Journal of Antivirals & Antiretrovirals
Open Access
ISSN: 1948-5964
ISSN: 1948-5964
Research Article - (2021)
Objectives: In tropical and subtropical nations dengue is a main public health matter. We try to find to analyze the clinical and hematological factors from a Complete Blood Count (CBC) which differentiate dengue infection. The purpose of the study was to categorize clinical features and hematological parameters and develop predictive model of high fever patients was treated as early marker and possible prognosticator factors of dengue.
Methods: Demographic data analysis with variables like gender, place, age and clinical data analysis of clinical parameters with dengue confirmation test have been done develop predictive model factors to differentiate Dengue Infection (DI) from CBC data of Acute Febrile Illness (AFI) patients in Delhi-NCR, Sonepat region from 2015 to 2018.
Results: Among 223 patients, 167 were confirmed with 100 primary and 67 secondary DI of maximum number male patients in the age group of 10-30 years from 2015 to 2018 while 56 had negative results. Badhkhalsa, Jakholi, Sewli and Rai were high dengue reported area in Delhi-NCR, Sonepat. There was a statistically significant value (p<0.05) of Total Leukocytes Count (TLC) cells/cmm during AFI phase from 2015 to 2018 using logistic regression and ROC graph. TLC (cells/cmm) had a higher area ± SE value from 2015 to 2018 (0.66 ± 0.07, 0.76 ± 0.10, 0.68 ± 0.07 and 0.79 ± 0.06) respectively which were statistically significant (p<0.05). Dengue diagnosis test of mean value of TLC (<4000 cells/cmm) from 2015 to 2018 were evaluated with a prevalence of dengue disease of 35.09%- 58.06%, sensitivity of 41.03%-100%, specificity of 24.10%-93.10% and accuracy rate of diagnosis evaluation of 62.07%-70.97% were related to danger sign DI in Delhi-NCR, Sonepat area.
Conclusion: As per our study we can conclude that due to non-specific clinical features and delayed of confirmation test, among the clinical parameters TLC could be the useful feature for quick finding of DI which is unique, simple, easily available, cost effective approach mainly in rural area.
Acute febrile illness; Dengue; Complete blood count; Logistic regression; ROC curve
Dengue is an arthropod-borne viral infection, vector-borne flavivirus disease transmitted by Aedes aegypti mosquito that poses a foremost public health threat in many regions [1]. The global recovery of dengue may be failure to control the climate change, Aedes aegypti mosquitos populations, uncontrolled population growth expansion, modern civilization and increased traveling [2,3]. Worldwide, dengue infects 50 million people every year and causes 20,000 to 25,000 deaths and out breaks has been reported in Delhi, India in 1996, 1999, 2003, 2009 with high number of suspected dengue cases were reported in rainy season [4,5].
Dengue Virus (DENV) is RNA (single stranded) virus of the family Flaviviridae [6]. Dengue is caused by antigenically distinct virus serotypes DEN 1-4 with common cause of AFI in the tropical and subtropical areas of the world [7]. DIs are asymptomatic and the clinical appearances occur in Dengue Fever (DF) and Dengue Haemorrhagic Fever/Dengue Shock Syndrome (DHF/DSS) are unusual above 15 years patients and are more collective in secondary infection [8].
AFI is the utmost public clinical disease among patients appearing to hospital in emerging countries, caused by a variability of bacterial, viral and parasitic causes. It is essential that clinicians be alert of the pervasiveness of dual infections to make a rapid diagnosis in order to recruit proper cure. For dengue laboratory confirmation is required with serology and virus-related separation actuality the most common analytical approaches used [9]. For dengue detection ELISA and PCR techniques are used to every develop city hospitals. A restriction of the laboratory diagnosis for dengue is that it may take numerous days or weeks for the outcomes to be finished and informed [10]. Although there is no particular clinical features depend on blood test to identify DI which can assist clinicians make better decisions by knowing patients’ blood test report [11-13].
Currently, Dengue Day 1 Test is used in rural area hospitals of many middle and low income countries as detection of denguespecific NS1 antigen and IgG/IgM antibody from serotypes DENV 1-4 to approve dengue primary and secondary infection [14,15]. An IgM/IgG ratio can be used to distinguish primary and secondary dengue virus infections by commercial company protocol [16]. Although serology is available in many private clinic and hospitals, but those tests are very costly and delay in tracking down results and in many regions, no laboratory testing is available at all [17,18].
Disease statistical forecast model can help to predict early DI using clinical features and hematological parameters by making quick decision in rural area [8,19]. Various types of models can be constructed on the particular method used and these can include multiple logistic regression, specificity, sensitivity [20-23]. There is need to improve the model and validate them in different populations. So, for a quick and preliminary test, CBC can be used to analysis of Hemoglobin (Hgb), TLC, neutrophils, lymphocytes, eosinophils, monocytes, Erythrocyte Sedimentation Rate (ESR), Haematocrit/Packed Cell Volume (PCV), RBC, platelets (PLT), Mean Corpuscular Volume (MCV), Mean Cell Hemoglobin (MCH) and Mean Corpuscular Hemoglobin Concentration (MCHC) as clinical features of dengue patients. Few scientists reported demographic study on clinical parameters and stated that thrombocytopenia (PLT<20,000 cells/mcL) and leucopenia (TLC<4000 cells/mcL) are transient and asymptomatic features in maximum number of dengue positive late stage patients bleeding, purpura which can give an idea for diagnosis of dengue [24,25]. But there is no predictive model research by statistical analysis like logistic regression, Receiver Operating Characteristic (ROC) graph, specificity, sensitivity and accuracy of diagnosis evaluation parameter which can reduce the spread of infection.
Our study focuses on the significance, simple, cost effective routine blood test in primary identification of DI. DF was not documented as a chief public health threat in Delhi and neighboring areas as Haryana before 1996 outbreak, so there was little indication and consciousness in this regard depend on demographic data analysis of recent four year. A potential observational study was approved to define the group of patients suffering from dengue symptoms with different clinical parameters and the pattern of presentation of dengue fever from September 2015 to December 2018 in Delhi- NCR, Sonepat Haryana.
Study area and data collection
A surveying data analysis was done total 223 patients in PR Institute of Medical Science and Research (PRIMSR) hospital in Delhi- NCR, Sonepat from 2015-2018. Individual patients’ data from different villages of Sonepat district, Haryana which is under Delhi- NCR (28.9268°N, 77.0967°E) were collected with AFI phase (<12 days) from the pathology laboratory of PRIMSR, SRM University, Delhi-NCR, Sonepat and Centre for Drug Design Discovery and Development (C4D). All the suspected patients’ data were reviewed for demographic, clinical, laboratory and outcome data. The descriptive data analysis has been done on gender, age, place and different symptomatic and clinical parameters of AFI patients.
Hematology and serology analysis
The findings of primary and secondary infection of dengue were carried out on the basis of clinical, epidemiological and laboratory data from other AFI phase of less than 12 days illness. The CBC parameters such as Hgb, TLC, neutrophils, lymphocytes, eosinophils, and monocytes, ESR, PCV, RBC, PLT, MCV, MCH and MCHC were analyzed. The serological data were analyzed depend on specific Dengue Day1 Test-NS1, IgG/IgM result which was used to detect dengue at the pathology laboratory of PRIMSR [11,18,26].
Statistical analysis
The collected data from patients was analyzed using SPSS statistical software (version 20) which is further represented as descriptive clinical data analysis; logistic regression and ROC analysis model with P value (p<0.05; statistically significant); Bayesian analysis model for disease diagnosis evaluation to predict prevalence of disease, sensitivity, specificity, positive predictive value, negative predictive value and accuracy of test [13,27-29].
Descriptive data analysis of dengue
Among 223 patients, 167 were confirmed with 100 primary and 67 secondary DI of maximum number male patients in the age group of 10-30 year (Figures 1 and 2) (Table 1). The ultimate study sample included that 62.5% (40/64) dengue positive and 37.5% (24/64) dengue negative cases in 2015; 37.5% (24/31) dengue positive and 19.4% (6/31) dengue negative cases in 2016; 72.4% (50/69) cases were of dengue positive and 23.1% (19/69) negative in 2017; 88.1% (52/59) dengue positive and 7/59 (11.9%) dengue negative cases in 2018. From the sample of three years (2015 to 2018), it was found that males are highly infected with dengue virus than females; patients from age group 10-19 year and 20-29 year were most affected (Figures 1a-1d) (Table S1). The cases were highly reported from place Badhkhalsa, Jakholi, Sewli, and Rai in every year (2015-2018) and therefore we urges that the government or healthcare officials ensures awareness, surveillance and healthcare practices in these regions (Figures 3a-3d and 4a-4d) (Table S1).
| Dengue diagnosis test | Type of infection | 2015 | 2016 | 2017 | 2018 |
|---|---|---|---|---|---|
| NS1-Negative; IgG-Negative; IgM-Positive | Primary | 3 | 4 | 2 | 11 |
| NS1-Positive; IgG/IgM-Negative | Primary | 29 | 11 | 1 | 19 |
| NS1-Positive; IgG-Negative; IgM-Positive | Primary | 3 | 1 | 11 | 5 |
| Total=100 | 35 | 16 | 14 | 35 | |
| NS1-Negative; IgG-Positive; IgM-Negative | Secondary | 3 | 3 | 1 | 4 |
| NS1-Negative; IgG/IgM-Positive | Secondary | 1 | 5 | 7 | 0 |
| NS1-Positive; IgG/IgM-Positive | Secondary | 1 | 1 | 28 | 7 |
| NS1-Positive; IgG-Positive; IgM-Negative | Secondary | 1 | 1 | 1 | 4 |
| Total=67 | 6 | 9 | 37 | 15 |
Table 1: Demographic analysis of primary and secondary dengue infection.
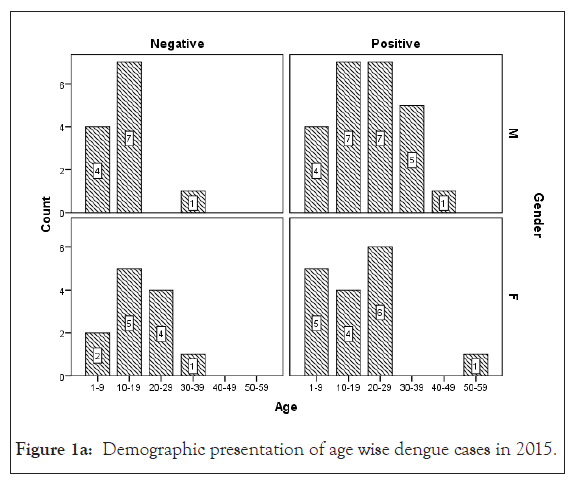
Figure 1a: Demographic presentation of age wise dengue cases in 2015
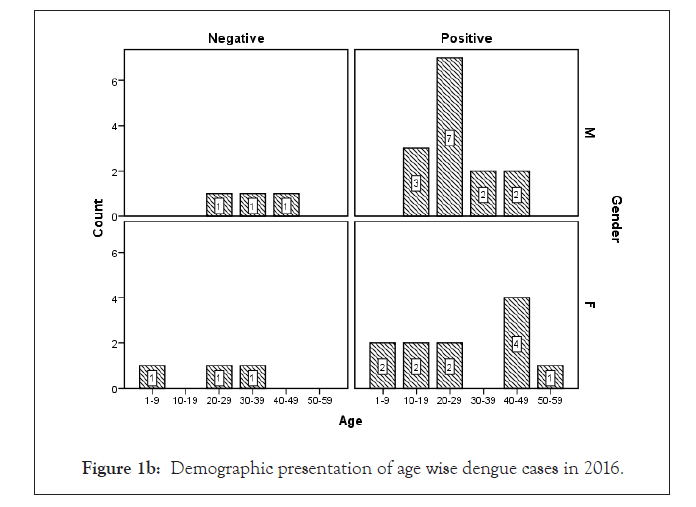
Figure 1b: Demographic presentation of age wise dengue cases in 2016.
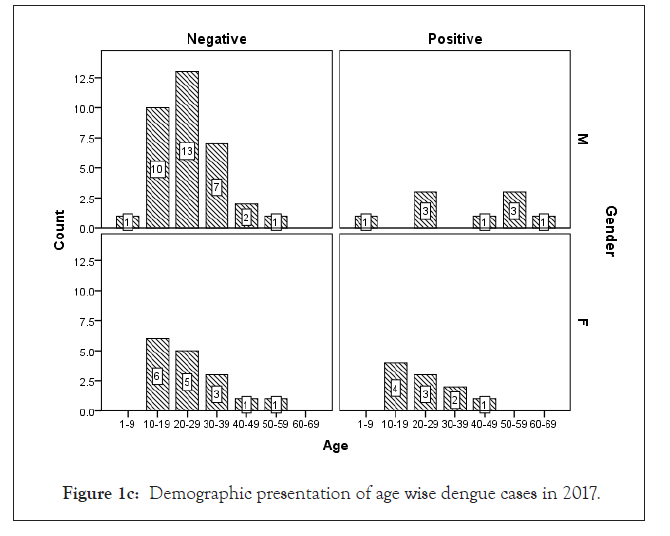
Figure 1c: Demographic presentation of age wise dengue cases in 2017.
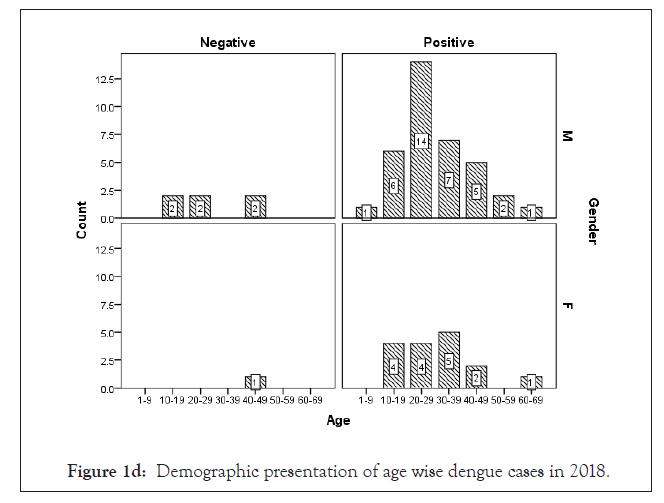
Figure 1d: Demographic presentation of age wise dengue cases in 2018.
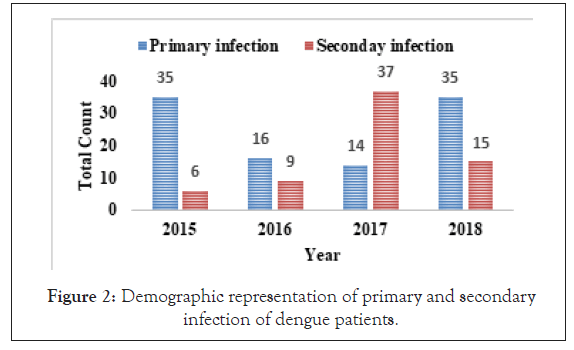
Figure 2: Demographic representation of primary and secondary infection of dengue patients.
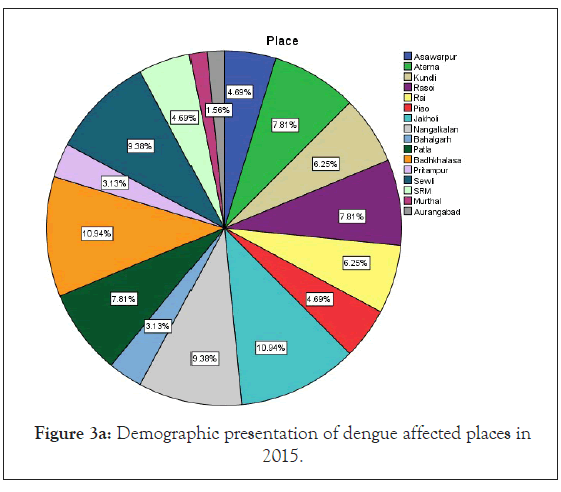
Figure 3a: Demographic presentation of dengue affected places in 2015.
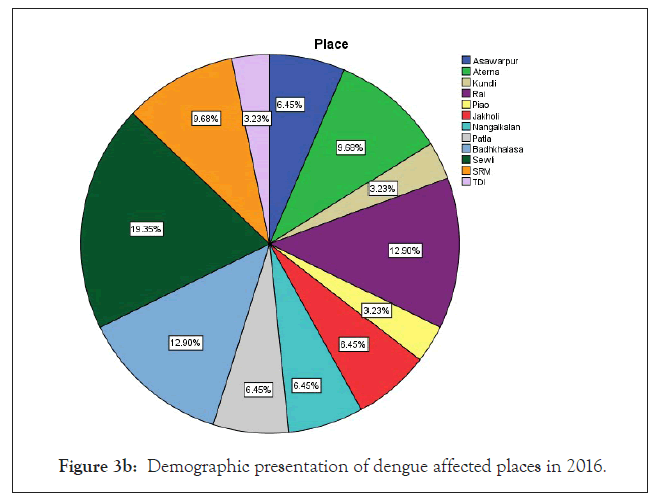
Figure 3b: Demographic presentation of dengue affected places in 2016.
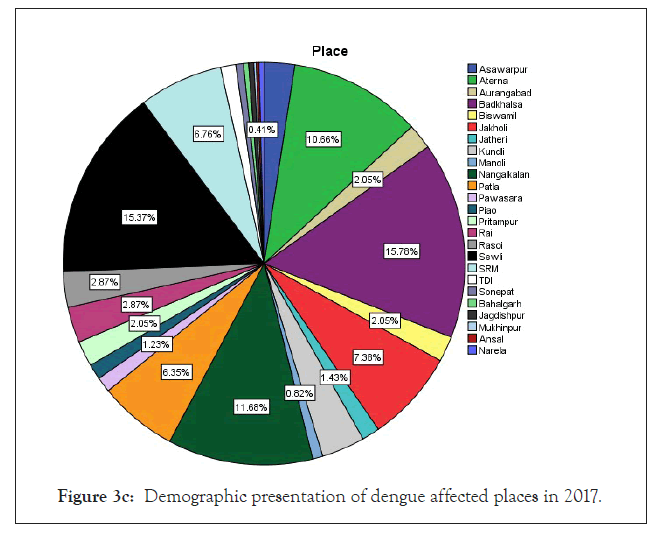
Figure 3c: Demographic presentation of dengue affected places in 2017.
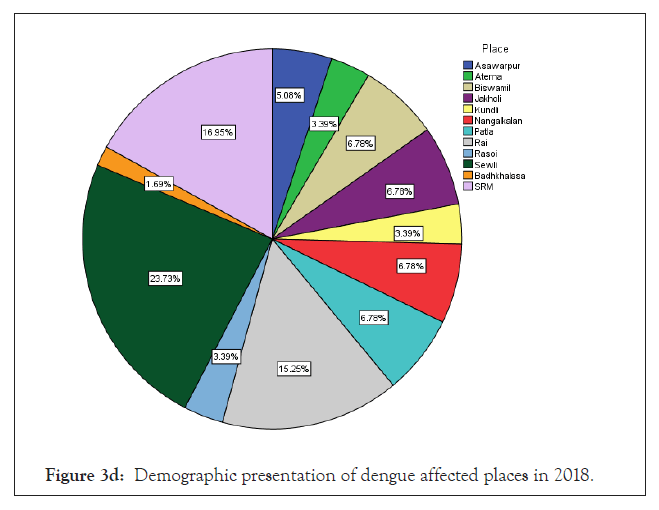
Figure 3d: Demographic presentation of dengue affected places in 2018
Figure 4a: Demographic presentation of date wise dengue cases in 2015.
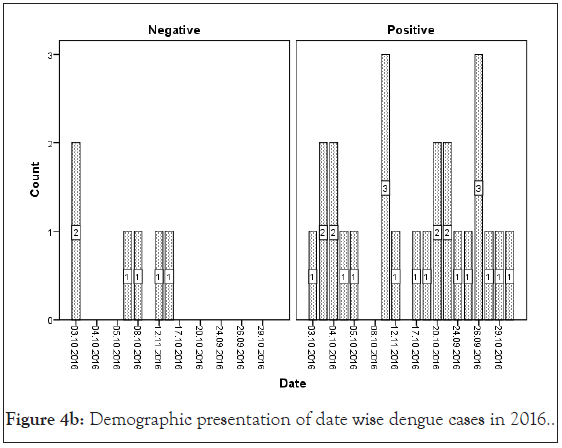
Figure 4b: Demographic presentation of date wise dengue cases in 2016
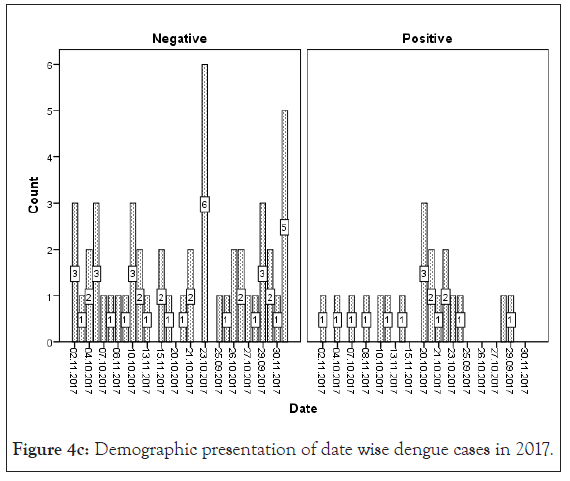
Figure 4c: Demographic presentation of date wise dengue cases in 2017.
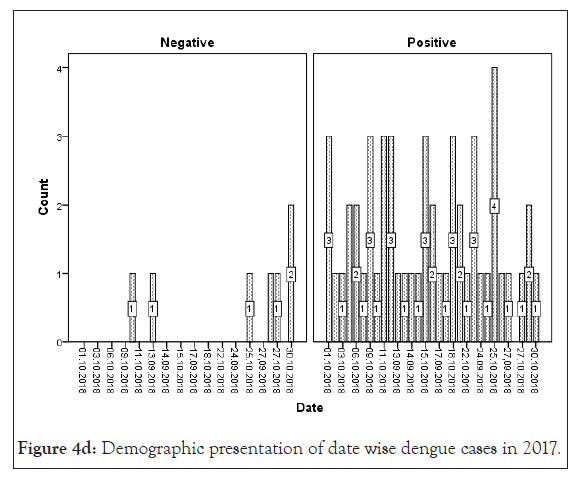
Figure 4d: Demographic presentation of date wise dengue cases in 2017.
Serological data analysis
The sample has been analyzed to classify dengue primary infection and secondary infection in Figure 2 and Table 1. It was found that dengue infected patients have high primary infection (NS1-Positive IgG/IgM-negative) results in 2015, 2016 and 2018 and maximum secondary infection (NS1-positive; IgG/IgM-positive) result in 2017 and throughout the year total 100 patients’ have primary and 67 patients’ have secondary DI from 2015 to 2018 (Figure 2 and Table 1).
| Year | Clinical parameters | Prevalence of disease (%) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | Accuracy (%) |
|---|---|---|---|---|---|---|---|
| 2015 | Hgb<12 g/dL (Female) | 76.92% | 70% | 83.33% | 93.33% | 45.45% | 73.08% |
| TLC<4000 cells/cmm | 35.09% | 100% | 54.05% | 54.05% | 100% | 70.18% | |
| Eosinophils>4% | 9.38% | 16.67% | 32.76% | 2.50% | 79.17% | 31.25% | |
| PLT<1,50,000 platelets /mcL | 20.63% | 100% | 48% | 33.33% | 100% | 56.73% | |
| MCH>32 pg | 22.92% | 90.91% | 45.95% | 33.33% | 94.44% | 56.25% | |
| 2016 | TLC<4000 cells/cmm | 58.06% | 94.44% | 38.46% | 68% | 83.33% | 70.97% |
| 2017 | TLC<4000 cells/cmm | 57.35% | 41.03% | 93.10% | 88.89% | 54% | 63.24% |
| 2018 | TLC<4000 cells/cmm | 50% | 100% | 24.14% | 56.86% | 100% | 62.07% |
| PLT<1,50,000 platelets/mcL | 81.36% | 85.42% | 0% | 78.85% | 0% | 69.49% |
Table 2: Sensitivity, specificity and accuracy of diagnosis test evaluation of dengue patients.
Hematological data analysis
Hematological analysis of CBC for all patients from 2015-2018 has been shown in Table S2. Our present study showed that TLC<4000 cells/mcL, lymphocytes <20%, monocyte <2% has been found in all the dengue positive patients. ESR>20 mm/hr in female and >15 mm/hr in male; PCV<40% in male; RBC<4.7 cells/cmm in male. PLT 1,00,000-1,49,000/mcL; MCV<83 fL; MCH<27 pg and >32 pg, MCHC>34.5 g/dL were reported in maximum number of dengue positive patients from 2015-2018.
Sensitivity and specificity analysis
In our present study we analysed clinical data to test predictive model with the best sensitivity, specificity, prevalence of disease, diagnosis evaluation accuracy rate results from 2015 to 2018. Using Bayesian analysis model, dengue diagnosis evaluation determined TLC<4000 cells/mcL with 100% sensitivity, 54.05% specificity and 35.09% prevalence of disease with 73.08% test accuracy rate in 2015; 94.44% sensitivity, 38.46% specificity and 58.06% prevalence of disease with 70.97% accuracy rate in 2016; 41.03% sensitivity, 93.10% specificity and 57.35% prevalence of disease with 63.24% accuracy rate in 2017; 100% sensitivity, 24.14% specificity and 50% prevalence of disease with 62.07% accuracy rate in 2018 (Table 2).
Logistic regression analysis
In our study the clinical data analyzed using logistic regression for the year 2015 to 2018 and by using SPSS software. TLC (2015 to 2018), Eosinophils (2015), RBC (2017), PLT (2015, 2018), MCH (2015) were found statistically significant (p<0.05) with good likelihood model ratio and chi-square value of clinical data parameters (Table 3).
| Logistic regression | Likelihood model | Chi-square | Significant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2015 | 2016 | 2017 | 2018 | 2015 | 2016 | 2017 | 2018 | |
| Hgb (gm/dL) | 10.75 | 8.61 | 12.26 | 7.47 | 1.35 | 0.24 | 2.65 | 2.04 | 0.72 | 0.97 | 0.44 | 0.72 |
| TLC (cells/mcL) | 6.52 | 4.94 | 6.63 | 3.53 | 24.07 | 5.41 | 13.85 | 10.92 | 0.0001* | 0.02* | 0.0001* | 0.004* |
| Neutrophils (%) | 10.66 | 5.83 | 7 | 5.42 | 1.17 | 0.46 | 0.048 | 0.65 | 0.55 | 0.49 | 0.82 | 0.72 |
| Lymphocytes %) | 11.45 | 6.77 | 9.76 | 7.13 | 1.39 | 1.37 | 1.95 | 0.22 | 0.49 | 0.5 | 0.37 | 0.89 |
| Eosinophils (%) | 6.21 | 3.43 | 6.04 | 3.67 | 5.9 | 0.43 | 0.051 | 1.04 | 0.01* | 0.5 | 0.82 | 0.3 |
| Monocytes (%) | 8.296 | 5.08 | 7.34 | 3.64 | 1.01 | 0.03 | 0.09 | 3.41 | 0.31 | 0.85 | 0.76 | 0.065 |
| ESR (mm/hr) | 13.02 | 9.09 | 9.01 | 4.88 | 3.87 | 3.85 | 2.71 | 6.06 | 0.27 | 0.27 | 0.25 | 0.1 |
| PCV (%) | 11.64 | 7.96 | 11.91 | 7.47 | 6.88 | 2.8 | 5.6 | 2.29 | 0.14 | 0.59 | 0.34 | 0.51 |
| RBC (cells/cmm) | 12.4 | 7.59 | 9.27 | 7.3 | 5.79 | 6.39 | 9.19 | 4.43 | 0.32 | 0.17 | 0.06 | 0.48 |
| PLT (Platelets/mcL) | 4.37 | 5.06 | 9.87 | 5.54 | 15.44 | 0.97 | 9.83 | 11.94 | 0.0001* | 0.8 | 0.08 | 0.03* |
Note: * means approximate value.
Table 3: Logistic regression analysis of different parameters of dengue patients.
ROC analysis
In our analysis ROC analysis was done with best logistic regression results to compare the useful parameters of test TLC (cells/mcL) in dengue model from 2015-2018, obtaining an area under the curve of 0.66 ± 0.07 in 2015; 0.76 ± 0.10 in 2016; 0.68 ± 0.07 in 2017 and 0.79 ± 0.06 in 2018 respectively which were statistically significant (p<0.05) (Figures 3a-3d and Table 4).
| Clinical parameters | Area ± SE | Significant | ||||||
|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2015 | 2016 | 2017 | 2018 | |
| Hgb (g/dL) | 0.48 ± 0.07 | 0.48 ± 0.12 | 0.38 ± 0.07 | 0.58 ± 0.10 | 0.8 | 0.86 | 0.13 | 0.47 |
| TLC (cells/mcL) | 0.66 ± 0.07 | 0.76 ± 0.10 | 0.68 ± 0.07 | 0.79 ± 0.06 | 0.03* | 0.04* | 0.02* | 0.01* |
| Neutrophils (%) | 0.44 ± 0.07 | 0.43 ± 0.13 | 0.51 ± 0.07 | 0.54 ± 0.11 | 0.44 | 0.61 | 0.89 | 0.72 |
| Lymphocytes (%) | 0.43 ± 0.07 | 0.55 ± 0.12 | 0.43 ± 0.08 | 0.54 ± 0.11 | 0.37 | 0.68 | 0.38 | 0.7 |
| Eosinophils (%) | 0.40 ± 0.07 | 0.52 ± 0.13 | 0.49 ± 0.07 | 0.53 ± 0.09 | 0.22 | 0.88 | 0.94 | 0.74 |
| Monocytes (%) | 0.44 ± 0.07 | 0.48 ± 0.13 | 0.51 ± 0.07 | 0.38 ± 0.11 | 0.47 | 0.9 | 0.81 | 0.32 |
| ESR (mm/hr) | 0.57 ± 0.07 | 0.56 ± 0.14 | 0.61 ± 0.07 | 0.43 ± 0.10 | 0.3 | 0.67 | 0.15 | 0.55 |
| PCV (%) | 0.40 ± 0.07 | 0.43 ± 0.12 | 0.38 ± 0.07 | 0.62 ± 0.11 | 0.21 | 0.58 | 0.15 | 0.28 |
| RBC (cells/cmm) | 0.44 ± 0.07 | 0.36 ± 0.13 | 0.40 ± 0.07 | 0.59 ± 0.07 | 0.44 | 0.3 | 0.23 | 0.43 |
| PLT (Platelets/mcL) | 0.35 ± 0.06 | 0.44 ± 0.12 | 0.33 ± 0.07 | 0.55 ± 0.11 | 0.04 | 0.63 | 0.03 | 0.64 |
| MCV (fL) | 0.47 ± 0.07 | 0.40 ± 0.12 | 0.39 ± 0.07 | 0.53 ± 0.11 | 0.67 | 0.45 | 0.18 | 0.79 |
| MCH (pg) | 0.63 ± 0.07 | 0.43 ± 0.12 | 0.56 ± 0.07 | 0.56 ± 0.10 | 0.08 | 0.54 | 0.42 | 0.59 |
| MCHC (g/dL) | 0.58 ± 0.07 | 0.51 ± 0.13 | 0.56 ± 0.07 | 0.44 ± 0.12 | 0.28 | 0.92 | 0.44 | 0.63 |
Note: The test result variable: TLC has at least one tie between the positive actual state group and the negative actual state group, * means approximate value.
Table 4: ROC analysis of significant clinical parameters of dengue patients.
Dengue diseased patient current with AFI without contained signs and indications and the clinical appearances may be similar to other infections later making a discrepancy analysis difficult in individual it from other infections such as (malaria, chikunguniya, typhoid etc. The etiological diagnosis becomes necessary in the clinical management and dengue virus causes epidemic and sporadic cases year-round [12,30].
Although it is problematic to control why the frequency of dengue cases rises next every years, but there are many aspects which can accredited as important like rainfall, climate change, prevention awareness by people and government, immune system of people, dengue serotypes incidence, lack of knowledge, costly and insufficient serological test kits, negligence of regular clinical test and visit to local hospitals. According to the National Vector Borne Disease Control Programme (NCVBDCP) data, the last five years that were 99913 (cases), 220 (death) in 2015; 129166 (cases), 245 (death) in 2016; 188401 (cases), 325 (death) in 2017, 101192 (cases), 172 (death) in 2018; 136422 (cases), 132 (death) in 2019 were reported [31]. Because of symptoms are not specific and can be found in other infections. Our study acknowledged the important variances of clinical structures and CBC parameters to simplify the individual of DI from the other sources.
AFI are very common throughout tropical season mainly from August to November in various areas of India [30]. An earlier study proposed that maximum dengue cases are reported in the month of July to October for every year [15]. Also Jain et al. reported dengue positive cases from August and November 2015 in a tertiary care center in New Delhi, India [32]. Our organization in 2015 the first situation of dengue was established as positive in the last week of September with AFI regularly. Delhi-NCR, Sonepat faces large number of dengue cases every year in month September to December. In our study the maximum dengue positive cases were recounted in the month of October (2015-2018) (Figure 4).
Neralwar et al. reported that a total of 1637 samples are tested where 161(29.92%) samples were NS1 positive, 83(15.42%) were both NS1+IgM Ab positive and 294 (54.64%) were IgM Ab positive [33]. As per WHO guidelines (1997), there will be a primary antibody response with production of specific IgM antibody as early as 3-10 days. The anti-dengue IgG antibody will seem in the first week of illness [34]. The IgM antibody in secondary DI is significantly minor and untraceable than primary DI and also both anti-dengue IgM and anti-dengue IgG antibodies to confirm secondary DI [35].
We found that Dengue day 1 test was a useful rapid test in diagnosis of dengue primary and secondary infection through identification of NS1 Ag, IgG/IgM and able to diagnose a large number of sample at a time. Majority of the positive cases were noticed in the age group 10-30 year and male were more prone to DI while comparing with female. We had detected maximum secondary infection have been shown in the age group of 10-29 year and primary infection in the age group of 20-29 year which numbers were very less based on IgG/IgM antibodies detection test due to its simplicity, high specificity and sensitivity and it can lead to error in interpretation of diagnosis. More number of dengue positive cases was observed in NS1 antigen detection test in the age group of 10-50 year during the primary stage of DI and can be used as new biomarker for early finding in peripheral blood before formation of antibodies within 10 days of AFI where acute primary infection were higher detection rate than acute secondary infection.
In earlier research, Makroo et al. and Neralwar et al. found that the age group of 21-30 year (32.44%) was affected maximum with dengue positive in India [33,36]. Another study showed of maximum dengue positive cases were reported in the age group 35-68 year with male patients [18]. Joshi et al. reported that DIs was mostly seen in younger age of 2-25 year [37]. Jakribettu et al. reported in Mangalore area that 69 were confirmed dengue cases where 69.56% (48/69) were male and 30.43% (21/69) were female children [35]. In our study we reported that a total number of 223 patients’ data were collected during 2015-2018 with AFI symptoms where 167 were dengue positive cases with 100 primary and 67 secondary infection, where males were highly infected with dengue virus than females patients between the age group 10-19 year and 20-29 year that were mostly affected (Figures 1 and 2) (Tables 1 and S1).
In previous study, Ramandeep et al. reported that Hgb (5.4-12.7 g/dl), TLC (1200-18500/cumm), PLT (20,000-80,000 cells/cumm) were low in dengue positive patients [38]. Also, Sharma et al. revealed that maximum dengue positive patients from AIIMS, New Delhi presented low count of Hgb (8.1 g/dl), TLC (1.4 × 109/l) and PLT (26 × 109/l) with high fever, body aches, retro-orbital pain symptoms [39]. Also, Joshi et al. revealed that leukopenia (36%) and thrombocytopenia (57%) could be used in early diagnosis and proper management for dengue [37]. Ralapanawa et al. found that leucopenia (TLC<5000 cells/mm3) was observed among 70.9% of dengue positive patients during the first 3 days of acute febrile phase of the illness [40]. Khandelwal et al. revealed that 26% of the dengue positive patients with age group of 1-14 year had leucopenia (<4000 cells/mcL), 8% patients had leukocytosis (>11,000 cells/ mcL) and 68% patients had low neutrophil count (<40%) [41]. Also Joshi et al. reported a higher proportion of male showed increased hematocrit/ PCV [42]. In our study we observed that a maximum number of dengue positive patients were analyzed with low count of TLC, lymphocyte, monocyte, PLT and high count of MCHC depend on 2015-2018 duration data analysis. In previous study, Jain et al. reported that lowest PLT, leukocytosis were identified as predictors of dengue disease on multivariable logistic regression analysis [32]. Also we can observed earlier research that leucocyte count increase to get normal on day 9-10 after therapy which is very important for clinical improvement [43]. In our research, as per Bayesian analysis model and Logistic regression model, TLC <4000 cells/cmm (leukopenia) was mostly statistically significant (p<0.05) among different clinical parameters with high ROC value (area ± SE) through 35%-58% prevalence of disease, 41%-100% sensitivity, 38%-94% specificity with 62%-71% accuracy of dengue disease diagnosis test evaluation from the year 2015-2018 (Figure 5a-5d) (Tables 2-4).
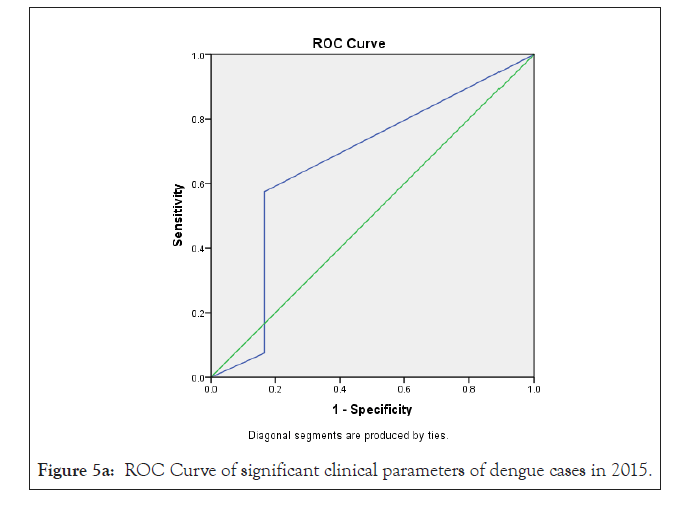
Figure 5a: ROC Curve of significant clinical parameters of dengue cases in 2015.
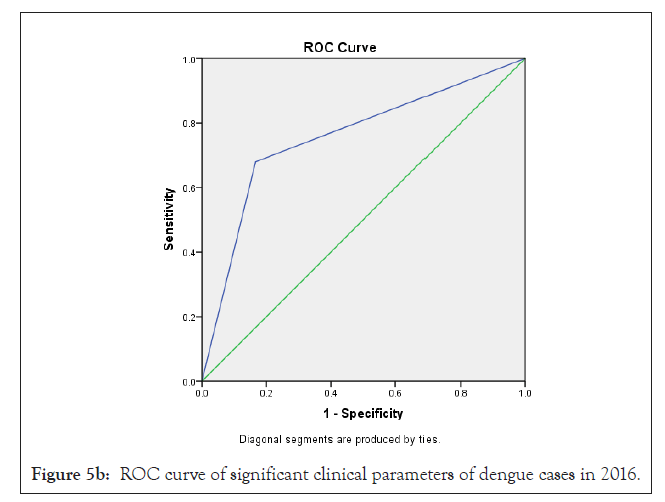
Figure 5b: ROC curve of significant clinical parameters of dengue cases in 2016
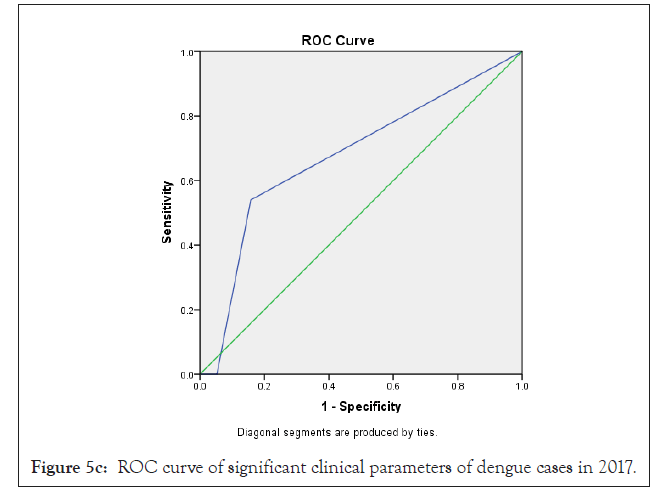
Figure 5c: ROC curve of significant clinical parameters of dengue cases in 2017.
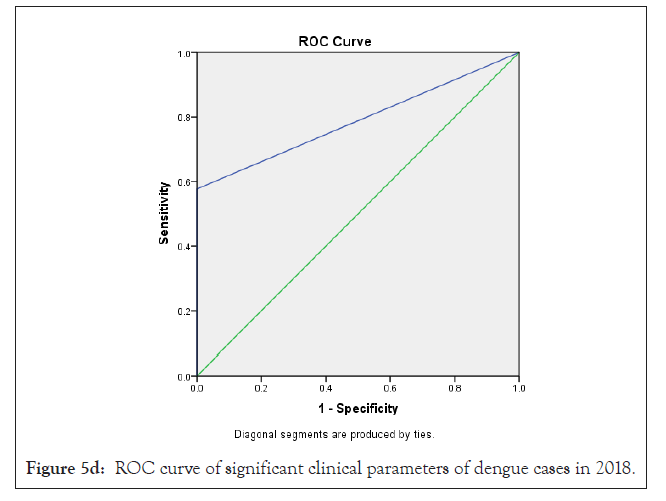
Figure 5d: ROC curve of significant clinical parameters of dengue cases in 2018.
The restriction of this study was that it was a surveying study. Much evidence which may be useful for contrast and the finding of DI was not available from the database. CBC parameters and supplementary effort needs to be completed using a detailed disease group to associate to the dengue group on doctor verdict as discrete case on the day to day source. The enrollment in the study was done from diagnosis and reviewed for history of fever which was matched the inclusion criteria which may be effect the selection bias of case and quality of the study. Boundaries of the learning included that it was a single-center, hospital-based study and that preferentially AFI patients demanding hospitalization were included.
Predictive model is of practical importance in hospitals or health organization having low cost and or limited access of laboratory resources depend on the clinical symptoms. The dengue forecast model presented in our study can be used to identify and detect the true dengue cases in early stages. We must say that dengue is still a serious health problem in Delhi-NCR, Sonepat area as the number of cases is rising from 2015 to 2018 due to rapid urbanization. As per our study, due to the amplified occurrence of previous infection of dengue, the percentage of secondary dengue cases were growing in Delhi-NCR, Sonepat area in every year. An earlier study recommended higher value of TLC can be used use as a dengue predictor, but we found that low amount of TLC was statistically significant from 2015 to 2018. Our analysis showed that the total white blood cell count was significantly lower in dengue positive cases from 2015 to 2018 as a threshold value of <4000 cells/mcL for leucopenia. Leucopenia is caused by bone marrow suppression by virus in acute phase illness due to decrease in polymorphs and inhibition of myeloid progenitor cells as bone marrow examination in the first 7 days. In 2015 we found that clinical parameters like high eosinophil, 100000-1,49,000 PLT/mcL range platelet count, high MCH count were statistically significant but not observed in 2016 to 2018 and which can be new parameters to predict recovery of DI. There were no previous study of eosinophil and MCH. So, we acknowledged significant clinical demonstrations and beneficial CBC parameters to qualify the distinction of dengue patients with AFI caused by other disease. These discoveries can be useful to local hospital circumstances as the CBC data can be composed from suspected patients and for more endorsement, a further examination should be considered for early analysis and treatment for the patients.
We thank to PRISMR Hospital, C4D and SRM University, Delhi- NCR, Sonepat to carry out the research.
Protection of human and animal subjects. The authors declare that no experiments were performed on blood sample of humans or animals for this study. We have collected data from PR Institute of Medical Science and Research Hospital. All authors declare that written informed consent was obtained from the patient for publication of this paper.
There is no conflict of interest among the authors
There is no financial support for conducting this research.
Citation: Paul A, Vibhuti A, Raj VS. (2021) Evaluation of Clinical and Hematological Parameters of Acute Febrile Illness Patients: A Dengue Predictive Model. J Antivir Antiretrovir. S17:001.
Received: 28-Jan-2021 Accepted: 12-Feb-2021 Published: 19-Feb-2021 , DOI: 10.35248/1948-5964.21.s17.001
Copyright: © 2021 Paul A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.