
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2020)Volume 3, Issue 2
Background: Chronic heart failure is a complex disease associated with various pathophysiological and biochemical
disorders. We assessed the 10 years prognostic role of a multimarker panel of markers for myocyte stress (GDF-15),
extra-cellular matrix remodeling (Galectin-3, mimecan, TIMP-1), inflammation (Galectin-3), myocyte injury (hs-TnT)
and angiogenesis (endostatin, IBP-4, IGF-BP-7, sFlt-1 and PLGF) head-to-head with the biochemical gold-standard
NT-proBNP.
Methods: Blood samplesfrom 149 patients with heart failure were analysed. After 10 years of follow-up (median
follow-up 104 months, IQR 43-117), data regarding rehospitalisation for chronic heart failure and all-cause-mortality
were acquired.
Results: Regarding Kaplan Meier analysis, all markers, dichotomized according to you denindex, were significant
predictors for all-cause-mortality (each p<0.05) and for the combined end point of all-cause-mortality and
rehospitalisation (each p<0,05). Including all markers in Cox Regression analysis, NT-pro-BNP, hs-TnT and IGF-BP7
were independent predictors for both end points (each p<0,05). Patients in whom all three markers were elevated had
a significant worse long-time-prognosis than patients without elevated markers (risk of all-cause-mortality 90,5% vs.
25%, risk of all-cause-mortality or rehospitalisation 97,6% vs. 43,7%).
In a Cox regression model with clinical relevant parameters (ejection fraction<30%, age, serum creatinine, gender)
and the multimarker panel (hs-TnT, NT-pro-BNP, IGF-BP7), all biomarkers remained independent significant
predictors for both end points beside ejection fraction<30% and male gender (each p<0.05).
Conclusion: In a 10-years-follow-up, a combination of three biomarkers with different pathophysiological background
(NT-pro-BNP, hs-TnT and IGF-BP7) increased the prognostic value and identified patients with a high risk of
mortality and rehospitalisation. Especially IGF-BP7 seems to play an important role regarding prognostication in
heart failure.
Biomarkers; Chronic heart-failure; Prognostication; Long-term follow-up; Multimarker panel
Heart failure is one of the major public health problems world wide [1]. It can be defined as a pathologic state of systolicordiastolic dysfunction, which leads to a difference in oxygen supply and demand. Despite improvements in therapy, morbidity and mortality are still high. Due to ageing population, the prevalence of heart failure is on the rise [1]. It also increases with a rising number of people suffering from cardiovascular comorbidities such as diabetes or arterial hypertension. In the last years, efforts were made to improve diagnosis and prognostication by identifying new biomarkers. Chronic heart failure is a complex disease with various pathophysiological and biochemical disorders that cannot be reflected by a single biomarker. The goal of the current study was toassess a multimarker panel of markers for myocyte stress (GDF-15), extra-cellular matrix remodeling (Galectin-3, mimecan, TIMP-1), inflammation (Galectin-3), myocyteinjury (hs-TnT) and angiogenesis (endostatin, IGF-BP4, IGF-BP7, sFlt-1 as antiangio genetic factors and PLGF as angio genetic factor) together with the biochemical gold-standard NT-proBNP. A 3 years follow-up of this panel was published in 2014 [2]. Herein, all markers except PLGF showed prognostic utility. The aim of the current study was to investigate the long-term prognostic role of these markers over a follow-up period of 10 years and to evaluate a simplified multimarker panel, which can identify patients with high risk of mortality or rehospitalisation.
Study population
149 patients with chronic heart failure were included in the current study. Patients between 18 and 80 years with dilated or ischemic cardio myopathy or valvular disease were included. All patients had to sign a consent form at the beginning of the study. Exclusion criteria were pulmonary embolismor stroke in the last 6 months, acute myocardial infarction, severe pulmonary hypertension and end stage renal disease. At the beginning of the study, patients were assessed by interview (NYHA stadium, drugs) as well as physical examination (edema, elevated JVP, pulmonaryrales). All investigations were performed by the same experienced examiner. For all patients, an echocardiography study (Phillips i.e33, Amsterdam, The Netherlands) was performed at the beginning of the study. Simpson’s method was used to quantify left ventricular ejection fraction. The estimated glomerular filtration rate (eGFR) was calculated according to the Modification of Diet in Renal disease (MDRD) formula [3].
Follow-ups were made after 2, 3, 5 and 10 years by telephone interviews and reviewing physician letters. Results of the 3 years follow-up were published in 2014 [2]. The primary end point of the current study was mortality from all causes. Survival confirmations as well as dates of death were obtained from hospital or death registries or by verification of relatives or the family doctor. The secondary end point was a combined end point of all-cause-mortality and rehospitalisation due to congestive heart failure. No patient was lost to follow-up. The study was approved by the institutional ethics committee and performed in agreement with good clinical practice guidelines and with the standards established for human experimentation by the Declaration of Helsinki.
Sample processing and biochemical analysis
Collected blood samples were stored on ice, centrifuged, distributed into 2 ml tubes and frozen at 80°C. All laboratory analyses were performed by laboratory staff of Roche Diagnostics in Penzberg, Germany, who were blinded to all patient data.
Hs-TnT analysis was assessed by electrochemi luminescence method (Roche Diagnostics, Mannheim, Germany) [4]. The analytical range was 0.003-10 ng/ml. For analysis of NT-pro BNP the Elecsys 2010 assay (Roche Diagnostics, Mannheim, Germany) was used [5]. The analytical range was 5-35000 pg/ml. GDF-15 (LOD 0.4 ng/ml), sFlt-1 (LOD 10 pg/ml) and PLGF (LOD 3 pg/ml) were measured by highly sensitive and highly specific pre clinical assays (Roche proprietary) with the Elecsys analyzer. Microtiter plate prototype assays from Roche were used to measure Mime can (LOD 0.39 ng/ml), TIMP-1 (LOD 0.08 ng/ml), and IBP-7 (LOD 0.1 ng/ml). Galectin-3 (BG Medicine, Waltham, USA; LOD 1.13 ng/ml), endostatin (R&D Systems, Minneapolis, USA; LOD 0.023 ng/ml) and IBP-4 (Abcam, Cambridge, UK; LOD 0.25 ng/ml) were assessed by commercially available assays.
Statistics
Data were analysed by using commercial statistical software (IBM SPSS Statistics Version 25). Normally distributed descriptive data was presented as mean (SD) while not normally distributed data was presented by medians and IQR. All markers were dichotomized for coxregression analyses, Kaplan meier curves and multimarker panel analyses. The optimal cut-off point was determined with the Youden Index. The Youden index, a measure of the ROC curve is defined as maxc {sensitivity (c)+specificity (c)-1} and gives equal weight to the sensitivity and specificity. If the cut-off point is identified via the Youden index, the overall correct classification rate (i.e. sum of sensitivity and specificity) can be maximized [6].
Regarding both end points, Kaplan Meier curves were calculated and the curves were compared by the log rank test. AUC-values were calculated for both end points by ROC analysis. For ROC analysis, all markers were utilized as continuous variables. Multi variable Cox proportional hazard analyses with all markers were performed as stepwise regressions with backward elimination to evaluate possible associations regarding all markers and both end points. Moreover, in a multimarker approach, patients were divided in 4 different groups, corresponding to the number of elevated parameters. After that, the overall survival and the incidence of the composite end point were compared graphically by generating Kaplan Meier curves. Furthermore, a multivariate Cox regression analysis with the markers and clinical parameters (age, male gender, severly reduced left-ventricular ejection fraction (<30%) and serum creatinine) was conducted.
Study population
Baseline characteristics of the study population (n=149) are depicted in Table 1. The majority of patients were male (87%) and had ischemic heart disease (61%). Average age at baseline was 61.8 years. Most of the patients suffered from hypertension (78%), whereas diabetes was present in a third of the study population. The vast majority (97%) of patients had a depressed LV-function (LVEF median=30%). Approximately half of patients were classified as NYHA class III or IV. Renal function was impaired (GFR<60 ml/min/1.73 m2) in 37,4% of the study population. Heart failure medication with ACE inhibitors, betablockers or mineral corticoid receptor antagonists was implemented according to guideline recommendations in the majority of the patients Table 1.
| Clinical Characteristics | All (n=149) | Alive (n=70) | Dead (n=79) |
|---|---|---|---|
| Age ± SD (years) | 62.2 ± 11.6 | 59 ± 11.9 | 65 ± 10.7 |
| Male; n (%) | 130 (87.2%) | 56 (80%) | 74 (93.7%) |
| Ischemic CMP; n (%) | 91 (61.1%) | 42 (60%) | 50 (63.3%) |
| BMI ± SD (kg/m2) | 28.6± 4.8 | 29.4 ± 5.2 | 27.8 ± 4.5 |
| Hypertension; n (%) | 116 (77.9%) | 54 (77.1%) | 62 (78.5%) |
| Diabetes; n (%) | 45 (30.2%) | 10 (14.3%) | 35 (44.3%) |
| Drug therapy; n (%) | |||
| ACE-1/ARB | 127 (85.2%) | 59 (84.3%) | 68 (86.1%) |
| β-Blocker | 122 (81.9%) | 58 (82.9%) | 64 (81%) |
| Diurectics | 129 (86.6%) | 48 (68.6%) | 73 (92.4%) |
| Mineralcorticoid receptor antagonist | 91 (61.1%) | 40 (57.1%) | 51 (64.6%) |
| Digitalis | 26 (17.4%) | 11 (15.7%) | 15 (19%) |
| EF; Median (± SD) | 30 ± 9.3 | 37 ± 9.2 | 30 ± 8.7 |
| NYHA III and IV; n (%) | 78 (52.4%) | 23 (32.8%) | 48 (60.7%) |
| eGFR; Median (ml/min/1.73 m2) | 69.9 | 80 | 57.5 |
| NT-proBNP (pg/ml); Median (± SD) | 1504 | 799.6 ± 3016.5 | 2282 ± 3621.9 |
| hs-TnT (pg/ml); Median (± SD) | 18 | 11 ± 619.3 | 24.4 ± 627.4 |
| PLGF (pg/ml); Median (± SD) | 21 | 19.7 ± 6 | 22.3 v 6.4 |
| sFlt-1 (pg/ml); Median (± SD) | 94.3 | 79 ± 60.2 | 108.1 ± 246.6 |
| GDF-15 (ng/ml); Median (± SD) | 1.9 | 1.4 ± 2.2 | 2.6 ± 7.2 |
| Galectin-3 (ng/ml); Median (± SD) | 19.9 | 17.8 ± 7.6 | 22.9 ± 12.6 |
| TIMP-1 (ng/ml); Median (± SD) | 213.6 | 203.8 ± 72.7 | 228.3 ± 97.9 |
| IGF-BP7 (ng/ml); Median (± SD) | 41.3 | 36.2 ± 11.6 | 46.5 ± 18.2 |
| IGF-BP4 (ng/ml); Median (± SD) | 60.1 | 50.7 ± 41.3 | 82.2 ± 76.7 |
| Mimecan (ng/ml); Median (± SD) | 6.1 | 1.5 ± 11 | 14.5 ± 16.2 |
| Endostatin (ng/ml); Median (± SD) | 268.5 | 226.7 ± 82.8 | 304.6 ± 106.1 |
Table 1: Baseline characteristics; bold: significant differences between dead and alive (p<0.05).
Prognostication in heart failure
Median follow-up time was 117 months (IQR 115-119 months). During the follow-up time 73 deaths (52.5% of the study population) were observed. The combined end point of all-cause- mortality and rehospitalization for congestive heart failure occurred in 96 patients (69.1%). No patients were lost to follow-up.
In a ROC analysis for the prognostication of all-cause-mortality, IGF-BP7 (AUC 0.76; 95% CI: 0.69-0.84), GDF-15 (AUC 0.76; 95% CI 0.68-0.84) and NT-proBNP (AUC 0.76; 95% CI: 0.68-0.83) showed the highest AUC-values shown in Table 2. The AUC-value of hs-TnT was 0.69 (95% CI: 0.6-0.78).
| Marker | AUC value | 95% CI |
|---|---|---|
| nt-pro-BNP | 0.76 | 0.68-0.83 |
| hs-Troponin | 0.69 | 0.6-0.78 |
| PLGF | 0.62 | 0.53-0.71 |
| sFlt-1 | 0.71 | 0.62-0.79 |
| GDF-15 | 0.76 | 0.68-0.84 |
| Galectin-3 | 0.67 | 0.58-0.76 |
| TIMP-1 | 0.64 | 0.55-0.73 |
| IGF BP-7 | 0.76 | 0.69-0.84 |
| IBP-4 | 0.68 | 0.59-0.76 |
| Mimecan | 0.73 | 0.65-0.80 |
| Endostatin | 0.74 | 0.66-0.82 |
Table 2: AUC values of all markers.
Regarding Kaplan Meier analysis, all markers were significant predictors for both end points (each p<0,05) shown in Table 3.
| Kaplan Meier analysis according to different proteins (p values derived from Long-rank test) | ||||||
|---|---|---|---|---|---|---|
| Marker | All cause mortality | Combination of all-cause mortality and rehopitalisation | ||||
| <cut point | >cut point | p value | <cut point | >cutppint | p value | |
| NT-proBNP | 78/25 | 71/54 | <0.001 | 78/42 | 71/62 | <0.001 |
| hs-TnT | 67/21 | 82/58 | <0.001 | 67/36 | 82/68 | <0.001 |
| PLGF | 83/36 | 66/43 | 0.007 | 83/52 | 66/52 | 0.023 |
| sFlt-1 | 76/26 | 73/53 | <0.001 | 76/40 | 73/64 | <0.001 |
| GDF-15 | 56/13 | 93/66 | <0.001 | 56/28 | 93/76 | <0.001 |
| Galectin-3 | 53/17 | 95/61 | <0.001 | 53/27 | 95/76 | <0.001 |
| TIMP-1 | 114/51 | 35/28 | <0.001 | 114/73 | 35/31 | <0.001 |
| IGF BP-7 | 100/37 | 49/42 | <0.001 | 100/59 | 49/45 | <0.001 |
| IGF BP-4 | 65/25 | 74/48 | <0.001 | 65/39 | 74/57 | 0.006 |
| Mimecan | 92/33 | 57/46 | <0.001 | 92/53 | 57/51 | <0.001 |
| Endostatin | 85/32 | 62/46 | <0.001 | 85/49 | 62/54 | <0.001 |
Table 3: Kaplan meier analysis according to different proteins (p values derived from Log-rank Test).
The marker with the best performance was IGF-BP7 with an event rate of 86% for all-cause-mortality and 92% for the combined end point if the marker was elevated (>Youden Index). Mimecan (event rate 81% for all-cause-mortality and 89% for the combined end point) and TIMP-1 (event rate 80% for all-cause-mortality and 89% for the combined endpoint) were also prognostically conclusive markers for both end points. NT-proBNP showed an event rate of 76% for all-cause-mortality and 87% for the combined endpoint. Especially for the combined endpoint, Endostatin (event rate 87%) and sFlt-1 (event rate 88%) were also relevant parameters. The event rate forhs-TnT was 71% for all-cause-mortality and 83% for the combined end point.
Multimarker model
In a Cox regression model with all markers shown in Table 4, NT-proBNP, hs-TnT and IGF-BP7 were the only independent predictors for both endpoints (each p<0,05).
| Cox regression with all markers (step 8) | ||||
|---|---|---|---|---|
| All cause mortality | All cause mortality and rehospitalisation | |||
| Marker | Exp (B) | p value | Exp (B) | p value |
| NT-proBNP | 0.470 (95% CI 0.236-0.840) | 0.011 | 0.563 (95% CI 0.349-0.906) | 0.018 |
| hs-Troponin | 0.468 (95% CI 0.264-0.828) | 0.009 | 0.537 (95% CI 0.337-0.857) | 0.009 |
| IGF BP-7 | 0.486 (95% CI 0.288-0.821) | 0.007 | 0.576 (95% CI 0.362-0.918) | 0.02 |
| GDF-15 | 0.467 (95% CI 0.220-0.991) | 0.047 | 1.05 (95% CI 0.489-2.252) | NS |
| Endostatin | 0.761 (95% CI 0.439-1.321) | NS | 0.576 (95% CI 0.372-0.893) | 0.014 |
Table 4: Cox regression analysis with all markers (step 8), marker categorized by Youden Index.
Furthermore, Endostatin was an additional independent predictor for the combined endpoint (p<0.05), but it was not significant for all-cause-mortality (p=n.s.). Therefore, NTproBNP, hs-TnT and IGF-BP7 were included in a multimarker model. Patients were divided in four groups, according to the number of elevated markers.
Regarding all-cause-mortality, the highest mortality rate with 90,9% could be observed in the group with all three markers elevated. In contrast only ten patients (21.7%) died in the group with no elevated marker. The combined endpoint of all-causemortality and rehospitalisation occurred in 97% of the patients with all three markers elevated, but only in 43,5% of patients with no elevated marker. The event rate increased step wise with the number of elevated markers depicted in Figure 1.
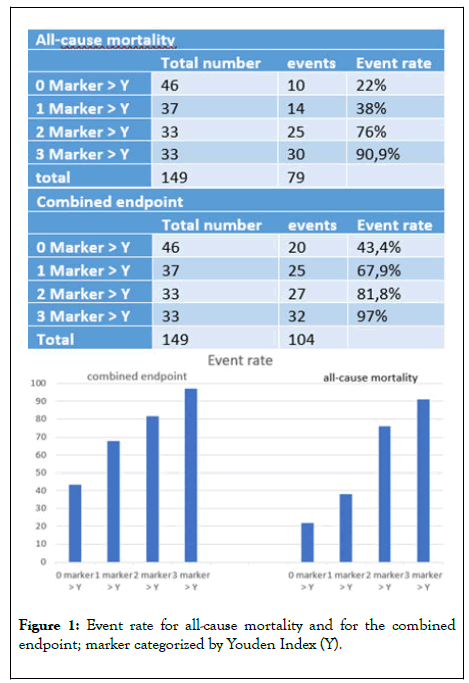
Figure 1: Event rate for all-cause mortality and for the combined endpoint; marker categorized by Youden Index (Y).
Kaplan Meier curves are depicted in Figure 2.
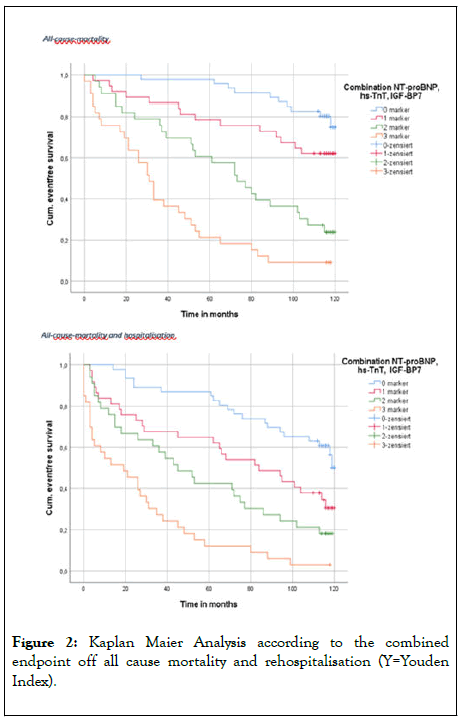
Figure 2: Kaplan Maier Analysis according to the combined endpoint off all cause mortality and rehospitalisation (Y=Youden Index).
In a multivariate Cox regression analysis with the three markers and clinically relevant parameters (age, male gender, severely reduced left-ventricular ejection fraction and serum creatinine), all clinical parameters except serum creatinine (p=n.s.), as well as elevated NT-proBNP, hs-TnT and IGF-BP7 were independent significant predictors for all-cause-mortality (each p<0,05). Regarding the combined end point, all three biomarkers, male gender and severely reduced left-ventricular ejection fraction predicted the risk of all-cause-mortality and rehospitalisation (each p<0,05), opposite to age and serum creatinine (p=n.s.) shown in Table 5.
| Cox regression with all markers (Step 2) | ||||
|---|---|---|---|---|
| All cause mortality | All cause mortality | |||
| Marker | Exp (B) | p value | Exp (B) | p value |
| NT-proBNP | 0.473 (95% CI 0.273-0.822) | 0.008 | 0.597 (95% CI 0.374-0.953) | 0.031 |
| hs-TnT (>Y) | 0.468 (95% CI 0.264-0.828) | 0.018 | 0.557 (95% CI 0.350-0.887) | 0.014 |
| IGF BP-7 (>Y) | 0.375 (95% CI 0.232-0.606) | <0.001 | 0.508 (95% CI 0.330-0.783) | 0.002 |
| Severly reduced EF (<30%) | 1.655 (95% CI 1.003-0.991) | 0.049 | 1.750 (95% CI 1.126-2.719) | 0.013 |
| Age | 0.761 (95% CI 0.439-2.730) | 0.021 | 1.017 (95% CI 0.998-1.036) | NS |
| Serum creatinine | 1.195 (95% CI 0.806-1.770) | NS | 1.190 (95% CI 0.842-1.683) | Ns |
| Male gender | 3.142 (95% CI 1.259-7.840) | 0.014 | 2.517 (95% CI 1.253-5.057) | 0.01 |
Table 5: Cox regression with clinical parameters-Marker categorized by Youden Index (Y).
The aim of the current study was to assess the role of nine biomarkers with different pathophysiological background in the long-term prognostication of patients with chronic heart failure, together with the well-established markers NT-proBNP and hs- TnT. After a follow-up of 10 years, all markers were significant predictors for both endpoints. In a previous analysis a multimarker approach with five different biomarkers, namely NT-proBNP, hs-TnT, GDF-15, TIMP-1 and IBP-4 was evaluated. 2 Actually, we performed a Cox regression analysis with all markers, where only hs-TnT, NT-proBNP and IGF-BP7 were significant and independent predictors for both endpoints. Therefore, they were integrated in a novel and simplified multimarker panel. Notably and in contrast to previous data, Youden index instead of Median was used as cut-off in the current data to improve the stratification of prognosis.2 If median instead of Youden index was used, the results would be similar, with still hs-TnT, NT-proBNP and IGF-BP7 as independent predictors for all-cause-mortality (data not shown).
Performance of the different biomarkers
NT-pro BNP is a marker for volume overload, increased myocardial wall stretch and neurohumoral activation [7]. According to the current ESC and AHA guidelines [8,9] NTproBNP is an important parameter for the initial diagnosis and the prognostication of heart failure. In patients with chronic kidney disease, NT-proBNP indicates an increased risk for dialysis and clinical adverse outcome [10]. Consistent with previous studies [11-13], in the current study NT-proBNP was an independent significant predict or for both end points.
Highly sensitive Troponin-T is not only a marker for acute myocardial injury, it also seems to be relevant for the diagnosis and prognostication of chronic heart failure [14,15]. In the current study it was an independent predictor for both endpoints, but comparing the event rate and the AUC value, it was inferior to NT-proBNP.
In order to improve the prognostication in heart failure, efforts have been made to identify additional biomarkers. Therefore, we assessed the prognostic potential of nine proteins in the longterm prognostication of heart failure, head-to-head with NT-proBNP and hs-TnT. Comparing all markers in a Cox regression analysis, only NT-proBNP, hs-TnT and IGF-BP7 were independent predictors for both endpoints.
IGF-BP7 is able to bind Insulin with a high affinity [16]. It is expressed in various tissues and is not only associated with diabetes, obesity, acute kidney injury and different types of cancer, but also with heart failure [17-20]. In a mouse model, high expression in myocytes and high plasma levels of IGF-BP7 were associated with cardiac hypertrophy. Interestingly, IGF-BP7 is also associated with diastolic function and related to diastolic filling, dilatation of the left atrium and echocardiographic parameters for diastolic dysfunction in patients with heart failure [20-22]. Furthermore, high levels of IGF-BP7 may be associated with lower exercise capacity.
In contrast to the three-years-follow-up, in the extended followup of the current study IGF-BP7 was the marker with the best performance, equivalent to NT-proBNP, and was an independent significant predictor for both endpoints. Our resultsare in accordance to previous studies that showed a positive correlation between an elevationof IGF-BP7 and increased mortality and rehospitalisation rate for heart failure [23,24]. All these studies enrolled predominantly men with systolic heart failure. Compared with the set wo previous trials, one strength of the current study is the longer follow-up period (104 months vs. 12 and 26 months). In the current study one blood analysis was performed at the beginning of the study, whereas serial measurements were made in the two previous studies. Instead of all-cause-mortality, cardiac mortality was used as endpoint in previous data. The current study showed for the first time, that a multi marker panel with IGF-BP7, NT-proBNP and hs-TnT increased the prognostic value and identified patients with a high risk of mortality and rehospitalisation in a long-term follow-up. Remarkably, patientswith an elevationof all markers had an event rate of 90.9% for all-cause-mortality and 97% for all-cause-mortality and rehospitalisation after 10 years follow-up.
The other markers, especially Endostatin, performed more than satisfactorily although they were no independent predictors for both endpoints. Endostatin, an angio genesis inhibitor, was an independent predictor for the combined endpoint but not for all-cause-mortality. This result is in contrast to the study of [25]. The number of enrolled patients was similar to the current study (151 vs. 149) but the median follow-up was shorter (31 months vs. 104 months). In contrast to the current study, there were less patients with ischemic heart disease (27% vs. 61%) and they analysed only Endostatin and NT-proBNP and used a different multivariate Cox model.
GDF-15, IBP-4 and TIMP-1 were the markers with the best performance in the 3 year follow-up [2]. Therefore these markers were included in a multimarker panel together with the wellestablished markers NT-proBNP and hs-TnT. In the current study these three markers showed prognosticutility. Surprisingly, they were not independent predictors for both endpoints. Notably, and in contrast to the previous study, Youden index was used as cut-off for all markers in the current study, which defines the maximum potential effectiveness of a biomarker. Nevertheless, if median instead of Youden index was used, the markers were also not independent predictors for both endpoints (data not shown).
GDF-15 was an independent predictor for all-cause-mortalilty, but not for the combined endpoint. A study investigated the prognostic role of GDF-15 in 910 patients enrolled in the HF ACTION Study. In accordance with the current data, they found that GDF-15 is an independent predictor for all-causemortality. In contrast to the current study, the median follow-up was shorter (30 months vs. 104 months) [26].
Regarding IBP-4 there are little data about prognostication in patients with heart failure. A study assessed the prognostic potential of IBP-4 in patients with acute ST-segment elevation myocardial infarction and found a positive correlation between higher IBP-4 levels and all-cause-mortality, cardiovascular mortality and major cardiac events [27]. Compared to the current data, patients had a higher LVEF (45,8% vs. 30%), less diabetes (9% vs. 30%) and hypertension (33.8% vs. 77,9%) and there was no information about heart failure medication. Moreover, there was no comparison with other relevant biomarkers.
High plasma levels of TIMP-1 had been related to echocardiographic indices of left ventricular hypertrophy and correlated inversely with systolic function in patients enrolled in Framingham heart study after myocardial infarction [28]. The prognostic potential of TIMP-1 is unclear, since previous studies yielded inconsistent results. Some studies suggest that TIMP-1 is not an independent predictor for all-cause-mortality and cardiac rehospitalisation in heart failure [29,30], whereas, and in contrast to our data, Frantz et al. Found that TIMP-1 is an independent significant predictor for all-cause-mortality [31]. Compared to the current study, there were less patients with systolic heart failure (59% vs. 87,9%) and the median of the left ventricular rejection fraction was higher (44% vs. 30%). Furthermore, the median follow-up was shorter (24 months vs. 104 months). Additionally, Frantz et al. used different markers (TIMP-1, MMP-9, NT-proBNP and TNF-α) and TIMP-1 was adjusted to different parameters, which may also explain the different results.
Further, four more proteins may be useful in the prognostication of heart failure, namely sFLt-1, Mimecan, Galectin-3 and PlGF. sFLt-1, a soluble protein, which regulates PlGF, has been associated with adverse outcome in patients with heart failure in previous studies [32,33]. Galectin-3 plays an important role in myocardial fibrosis. Imran et al. performed a meta-analysis of 18 studies with 32 350 patients and found a positive correlation between plasma levels of Galectin-3 and mortality [34]. A major difference between these studies and the current study is the use of different biomarkers. According to the current AHA guidelines, Galectin-3 is recommended as a predictor for the prognostication in heart failure [9]. In the current study, Galectin-3 wasn’t an independent predictor for both endpoints in the Cox regression analysis with all markers. Mime can is associated with arterial stiffness and coronary-artery disease [35]. There are two studies, that found no association between Mime can levels and outcome after adjustment to clinical parameters, but in contrast to the current study different biomarkers were used [23,36]. Regarding PlGF, an angio genetic factor, no diagnostic or prognostic utility was seen regarding previous data [2]. Notably, in the current study PlGF was a significant predictor for both endpoints. This may be attributed to the use of different cut-offs. In the current study the Youden Index was used as cut-off, whereas in a previous analysis the cutoff was identified by Median. Nevertheless, PlGF remained significant for all-cause-mortality but not for the combined endpoint if median instead of Youden Index was used as cut-off (data not shown), indicating that it may be associated with longterm prognosis of all-cause-mortality in patients with chronic heart failure. However, in accordance to a study [32], it was not an independent marker of mortality or the combined endpoint.
Multimarker model
A multimarker approach with the three markers with the best performance (NT-proBNP, hs-TnT and IGF-BP7) increased the prognostic value compared to NT-proBNP and hs-TnT alone. Notably, these biomarkers are representing different pathophysiological pathways. NT-proBNP is a marker for volume overload, elevated hs-TnTreflectesmyocardic injury and IGF-BP7 is associated with angiogenesis. The rate of all-cause-mortality and the combined endpoint increased stepwise with the number of elevated markers. Elevation of all three markers was associated with the worst prognosis (even trates for both endpoints>90%). In contrast to our previous study, we included three instead of five markers in our multimarker model. Using three instead of five markers is more cost-effective and much easier in the clinical practice [36]. The use of the previous multimarker model with 5 markers offered no additional benefit for the prognostication of mortality or the combination of rehospitalisation and mortality over a follow-up of 10 years (data not shown). Thus, a multimarker model consisting of three different biomarkers may improve prognostication in heart failure. Further larger and prospective studies are necessary to investigate the prognostic value of the seproteins as biomarkers, especially in the proposed multimarker model.
The current study investigated the prognostic value of nine biomarkers in a long term follow-up together with the wellestablished markers NT-proBNP and hs-TnT. Over a follow-up period of ten years, all markers were associated with all-causemortality and rehospitalisation because of heart failure. Among these biomarkers, NT-proBNP, hs-TnT and IGF-BP7 showed the best performance and were independent significant predictors for both endpoints. A multimarker panel of these three markers with different pathophysiological background increased the prognostic value and identified patients with a high risk for mortality and rehospitalisation.
The current study is a single center study and enrolled predominantly men with systolic heart failure,where as women and patients with diastolic heart failure may be under represented. The statistical power may also be limited by the small patient collective. Therefore, the applicability in the clinical practice may be reduced. On the other hand, one strength of the current study is the long follow-up (10 years). Furthermore, and in contrast to previous data Youden index instead of median was used as cut-off in the current study. That’s why the comparability between previous data and the current data maybe limited. Nevertheless, the use of Youden index as cut-off increases the stratification of prognosis. Regarding all-cause-mortality, there are similar results if median instead of Youden index was used.
Citation: Bauer S, Strack C, Hubauer U, Ucer E, Wallner S, Luchner A, et al. (2020) Evaluation of a Multimarker Panel in Chronic Heart Failure: A 10 Years Follow-Up. J ClinChem Lab Med. 3:145. DOI: 10.35248/clinical-chemistry-laboratory-medicine.20.3.145
Received: 14-Jul-2020 Accepted: 28-Jul-2020 Published: 04-Aug-2020
Copyright: © 2020 Bauer S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.