Journal of Thyroid Disorders & Therapy
Open Access
ISSN: 2167-7948
ISSN: 2167-7948
Research Article - (2021)Volume 10, Issue 6
Hashimoto’s disease (HD) is an autoimmune thyroid disease often leading to hypothyroidism. The current therapy for these patients consists in traditional Hormone Replacement Therapy (HRT) to compensate inadequate thyroid function. However, clinical management of this and other autoimmune disorders usually dismisses the etiologic component, even when the gold-standard pharmacological treatment is not effective and/or the patient remains asymptomatic. Here, we propose to dig into the etiopathogenic factors as an attempt to unravel patient’s negative response to therapy and to improve therapeutic and preventive approaches as standard medical practice as well as to complement HRT. These factors may include genetic susceptibility, intestinal permeability defects, other pathologies like celiac disease, gluten intolerance and biopsychosocialstress, gender, sex hormones and micronutrients (vitamin D, iodine and selenium). Exposure to environmental toxic substances (heavy metals and others) and infectious agents (both viral and bacterial) may also trigger HD development. Finally, given the implication of multiple mechanisms of etiology and pathogenesis, a multidisciplinary strategy may be required to deliver integrated and successful patient care.
Hashimoto’s disease; Autoimmune disease; Thyroid destruction; Genetic susceptibility; Environmental factors
Hashimoto’s disease (HD); Anti-Thyroid Peroxidase Antibodies (anti-TPO); Anti-Thyroglobulin Antibodies (anti-Tg); Hormone Replacement Therapy (HRT); Thyroglobulin (Tg); Small Intestinal Bacterial Overgrowth (SIBO); Bisphenol A (BPA); Glutathione-S-Transferase (GST); Celiac Disease (CD); Non-Celiac Gluten Sensitivity (NCGS); T helper (Th); Thyrotropin- Releasing Hormone (HRT)
Autoimmune thyroiditis or Hashimoto's Disease (HD) is an autoimmune disease characterized by an autoantibody-mediated destruction of the thyroid gland (anti-thyroid peroxidase (anti- TPO) and anti-thyroglobulin (anti-Tg) antibodies) via and immune response mediated by T-lymphocytes. Although this leads to distinct levels of thyroid hypofunction, clinical manifestations may range from diffuse or nodular goiter with euthyroidism, subclinical hypothyroidism and permanent hypothyroidism [1].
HD was named after the Japanese physician Hakaru Hashimoto who made its first description in 1912 and referred to it as lymphomatous goiter [2]. Nowadays, autoimmune hypothyroidism has achieved estimated rates of incidence as high as 2.2/100,000/year (males) and 498.4/100,000/year (females) worldwide and they will continue to increase. The same trend is observed for all types of autoimmune thyroid disease, for which women have a higher incidence compared to men [3].
The hypothesis
As a result of this exponentially increased incidence, much attention has been drawn to the etiopathogenesis of autoimmune diseases [4]. However, the current diagnostic and therapeutic approach for HD takes into consideration the hormonal deficit upon glandular destruction almost exclusively, disregarding the possible pathogenic mechanisms that may be involved in the initiation and maintenance of the disease [5]. This way, conventional Hormone Replacement Therapy (HRT), including periodical follow-up and dose adjustment, still constitutes the first-line treatment, despite its poor efficacy in some patients [6-7]. In most patients, traditional HRT is able to relieve hypothyroidism symptoms. Nevertheless, an important percentage of them remain symptomatic in spite of proper patient adherence [6-7]. Indeed, some studies have reported that in up to 15% of the patients with normal Thyroid Stimulating Hormone (TSH) values, T3 levels fall below the normal range, which may be an explanation for the persistence of symptoms [8-9]. A possible mechanism behind this observation may be a deficient T4 thyroid hormone conversion into biologically active T3 in peripheral tissues, the so-called “functional hypothyroidism”. As a result, despite minimal intracellular T3 concentration, in the absence of plasmatic T3 fluctuations, the hypothalamuspituitary- adrenal axis feedback mechanism will not be triggered, thereby TSH concentration will remain stable [10].
Hence, we hypothesise that HD is caused by a combination of etiologic and susceptibility factors and that these factors are associated to the development and persistence of the disease and also in the persistence of symptoms in apparently correctly treated patients. In light of this, we propose to explore the etiologic factors surrounding HD to improve disease prophylaxis, to better understand treatment-refractory clinical cases, and to eventually design more precise and personalized therapeutic approaches, alone or in combination with hormone replacement methods. This could be the basis of a novel integrative therapeutic approach and an effective complement to the usual repositioning treatment.
Evaluation of the hypothesis
In general, three main factors have been involved in the development of autoimmune diseases: A genetically altered immune system, a reactive mucous membraneassociated immunity (mucosal immunity) against environmental antigens, and an increased intestinal permeability that promotes their entry into the systemic circulation [11-12] (Figure 1). Similarly, HD is driven by the following suggested key factors (Figure 2).
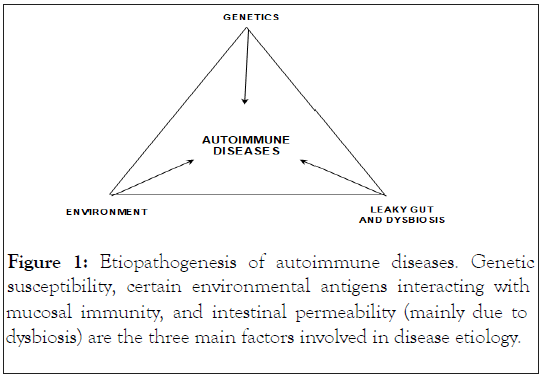
Figure 1: Etiopathogenesis of autoimmune diseases. Genetic susceptibility, certain environmental antigens interacting with mucosal immunity, and intestinal permeability (mainly due to dysbiosis) are the three main factors involved in disease etiology.
Figure 2: Key factors involved in Hashimoto’s disease. Several and non-mutually exclusive factors may be linked to the disease origin and development. CD, celiac disease.
Genetic factors: Multiple susceptibility genes may be involved in HD pathogenesis. Some of them are common among other autoimmune diseases, while certain genes are specific for thyroid autoimmunity. The first genetic locus identified in association with HD was the region of the major histocompatibility complex (or HLA). In Caucasian individuals, different HD subtypes have been associated with certain HLA. Alleles including DR3, DR5, DQ7, DQB1*03, DQw7 or the DRB1*04-DQB1*0301 haplotype. The associations with DRB4*0101, HLA-A2 and DRw53 and with DRw9 have been proven in the Japanese and Chinese population, respectively [13]. CTLA-4, PTPN22 and VDR genes also play an important role in the etiology of HD, other autoimmune diseases and, in the case of VDR, organspecific pathologies such as type-1-diabetes or Addison's disease. Nevertheless, Thyroglobulin (Tg) gene is the only HD-associated thyroid specific gene known so far, which also confers susceptibility to Graves-Basedow disease [13].
Intestinal permeability, dysbiosis and small intestinal bacterial overgrowth (SIBO): Intestinal permeability occurs when the intestinal barrier function is lost. In physiological conditions, it selectively allows nutrient, electrolyte and water absorption while preventing the entry of intraluminal toxins, foreign antigens and microorganisms [14]. Physical components, such as intercellular tight junctions, as well as biochemical and immunological factors are highly coordinated to maintain this intestinal barrier system. However, when integrity is compromised, harmful substances may cross the epithelium, disseminate through the bloodstream and lymphatic system and eventually affect tissue homeostasis [14]. Intestinally derived inflammatory mediators could lead to a systemic inflammatory and autoimmune response. Thus, increased intestinal permeability due to multiple putative causes has been postulated an essential trigger of an autoimmune response [14-15] (Table 1).
| Risk factors associated with increased intestinal permeability [12,15,17] |
|---|
| Intestinal inflammation |
| Oxidative stress |
| Stress |
| Nonsteroidal anti-inflammatory drugs |
| Alcohol consumption |
| Cow’s milk intolerance |
| SIBO |
| Sepsis |
| Pancreatic insufficiency |
| Intestinal infections |
| Obstructive jaundice |
| Surgery |
Table 1: Risk factors associated with increased intestinal permeability.
A healthy gut microbiota composition is also crucial to prevent intestinal permeability, although it may be altered by a diet rich in saturated fats and poor in fibre, the presence of environmental toxins and psycho-emotional stress [14]. Immune and dendritic cell dysregulation is one important consequence of dysbiosis-associated increased permeability. In fact, dendritic cells have a role in oral tolerance through the induction of specific integrin and chemokine receptors and their involvement in autoimmune thyroiditis have been shown [16]. Interestingly, the presence of intestinal dysbiosis have been associated to other conditions such as inflammatory bowel disease and/or type 1 diabetes [16]. Small Intestinal Bacterial Overgrowth (SIBO) is characterized by the presence of an abnormally high number of coliform bacteria in the small bowel, in conjunction with several gastrointestinal symptoms as diarrhoea, constipation and meteorism [17]. An increased prevalence of SIBO has been demonstrated in patients with autoimmune diseases such as multiple sclerosis, type 1 diabetes, and rheumatoid arthritis [18-20]. SIBO has been associated with GI’s dysmotility and hypochlorhydria and a can also induce intestinal permeability. Therefore, perpetuating the leakage of luminal toxins and inflammatory mediators associated with autoimmune disorders [21]. Additionally, a worsening of thyroid function parameters and an increase in anti-TPO antibodies it has also been observed in the presence of SIBO, therefore contributing with the induction of autoimmunity and hypothyroidism [22]. Furthermore, hypothyroidism and levothyroxine treatment have been both linked to the induction of SIBO and gastrointestinal dysmotility [23-24].
Infectious agents: Microorganism infections may be the leading cause of HD. Several types of microorganisms have been identified and at least 5 different mechanisms have been proposed for microorganism-induced autoimmunity. The main ones are thyroid protein molecular mimicry and the thyroid’s gland immune attack mediated by superantigens [25].
The Epstein Barr Virus (EBV) is one of the most important players in autoimmune thyroiditis onset and persistence of symptoms [26-29], both during the primary infection and upon reactivation and/or viral chronification. Increased activation of EBV in HD patients seems related to a CD8 T-cell deficiency, which underpins disease progression [30]. Additional microorganisms have been also involved such cytomegalovirus, Herpes Simplex and hepatitis C virus, B19 parvovirus, Yersinia enterocolitica, Borrelia Burgdorferi, Candida albicans and Toxoplasma gondii [31-38].
Exposure to environmental toxic substances: A growing pile of evidence supports the role of toxic substances as other important environmental triggers of autoimmune thyroiditis [39]. Exposure to Polychlorinated Biphenyls (PCB), dioxin and heavy metals has been linked to high levels of anti-Tg and anti- TPO autoantibodies [39]. Detection of Bisphenol A (BPA) in plasma has also been correlated with anti-TPO production [40]. In urine, an increased phthalate and BPA concentration has been associated with an alteration of circulating thyroid hormones [41]. Finally, exposure to phthalates and an exacerbation of autoimmune thyroid disease occurred concomitantly [42].
Environmental toxins may damage the intestinal mucosa and directly interact with follicular thyroid and immune cells. From a molecular point of view, although heavy metals exhibit distinct toxicity mechanisms, they all ultimately induce oxidative damage through the formation of Deoxy Ribonucleic Acid (DNA) and/or protein adducts, thereby impairing key respiratory enzyme activities [43]. Plasma and urine levels of mercury are not proportional to its detrimental effects in some autoimmune disorders; however in HD they are directly connected to lowered thyroid hormone concentration and the presence of anti-Tg antibodies in plasma [44-47]. Thyroid peroxidases, which catalyze the synthesis of T3, T4 are potent thyroid antioxidants which function depends on selenium concentration. Due to its extremely high affinity, mercury binds to selenium acting as an important chelator of selenium. Therefore, in the event of chronic mercury intoxication, selenium bioavailability may be reduced, consequently causing extensive cytotoxicity [48]. In addition, mercury impairs the protective antioxidant function of glutathione [49]. Autoantibodies are typically found in patients with other autoimmune diseases who have been chronically exposed to mercury. Specifically, it seems that inorganic mercury would enhance the production of autoimmune disease markers to a greater extent than organic forms [44]. However, the impact of mercury exposure on different clinical variables is a question that still remains unanswered, with studies showing conflicting results [50]. In recent years, increased susceptibility to mercury and BPA prenatal exposure have been attributed to certain genetic polymorphisms, being the Glutathione-S-Transferase (GST) GSTT1-/- and GSTM1-/- polymorphisms especially relevant as they hamper toxin excretion [51,52].
Vitamin D deficiency: A recent meta-analysis demonstrates a clear vitamin D deficiency in patients with HD [53], which may favour autoimmunity mechanisms such as the loss of tolerance to self-antigens, the production of autoantibodies and direct cell damage [54].
Celiac disease/Gluten intolerance: There is an increased prevalence of Celiac Disease (CD) in patients with autoimmune thyroid disease as revealed by a recent meta-analysis [55]. This could be partially explained by the increased immunosensitivity of celiac patients, as part of an autoimmune polyglandular syndrome, by a CD malabsorption-associated iodine and selenium deficiency or due to antibodies affecting both intestinal and thyroid tissues [4]. In addition, in non-celiac patients, gluten (gliadins and glutenins) and other wheat proteins are capable of generating intestinal permeability by directly damaging the intestinal mucosa [56-57]. The Non-Celiac Gluten Sensitivity (NCGS), also known as gluten intolerance or sensitivity to gluten, shares some clinical symptoms with irritable bowel syndrome in addition to some systemic ones such as headaches, arthromyalgia, asthenia, and mood alterations. Its prevalence ranges between 0.6%-6% of the population and diagnosis is established once CD and wheat allergy have been ruled out, i.e. patients do not present typical antibodies or pathognomonic lesions of CD in the intestinal mucosa. Still, a gluten-free diet leads to regression of the symptoms [58]. Interestingly, NCGS has been associated with the onset of HD and the consequent elevation of antithyroid antibodies. In fact, some of the symptoms of NCGS may also occur during the course of HD [59].
Excess of iodine and/or low Selenium:Iodine ratio: Iodine is absolutely required for thyroid hormone synthesis. It comes as no surprise, therefore, that insufficient iodine intake is directly correlated with a weakened hormone production. Excess iodine, however, may also lead to adverse effects depending on the underlying thyroid function, as well as the extent and duration of the excessive intake. In this regard, the rise in thyroid autoantibodies, and even thyroid autoimmune disease, may be triggered by iodine supplementation [60-63]. A similar response to iodine has been reported in studies using mice genetically predisposed to thyroid autoimmunity.
The mechanism of HD occurrence in response to iodine supplementation is not fully understood. Several mechanisms have been hypothesized such as greater immunogenicity of iodinated thyroglobulin and a direct toxic effect of iodine on thyroid cells through the increased generation of oxygen free radicals, which are byproducts of iodine peroxidation during thyroid hormone synthesis [60-63]. Glutathione peroxidase and thioredoxin oxidase are selenium-dependent enzymes that protect tissues from the harmful effect of reactive oxygen species (especially hydrogen peroxide) [63]. An excess of iodine in the context of selenium deficiency seems to reduce the activity of these and other selenoproteins [64-65] and, consequently, to enhance the accumulation of free radicals and to promote an inflammatory process within the thyroid tissue [63]. Nevertheless, their enzymatic activity may be reversed upon selenium administration [64]. A low (Selenium:Iodine) ratio also affects autoantibody production and causes a shift in the population of immune cells towards a deficit of regulatory T lymphocytes. Again, both effects may be restored by selenium supplementation [64,66-68].
Gender and hormonal factors: Gender greatly influences the development of autoimmune diseases. Numerous epidemiological studies have shown a prevalence of up to 3:1 (female:male) [69]. In a report by the National Health and Nutrition Examination Survey (NHANES), the prevalence of anti-TPO and anti-Tg levels above the normal range was significantly higher in women than in men (42.1% vs. 36.1% and 9.6% vs. 5.8%, respectively) [70]. This gender gap could be explained by a number of factors like the differences between male and female immune systems that make women more prone to react to certain antigens, which may in turn be sustained by hormonal differences. Therefore, gender bias in autoimmunity may also be explained by differences in reproductive function (puberty, pregnancy and menopause) [70]. Estrogens affect immunity by modulating the development and function of lymphocytes and promoting cytoprotection. During the onset of HD, an adequate interaction among thyroid, antigen-presenting and T cells is necessary [71]. As a result, specific cytokines produced by the innate immune system are able to influence the differentiation of bipotential T helper (Th) cells preferentially towards Th1 (cellular immunity) and not Th2 (humoral immunity) cells [69]. At this stage though, estrogens are capable of suppressing the Th1-mediated responses while Th2 activity is enhanced [70]. Hence, estrogens may have a protective role in Th1-dependant diseases like HD, yet they would tend to worsen antibody-mediated pathologies [70]. During pregnancy, there is an exponential increase in estradiol, which would reduce the Th1-mediated response. An increase in regulatory T lymphocytes also takes place, which induces immune tolerance and suppression of autoimmunity [70]. Most notably, reduced anti-TPO and anti-TG levels during pregnancy have been demonstrated, reaching the lowest values during the third trimester [72]. However, during the postpartum estrogen levels drop thus leading to higher Th1-mediated disease risk. Certainly, HD is more frequently diagnosed at this stage [73-74].
Prolactin is known for its proinflammatory effects and it also fluctuates during pregnancy. Although an exacerbation of the disease would be expected, studies have found no correlation between hyperprolactinemia and HD [75]. Other sex hormones, namely progesterone and androgens, exert anti-inflammatory and immunosuppressive effects, respectively [70]. Consequently, they would have a protective role against autoimmunity. Nevertheless, a positive effect of androgens on Th2 responses has also been described [76]. Other factors may influence autoimmune disease susceptibility such as paternal/maternal inheritance (including mitochondrial inheritance, genomic imprinting and sex chromosome inactivation), genetic predisposition and environmental stimuli through epigenetic mechanisms. The role of microchimerism, i.e. the trafficking of cells between the mother and the fetus, in HD needs to be further explored [70].
Stress: Chronic biopsychosocial stress negatively shapes health over the life span by contributing to chronic inflammation processes and infection risk [77]. However, the exact mechanisms by which stress would interact with autoimmune disease pathophysiology have not been fully elucidated. Some studies suggest that, in cases of high stress-mediated hormonal activity, T-cell balance may be skewed toward Th2 (thus increasing Graves-Basedow’s disease risk), whereas Th1 function may be deficient. In other words, immunitymay be mainly humoral [78-79]. On the other hand, other studies consider that, upon stress relieve, the decrease of the immunosuppressive effects may induce a rebound autoimmune reaction that could affect the thyroid gland. In any case, it seems that stress would not be directly related to HD [80-82]. With regard to thyroid function, cortisol rise observed in chronic stress may inhibit the production of thyrotropin-releasing hormone (TRH), which may lead to central hypothyroidism.
Moreover, the peripheral conversion of T4 to T3 would also be reduced and shifted toward the production of biologically inactive reverse Triiodothyronine (T3), causing peripheral functional hypothyroidism [83-84].
We reckon that autoimmune thyroiditis disease course would be better handled by including the examination of etiopathogenic factors into its clinical assessment and patient management. We have shown that the extensive list of considerations may include genetic susceptibility, gastro-intestinal conditions (intestinal permeability defects, dysbiosis and SIBO), other pathologies like celiac disease/gluten intolerance, and specific micronutrients imbalance (vitamin D, iodine and selenium). Biopsychosocial stress, hormonal imbalances and gender differences and exposure to environmental toxic substances (heavy metals and others) and infectious agents (both viral and bacterial) also contribute to HD development and chronification. Finally, given the implication of multiple mechanisms of etiology and pathogenesis, a multidisciplinary strategy may be required to deliver integrated and successful patient care. Further studies are still needed in order to confirm the number of associations between these factors and the development and progression of HD. The study design should take into account the multiple and diverse factors as well as the multisystemic nature of the disease.
We would like to thank Neus Cantariño for her valuable help in editing this manuscript.
Citation: García I, Abanades S (2021) Etiopathogenetic Mechanisms in Hashimoto’s Autoimmune Thyroid Disease. Thyroid Disorders Ther 10:262.
Received: 15-Nov-2021 Accepted: 29-Nov-2021 Published: 06-Dec-2021
Copyright: © 2021 García I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.