
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2021)
Hydroquinone (HQ) is a major benzene metabolite, which is produced after benzene biotransformation mainly in liver, lungs and bone marrow. However, the effects of mechanisms, in particular related to its genotoxicity in humans are not well understood. Argyreia nervosa (AN) is traditionally used in rheumatic, cerebral disorders, syphilis, and wound healing as immunomodulatory agent. The aim of this study was to access the protective effect of quercetin present in hydroalcoholic extract of Argyreia nervosa leaves against HQ-induced genotoxicity in rats and to identify and clarify the mechanisms, using albino rats of wistar strain bone marrow cells. The data suggested that HQ caused DNA strand breaks, DNA Protein Crosslink’s (DPC) and chromosomal breaks and significant increase in the frequency of micronuclei was found in the Micronucleus Test (MNT) and chromosomal aberrations assay. To elucidate the Oxidative DNA damage mechanism, parameters such as antioxidant enzymes like LPO, SOD, GSH and catalase levels were chosen to monitor the levels of Reactive Oxygen Species (ROS) and Glutathione (GSH) respectively. The present study showed that HQ induced the increased levels of ROS and depletion of GSH in rats; quercetin exerted an antigen toxic effect on HQ- induced genotoxicity, by significantly reducing the number of structural aberrations in chromosomes. Our results demonstrate that quercetin exerts a cytoprotective effect against HQ-induced oxidative stress, DNA damage and genotoxicity.
Argyreia nervosa; Hydroquinone; DNA protein crosslink’s; Micronucleus test; Reactive oxygen species; Glutathione
Genotoxicity refers to interaction with, or damage to DNA and other cellular components which regulate the fidelity of the genome. It is a broad term that, as well as mutation includes damage to DNA such as the production of DNA adducts, by the chemical itself or its metabolites [1]. Cells have the capacity to protect themselves from such potentially lethal or mutagenic genotoxic effects by many repair processes and therefore many genotoxic events do not become evident as mutations [1,2]. However, the capacity to damage the genome (genotoxicity) is an indicator of potential mutagenicity. Thus, some methods that measure genotoxicity may not provide direct evidence of heritable mutation.
Objective of genotoxicity testing
The objective of genotoxicity testing is to exclude or identify potential mutagenic hazards to humans, and, for those substances that are positive, to aid in the elucidation of the Mode of Genotoxic Action (MoGA). This guidance therefore presents a strategy for genotoxicity testing since this term encompasses all the assays included in the strategy. Consequently, it is important to generate information on three types of genetic damage, namely gene mutation, changes to chromosome structure (i.e. clastogenicity) and number (i.e. aneuploidy), to provide comprehensive coverage of the mutagenic potential of a chemical [2].
Etiology of genotoxicity
Genotoxic is a chemical or agent that can cause DNA or chromosomal damage. Such damage in a germ cell has the potential to cause a heritable altered trait (germline mutation). DNA damage in a somatic cell may result in a somatic mutation, which may lead to malignant transformation (cancer) are mutagens; they can cause mutations. Geno toxins include radiation (UV and Ionizing radiation), chemical geno toxins (Hydroquinone, cyclophosphamide, ampicillin trihydrate, ethionamide) [3].
Mechanisms involved in genotoxicity
A genotoxic carcinogen typically induces tumours in multiple organs of rodents, may be carcinogenic to more than one species and to both males and females. In addition, there is often evidence of a dose-response relationship for tumour induction, of the type that does not suggest evidence of a threshold [3-5].
Importance of genotoxicity
1. Many in vitro and in vivo tests for genotoxicity have been developed that, with a range of endpoints, detect DNA damage or its biological consequences in prokaryotic or eukaryotic cells.
2. These assays are used to evaluate the safety of environmental chemicals and consumer products and to explore the mechanism of action of known or suspected carcinogens.
3. All the regulatory authorities require information on the genotoxic potential and safety profile of the new chemical entity [3-5].
The Organization for Economic Co-operation and Development (OECD) develops and coordinates environmental health and safety activities on an international level [4]. These activities include (a) Harmonizing chemical testing and hazard assessment procedures (b) Harmonizing classification and labelling (c) Developing principle for Good Laboratory Practices (GLPs) (d) Co-operating in the investigation of existing chemicals and (e) Sharing and exploring possible cooperative activities on risk management (Figure 1).
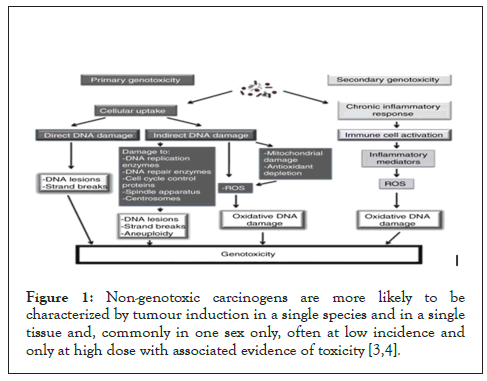
Figure 1: Non-genotoxic carcinogens are more likely to be characterized by tumour induction in a single species and in a single tissue and, commonly in one sex only, often at low incidence and only at high dose with associated evidence of toxicity [3,4].
Hydroquinone
Hydroquinone (HQ) is used as a developer in black-and-white photography, as an antioxidant by the rubber industry, and as a polymerization inhibitor of unsaturated monomers [6]. HQ also serves as an intermediate in the manufacture of food antioxidants (butylated hydroxyanisole and tert-butyl hydro quinone) and has been quantified in mainstream smoke from unfiltered cigarettes in amounts ranging from 88 μg to 155 μg per cigarette. HQ and products containing hydroquinone are used as skin-bleaching agents for hypermelanosis, freckles and post inflammatory hyperpigrnentation [7].
HQ is rapidly and extensively absorbed from the gut and trachea of animals. Absorption via the skin is slower but may be more rapid with vehicles such as alcohols. HQ distributes rapidly and widely among tissues. It is metabolized to p-benzoquinone and other oxidized products, and is detoxified by conjugation to monoglucuronide, monosulfate, and mercapturic derivatives [1,3,7]. The excretion of HQ and its metabolites is rapid, and occurs primarily via the urine. HQ and its derivatives react with different biological components, such as macromolecules and low relative molecular mass molecules, and have effects on cellular metabolism [7].
HQ can be metabolized to benzoquinones, which are potentially hematotoxic, genotoxic, and carcinogenic compounds that can also induce the formation of free radical, predisposing cells to oxidative damage. HQ exposure has been reported by the National Toxicology Program (NTP) to produce renal tubule adenomas and to exacerbate spontaneous Chronic Progressive Nephropathy (CPN) in male F344 rats [8]. Ames’ assay demonstrated that HQ was not mutagenic for salmonella typhimurium strain TA98, TA100, TA 1535 and TA 1537. HQ was mutagenic in many mammalian cells invitro using a variety of end-points.
Argyreia Nervosa (AN) (Syn: Argyreia speciosa) belongs to family convolvulaceae and commonly known as elephant creeper in english, chandrapada, samudrapala in telugu, samundar-ka pata in Hindi and vriddhadaruka in ayurveda. In ayurvedic preparations Argyreia nervosa used as rasayana drug. Argyreia nervosa is traditionally used in Rheumatic disorders, cerebral disorders, and inflammation, to treat wounds, syphilis, and diarrhea. Pharmacological activities such as anti-oxidant, anti-inflammatory, anti-rheumatic, immunomodulatory, adapt genic and hepatoprotective have also been reported. The present study was designed to authenticate the traditional claim of HAEAN in cytoprotective activity against oxidative damage by HQ [9]. Although several studies have proven the efficacy of quercetin as an antioxidant and anti-infiammatory agent, it has never been studied in a cytoprotective role against HQ toxicity.
Plant material and hydroalcholic extraction
The leaves of Argyreia nervosa (AN) 1 kg were collected from tirumala hills in tirupathi, chittoor district, Andhra Pradesh, India. The plant was authenticated by Dr. Madhava Chetty, Professor, department of botany, Sri Venkateshwara University, Tirupati. A voucher specimen of same was already present (Voucher specimen No. 1211) in the department of botany, S.V. University, Tirupati. The collected leaves were shade dried at room temperature and coarsely powdered, and macerated in 70% alcohol for 7 days with an intermittent vigorous shaking. Then resulted extract was dried by flash evaporation to obtain dark green residue and the percentage yield was 32.8% w/w [2,9].
Preliminary phytochemical analysis
Standard qualitative screening test of the extract was carried out for various plant constituents. The crude extract was screened for the presence or absence of secondary metabolites using standard procedures [3,7].
Animals
Pathogen free adult albino rats weighing 120-150 gm were used and housed 4 per cage in polypropylene cages less than 12 hrs light/ dark cycles in rooms maintained at 25 ± 30ºC fed with standard laboratory chow and water ad libitum. Animals were acclimated to their surroundings for 10 days to eliminate the effects of stress prior to initiation of experiments [10]. All the experimental protocols were approved by the Institutional Animal Ethical Committee (IAEC) of Sri Padmavathi School of Pharmacy (SPSP: 1016/PO/ Re/S/06/CPCSEA/2018/010). All the guidelines and principles of the protocol were in compliance with the guidelines and principles laid down by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), govt. of India, and New Delhi [11].
Acute oral toxicity study
The acute oral toxicity test of the extract was carried out by using albino rats of either sex weighing between 150-200 gas per revised OECD (Organization for Economic Cooperation and Development) guidelines 423. The treated animals were monitored for 14 days, for mortality and general behaviour. No death was observed till the end of the study. The extract was found to be safe up to the dose of 5000 mg/kg, hence 1/10th of the tested dose, 500 mg/kg dose was chosen as the experimental dose [12].
Experimental design
The rats of either sex were divided into 4 groups of six animals each. Normal rats (group I) were given vehicle only (2 ml water) to study normal parameters, group II were given only 500 mg/kg of hydroquinone to assess the normal parameters in disease control. While III and IV groups received 500 mg/kg of hydroquinone and alcoholic extract of ANR 500 mg/kg and 100 mg/kg of ascorbic acid respectively [9,11-13]. Bone marrow and liver, kidney tissue samples were collected for further genotoxic examination.
Micronucleus Test in rat bone marrow (MNT): The MNT was performed following the protocol of Natarajan and darroudi with some modifications. Studies were initiated by seeding cells onto tissue flasks and incubated at 37ºC for 24 h. Then they were treated with cyclophosphamide for 1 h. After washing twice with PBS, the cytokinensis blocking agent cytochalasin B was added in fresh medium for another 24 h [13]. Following exposure to cold hypotonic solution (5.6 g/l KCL) for 20 min, the cells were gently fixed in a methanol glacial acetic acid (3:1) solution twice. After air drying on conventional slides, the cells were stained with 2% Giemsa solution. According to the established criteria, a total of 1000 Binucleated Cells (BNC) was scored for the evaluation of the frequencies of MN [14].
Chromosomal analysis: Chromosome analysis was performed as described previously. Rat bone cells were collected from HQ treated animals. Rat bone marrow cells were cultured for 72 h at 37ºC for chromosomal analysis. After 70 h of incubation, 90 mL of colcemid was added into the culture and incubated for 2 h, and centrifuged at 1000 xg for 10 min at RT. The supernatant was carefully removed, the cells resuspended in a hypotonic solution (0.075 M KCl) at 37ºC for 20 min, centrifuged, and fixed with ice cold methanol/glacial acetic acid (3:1 v/v). Fixation and centrifugation were repeated several times until the supernatants were clear [14,15]. Cells were pelleted and resuspended in a minimal amount of fresh fixative to obtain a homogeneous cell suspension. The cell suspension was dropped onto slides and left to air-dry. Slides were stained with 2% giemsa solution. All slides were initially screened at 10 x and the metaphase spreads were scored at 100 x magnification under oil immersion. Triplicate cultures were maintained for each group altogether 300 metaphases per each group were noted. Frequency and types of structural chromosome aberrations (chromatid breaks, chromosome breaks, gaps, and tetraploid chromosomes) were scored [3,16].
Estimation of tissue antioxidant parameter
Superoxide Dismutase (SOD): SOD was estimated by the method of misra and Fridovich 0.5 ml of sample was diluted with diluted with 0.5 ml of distilled water, to this 0.25 ml ethanol; 0.5 ml of chloroform (all reagents chilled) was added. The mixture was shaken for 1 min and centrifuged at 2000 rpm for 20 min. The enzymatic activity in supernatant was determined. To 0.05 ml of carbonate buffer (0.05 M, pH 10.2) and 0.5 ml of EDTA (0.49 M) was added [7,10,17-19]. The reaction was initiated by the addition of 0.4 ml of epinephrine and the change in optical density/min was measured at 480 nm. SOD activity was expressed as units/ mg protein change in optical density/min. 50% inhibition of epinephrine to adrenochrome transition by enzyme is taken the enzyme unit. Calibration curve was prepared by using 10-125 units of SOD.
Catalase: 2.5 ml of Phosphate buffer and 0.1 ml of serum was incubated at 250◦C for 30 minutes. The absorbance was measured at 240 nm then 650 μl of hydrogen peroxide solution was added to initiate the reaction. The change in absorbance was measured for 3 minutes and activity was expressed as the μmoles of H2O2 degraded/ml/min [20].
Reduced glutathione: To citrated blood, 5% Trichloro Acetic acid (TCA) solution was added and centrifuged at 3000 rpm for 20 minutes. To 0.1 ml of supernatant, 1 ml of sodium phosphate buffer and 0.5 ml of DTNB reagent were added [21]. The absorbance of yellow color developed was measured at 412 nm. The values were expressed in mm/ml [3,6,22].
Lipid peroxidation: 0.1 ml of Plasma was treated with 2 ml of 37% Thiobarbituric Acid (TBA), 25N Hydrochloric acid (HCL) and 15% Trichloro Acetic acid (TCA) in 1:1:1 ratio and placed in water bath for 15 min, cooled and centrifuged and clear supernatant was measured at 535 nm against reference blank and the values were expressed in μM/ml/min.
Histopathology: The liver, kidney, that is collected after sacrificing the animals was blotted free of blood and other tissue fluids using the filter paper and stored in 10% formal saline solution before determining the histopathology [22].
To determine the histopathology, the tissue was fixed with bovine fluid (picric acid, formalin and acetic acid in the ratio of 75:52:5) and after 24 hours it was washed thoroughly in 70% alcohol and subjected to dehydration in ascending grades of alcohol (70% and 100%) followed by treatment of the tissue with the mixture of xylene and toluene of equal proportion in a successive manner of 10%, 50%, 70%, 90% paraffin wax in toluene and finally with 100% paraffin wax at a temperature of 60-70oC. Finally the tissue was embedded in the wax [2,20].
Statistical analysis: All the data was expressed as mean ± SEM. Statistical significance between more than two groups was tested using one-way ANOVA followed by Bonferroni multiple comparison test using the computer based fitting program (Prism, Graph pad 5). The significance level was set at P<0.001 for all tests.
By performing preliminary phytochemical analysis to HAEAN, the total phenolic content was found to be 1.082 mg/g of GAE (Gallic Acid Equivalent), flavonoid content was 0.784 mg/g of IE (Isoquercetin Equivalent) and the tannin content was 1.301 mg/g TAE (Tannic Acid Equivalent) (Table 1) and (Figures 2 and 3).
| S. No | Treatment | % of PCE | MnPCE/1000 PCE cells |
|---|---|---|---|
| I | Normal | 50.42 ± 1.405 | 4.516 ± 0.4574 |
| II | Control (Hydroquinone- 500 mg/kg; p.o) | 27.12 ± 0.8362+++ | 18.76 ± 0.4267+++ |
| III | Test -1 (Hydroquinone 500 mg/kg; p.o + Argyreia nervosa 500 mg /kg; p.o.) | 37.55 ± 0.8393** | 14.94 ± 0.4335** |
| IV | Test- 2 (Hydroquinone 500 mg/kg; p.o.+ Ascorbic acid 100 mg/ kg; p.o.) | 31.59 ± 0.4213* | 16.44 ± 0.4602* |
Note: All values are shown as mean ± SEM and n=6, +++P<0.001 when compared with normal control group. **P<0.001 when compared with disease control group.
Table 1: Percentage of Polychromatic Erythrocytes (PCE)-Micronucleated Polychromatic Erythrocytes (Mn-PCE).
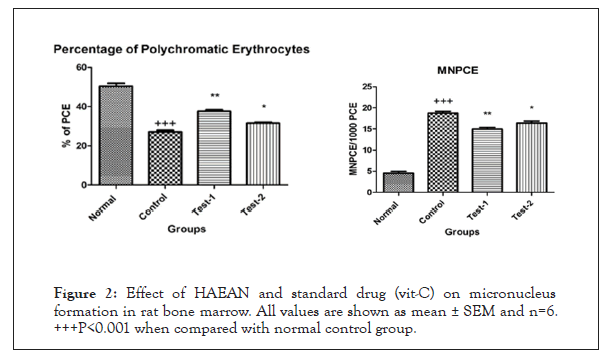
Figure 2: Effect of HAEAN and standard drug (vit-C) on micronucleus formation in rat bone marrow. All values are shown as mean ± SEM and n=6. +++P<0.001 when compared with normal control group.
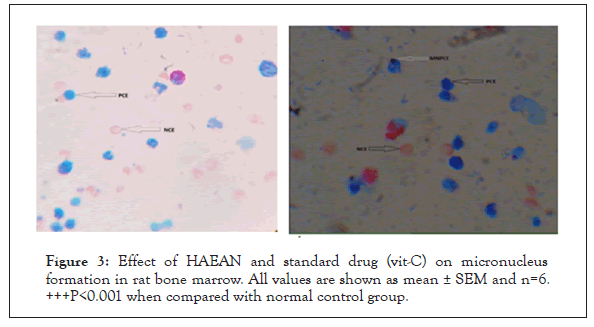
Figure 3: Effect of HAEAN and standard drug (vit-C) on micronucleus formation in rat bone marrow. All values are shown as mean ± SEM and n=6. +++P<0.001 when compared with normal control group.
Effect of HAEAN and VIT-C on micronucleus formation in rat bone marrow- percentage of Polychromatic Erythrocytes (PCE)-Micronucleated Polychromatic Erythrocytes (Mn- PCE)
Significant (P<0.0001) decrease in percentage of Polychromatic Erythrocytes (PCE) and rise in Micronucleated Polychromatic Erythrocytes (Mn-PCE) in rat bone marrow of control groups when compared with normal groups. There was a significant (P<0.001) elevation of percentage of Polychromatic erythrocytes and decreased Micronucleated Polychromatic Erythrocytes (Mn- PCE) was observed in Argyreia nervosa (500 mg/kg; p.o) and ascorbic acid 100 mg/kg; p.o.) treated groups when compared with disease control group (Table 2).
| S. No | Treatment | Chromosome breaks |
Chromatid breaks |
Chromatid gaps |
Quadri-radials | Percent of aberrant cells |
Mitotic index |
|---|---|---|---|---|---|---|---|
| I | Normal | 8 | 10 | 7 | 0 | 5 ± 1.16 | 18.3 ± 4.4 |
| II | Control (Hydroquinone- 500 mg/kg; p.o) |
28 | 44 | 17 | 6 | 14.8 ± 3.80 | 10 ± 2.8 |
| III | Test Hydroquinone 500 mg/kg; p.o. + Argyreia nervosa 500 mg /kg; p.o.) |
17 | 11 | 10 | 1 | 8.8 ± 2.11 | 16.6 ± 1.8 |
| IV | Standard Hydroquinone (500 mg/kg; p.o.+ Ascorbic acid 100 mg/ kg; p.o.) |
12 | 9 | 8 | 0 | 5.6 ± 1.38 | 17.4 ± 3.8 |
Note: All values are shown as mean ± SEM and n=6, +++P<0.05 when compared with normal control group. ***P<0.05 when compared with disease control group
Table 2: Effect of HAEAN and standard drug (vit-C) on chromosomal aberration in rat bone marrow.
Effect of HAEAN and standard drug (vit-C) on chromosomal aberration in rat bone marrow
Percent of aberrant cells: Total number of cells with chromosome aberration excluding gaps/total number of cells scored X 100.
A: Normal metaphasespread. B: Metaphase with Quadriradial.
C: Metaphase with chromosome gap.
D: Metaphase with chromosome and chromatid break.
Black arrow represent chromatid break, Red arrow represent chromosomal break.
There was a notable (p<0.05) increase in chromosome breaks, chromatid breaks, chromosomal gaps, quadriradials, percentage of aberrant cells and decrease in mitotic index in rat bone marrow of control groups when compared with normal group (Table 3) and (Figure 4).
| S. No | Groups | SOD (U/mg protein) liver | Catalase (µM H2O2 | GSH (µg of GSH/mg protein) | LPO (nm of MDA/mg protein) |
|---|---|---|---|---|---|
| Consumed/mg protein) | |||||
| 1 | Normal | 11.77 ± 0.207 | 109.5 ± 2.187 | 37.16 ± 0.6910 | 14.37 ± 1.163 |
| 2 | Disease control | 3.466 ± 0.308 | 36.90 ± 2.840 | 5.082 ± 0.6721 | 96.38 ± 17.32 |
| 3 | Standard | 7.972 ± 0.246 | 94.26 ± 3.81 | 12.37 ± 0.5972 | 32.74 ± 1.648 |
| 4 | Test | 5.729 ± 0.466 | 60.74 ± 2.414 | 11.02 ± 0.1543 | 56.84 ± 1.596 |
Note: All values are shown as mean ± SEM and n =6, a=**=p< 0.05 when compared with normal b=*=p<0.05 when compared with diseased control.
Table 3: Antioxidant enzyme levels in liver homogenates.
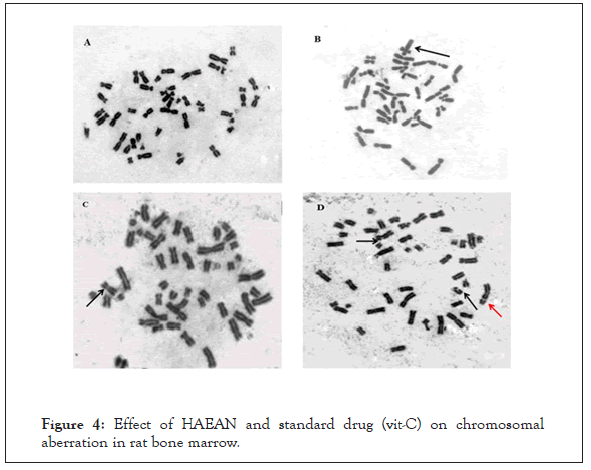
Figure 4: Effect of HAEAN and standard drug (vit-C) on chromosomal aberration in rat bone marrow.
The test and standard groups administered with Argyreia nervosa (500 mg/kg; p.o) and ascorbic acid (100 mg/kg; p.o.) are significantly decreased in chromosome breaks, chromatid breaks, chromosomal gaps, quadriradials, percentage of aberrant cells and rise in mitotic index when compared with that of disease control group (p<0.05).
Antioxidant studies
Antioxidant levels in liver and kidney
Effect of HAEAN and standard drug (vit-C) on in vivo antioxidant levels of liver and kidney homogenates.
Effect of HAEAN and standard drug (vit-C) on in vivo antioxidant SOD levels of liver and kidney homogenates
Disease control group showed prominent (p<0.001) reduction in the SOD levels when compared with of normal control group. Increased SOD levels were found to significant (p<0.001) in test and standard groups treated with Argyreia nervosa 500 mg/kg; p.o) and ascorbic acid 100 mg/kg; p.o). When compared with that of disease control group in catalase levels. When compared with the Argyreia nervosa treated groups, ascorbic acid treated groups showed increase in the liver and kidney SOD levels (Table 4).
| S. No | Groups | SOD (U/mg protein) | Catalase (µM H2O2 Consumed/mg protein) |
GSH (µg of GSH/mg protein) | LPO (nm of MDA/mg protein) |
|---|---|---|---|---|---|
| 1 | Normal | 10.11 ± 0.278 | 44.46 ± 1.238 | 27.09 ± 1.099 | 25.81 ± 0.848 |
| 2 | Disease control | 2.767 ± 0.194 | 11.97 ± 1.825 | 5.825 ± 0.467 | 189.5 ± 3.110 |
| 3 | Standard | 6.006 ± 0.231 | 30.24 ± 1.923 | 15.21 ± 0.612 | 43.78 ± 2.011 |
| 4 | Test | 4.137 ± 0.188 | 25.32 ± 1.886 | 11.781 ± 0.104 | 61.60 ± 1.702 |
Note: All values are shown as mean ± SEM and n=6, a= ** =p<0.05 when compared with normal b= * =p<0.05 when compared with diseased control.
Table 4: Effect of HAEAN and Vit-C on antioxidant enzyme levels in kidney homogenates.
Effect of HAEAN and standard drug (vit-C) on in vivo antioxidant catalase levels of liver and kidney homogenates
Disease control group showed prominent (p<0.001) reduction in the catalase levels when compared. The test and standard groups administered with Argyreia nervosa (500 mg/kg; p.o) and ascorbic acid 100mg/kg; p.o.) are significantly increased in catalase levels in liver and kidney when compared with that of disease control group (p<0.001). When compared with the Argyreia nervosa treated groups, ascorbic acid treated groups showed increase in the liver and kidney catalase levels (Figure 5).
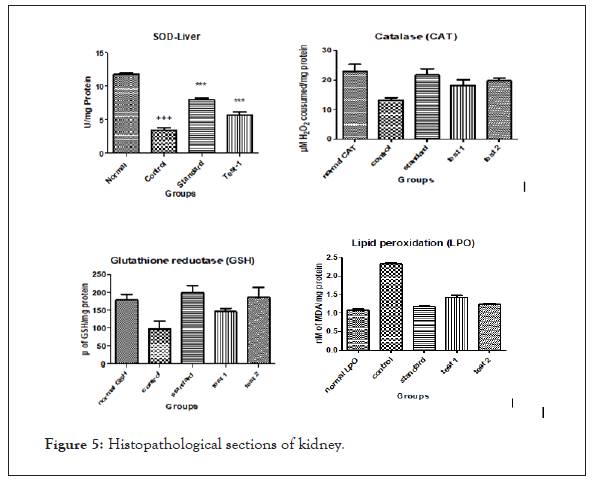
Figure 5: Histopathological sections of kidney.
Effect of HAEAN and standard drug (vit-C) on in vivo antioxidant GSH levels of liver and kidney homogenates
Significant (P<0.001) reduction in levels of GSH were seen in disease control group when compared to that of normal control group. There was a significant (P<0.001) elevation of reduced glutathione levels was observed in Argyreia nervosa (500 mg/kg; p.o) and ascorbic acid 100mg/kg; p.o.) treated groups when compared with disease control group. Ascorbic acid treated groups showed increased levels of reduced glutathione when compared to Argyreia nervosa treated groups.
Effect of HAEAN and standard drug (Vit-C) on in vivo lipid peroxidation levels of liver and kidney homogenates
There was a notable (p<001) rise in the malondialdehyde levels in the control groups when compared to the normal group depicting the lipid peroxidation.
Histopathological studies
Histopathology of liver, kidney of animals had shown damages in the architecture of the hepatic and renal tubular cells in the control group when compared to the normal, test (HA of AN) and standard treated groups (Figure 6).
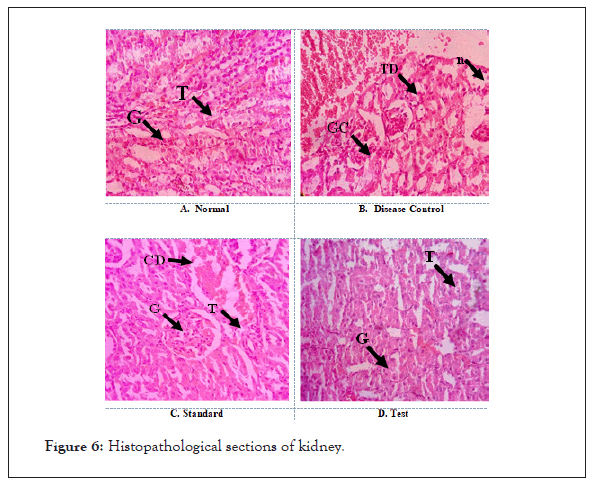
Figure 6: Histopathological sections of kidney.
A. Normal: Shows normal architecture with appearance of Glomeruli (G) and Tubular cells (T).
B. Disease Control: Section shows severe degeneration of Tubular cells (TG) with Glomerular Congestion (GC). Dilation of cystic and severe Necrosis (N) was also observed.
C. Standard: Protection from the drug induced renal damage is evident with normal appearance of Glomeruli (G) and Tubular cells (T). Mild Cystic Dilation (CD) was observed. No sign of necrosis indicates the protective effect of standard drug.
D. Test: Protection from the drug induced renal damage is evident with normal appearance of Glomeruli (G) and Tubular cells (T). No signs of cystic dilation and necrosis were observed.
Treatment with Argyreia nervosa (500 mg/kg; p.o) and ascorbic acid 100mg/kg; p.o.) treated groups showed significant (p<0.001) decreased levels when compared with disease control groups (Figure 7).
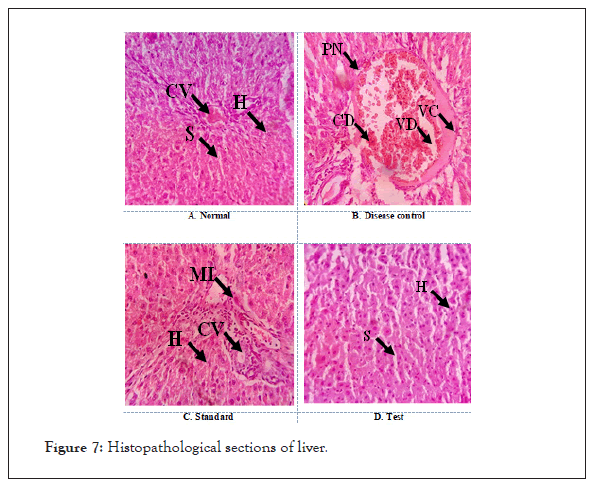
Figure 7: Histopathological sections of liver.
A. Normal: Section shows normal architecture with distinct Central Vein (CV), Sinusoidal spaces (S) and good number of functional Hepatic cells (H).
B. Disease control: Hepatic damage is evident with the disarrangement of hepatic cells, presence of Pyknotic Nuclei (PN), Cellular Decongestion (CD), vascular congestion and Vascular Degeneration (VD).
C. Standard: Preservation of hepatic organization is evident with functionally well-defined Hepatic cells (H), Central Vein (CV) and sinusoidal spaces. No signs of degenerations were observed, but Mild Inflammation (MI) was observed in comparison with normal control.
D. Test: Better preservation of hepatic organization is evident with functionally well-defined Hepatic cells (H), and sinusoidal spaces.
No signs of hepatic epithelial damage and pyknosis were observed.
Genotoxicity refers to interaction with, or damage to, DNA and/ or other cellular components which regulate the fidelity of the genome. Hydroquinone HQ is used as a developer in black-and- white photography, as an antioxidant by the rubber industry, and as a polymerization inhibitor of unsaturated monomers (Varagnat, 1981). HQ also serves as an intermediate in the manufacture of food antioxidants (butylated hydroxyanisole and tert-butyl hydro- quinone) and has been quantified in mainstream smoke from unfiltered cigarettes in amounts ranging from 88 to 155 μg per cigarette.
In our present study Hydroquinone was administered through oral route in which incidence of micronucleated polychromatic erythrocytes and chromosomal aberrations such as chromosome breaks, chromatid breaks, and chromosomal gaps were found to be increasing significantly and decreased tissue anti-oxidant levels were observed.
Evaluation of micronucleus frequency and chromosomal aberration in vivo are the primary models in the battery of genotoxicity tests and is recommended by the regulatory agencies around the globe to be conducted as part of product safety assessment. The assay, when performed appropriately, detects both clastogenicity (chromosome breakage) and aneugenicity (chromosome lagging due to dysfunction of mitotic apparatus).
Micronuclei, also haematologically known as howell-jolly bodies, are generally smooth, round remnants of nuclear chromatin seen in erythrocytes and are not to be confused for heinz or heinz ehrlich bodies resulting from oxidative injury to and precipitation of haemoglobin. In vivo, the process of erythropoiesis (production of erythrocytes) has been exploited in the micronucleus test.
The mammalian in vivo chromosome aberration test is used for the detection of structural chromosome aberrations induced by test compounds in bone marrow cells of animals, usually rodents. Structural chromosome aberrations may be of two types, chromosome or chromatid. In bone marrow cells, chromatid type aberrations can be stored in the first metaphase after treatment. Dicentric chromosomes, metacentric chromosomes, and chromosome fragments can be detected as well. An increase in polyploidy may indicate that a chemical has the potential to induce numerical aberrations. With the majority of chemical mutagens, induced aberrations are of the chromatid-type, but chromosome- type aberrations also occur. Chromosome mutations and related events are the cause of many human genetic diseases and there is substantial evidence that chromosome mutations and related events causing alterations in oncogenes and tumor suppressor genes are involved in cancer in humans and experimental systems.
To explore the mechanism of HQ genotoxic activity, further examinant were done to examined the level of ROS and the formation of 8-OHdG for HQ to induce oxidative DNA damage. By performing in-vivo antioxidant studies, results clearly showed that the formation of intracellular ROS was significantly increased in cells exposed to HQ at higher concentrations. ROS can damage DNA and lead to genotoxicity it is possible to infer that ROS may play an important role in the induction of HQ-DNA damage. Several previous studies demonstrated that HQ induced the generation of oxygen radicals, such as H2O2 and O in the presence of Myeloperoxidase (MPO) [22].
Among the variety of DNA lesions, 8-OHdG has been used the most extensively as a reliable marker of oxidative DNA damage produced by ROS. The 8-OHdG adduct can cause misreading of the DNA sequence during replication associated with G to T base trans versions and is therefore likely to be involved in mutagenesis and carcinogenesis from these results, we can infer that oxidative DNA damage generated directly or indirectly by HQ underlies the mechanisms of HQ induced DNA damage.
GSH performs several important biological functions, including quenching of ROS, and protection of cells from toxic compounds such as quinines The involvement of GSH in the HQ induced oxidative stress was examined through level of GSH. In the present study, a decrease of intracellular GSH was observed in rat kidney and liver tissues exposed to concentrations of HQ. These observations indicate that HQ can enhance oxidative stress by depleting intracellular GSH.
HQ induced DNA damage is mediated by the formation of ROS and depletion of GSH, which cause oxidative DNA damage, formation of DNA strand breaks by forming micronuclei and chromosomal aberrations conclude that the correlation between the alteration of intracellular GSH content and the formation and disappearance of HQ induced DNA damage, which clearly shows that GSH could be responsible for cellular defense against HQ induced DNA damage [1,20-22].
Phytochemical studies of hydroalcoholic extract of argyreia nervosa showed the presence of flavanoids, tannins, and polyphenols. Flavonoids like quercetin and kaempferol, are abundantly present in the argyreia nervosa. Polyphenols such as quercetin have become the subject of considerable attention, mainly because of their broad spectrum of beneficial effects. Although the protective effects of quercetin against various oxidative insults are well documented in literature, studies to alleviate hydroquinone induced toxicity are not well studied.
The present study, explores quercetin treatment attenuated the hydroquinone-induced DNA damage indicating its anticlastogenic potential. Anticlastogenic activity of quercetin might be due to: (i) Hydroxy group at third position of quercetin which might react with free radicals and thereby prevent the attack of free radicals on DNA, (ii) Its ability to bind with the DNA thereby preventing the reaction of free radicals with DNA, (Sengupta et al., 2006) (iii) Its free radical scavenging activity and inhibition of lipid peroxidation (iv) Higher diffusion into the membranes, which permits scavenging of oxyradicals at several sites throughout the lipid bilayer [5-8].
In the present study, the groups treated with argyreia nervosa showed significant increase in antioxidant levels like SOD, catalase and GSH, whereas decreased MDA levels when compared with control groups due to the predominance of QUR as a compound of polyphenols and flavanoids in preventing both metal and non- metal-induced oxidative damage is partly attributed to its free 3-OH substituent which is believed to increase the stability of the flavanoid radical. The catechol group is also directly connected to the chelating action of QUR, as has been proven by various studies in which quercetin suppresses lipid peroxidation by the scavenging action of its free radical.
Research reveals that flavonoids and vitamin-C have promising anti-oxidant potential. Oxidative damage in the cell or tissue occurs when the concentration of ROS (superoxide radical, hydroxyl radical and hydrogen peroxidase) generated exceeds the antioxidant capability of the cell by sies and stahl or when the antioxidant capacity of the cell decreases. Levels of non-enzymatic antioxidants (vitamin-C) and enzymatic antioxidants (GR and G-6-PDH) are the main determinants of the antioxidant defense mechanism of the cell [8,9-11].
From the histopathological slides, it was evident that the hepatic cell and the renal tubular cells were degenerated and damaged in the disease control rats. Whereas the architecture of hepatic cells and nephrons of kidneys were maintained normal in group-I rats.
As the normal rats, the histopathology of rats in group-III and group-IV treated with test and standard groups (HAEAN and Vit-C) respectively restored the architecture of hepatic cells and nephrons of kidneys is due to the presence of quercetin which scavenges the free radical generation by HQ thereby proven to be cytoprotective [12,22].
The present study was an attempt to evaluate the protective effect of quercetin on hydroquinone induced genotoxicity in rats with specific reference to oxidative stress and genotoxicity. Quercetin offered cytoprotection by maintaining cell viability, combated hydroquinone induced oxidative stress by restoring antioxidant enzyme activity, reducing LPO levels and reduced hydroquinone induced chromosomal aberration and DNA damage.
Genotoxins are the agents that can interact with the DNA, thus damaging its structure and causing mutations may lead to cancer. Identification of the genotoxic agents helps us understand the mechanism of the mutation and genotoxicity thereby paving way to better prevent the frequency of hazardous mutation and genotoxicity. In the present study, phytochemical studies of hydroalcoholic extract of Argyreia nervosa showed the presence of flavonoids, tannins, and polyphenols. Research revealed that flavonoids like quercetin has showed good antioxidant, antigen toxic, and immunomodulatory properties and offered cytoprotection by protecting the cells against hydroquinone-induced cytotoxicity by maintaining cell viability and reduced oxidative damage. Future studies can be focused on validating the mechanism of action responsible for the various beneficial effects and also on understanding which plant based compounds are responsible for the reported effects. Also, the outcome of such chemical studies may further expand its existing therapeutic potential.
Citation: Divya O, Pushpakumari B, Kumar AD, Nihafiaz S, Sameer P (2021) Evaluation of Cytoprotective and In vivo Anti-Oxidant Activity of Argyreia nervosa Leaves Extract against Hydroquinone Induced Genotoxicity. J Clin Toxicol. S20:003.
Received: 24-Nov-2021 Accepted: 08-Dec-2021 Published: 15-Dec-2021
Copyright: © 2021 Divya O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.