: 19-May-2023, Manuscript No. VMID-21-24304; Editor assigned: 22-May-2023, Pre QC No. VMID-21-24304 (PQ); Reviewed: 05-Jun-2023, QC No. VMID-21-24304; Revised: 24-Oct-2023, Manuscript No. VMID-21-24304 (R); Published: 26-Mar-2024 , DOI: 10.35248/2161-0517.24.13.281
Copyright: © 2024 Yan Y, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.