Journal of Clinical & Experimental Dermatology Research
Open Access
ISSN: 2155-9554
ISSN: 2155-9554
Research Article - (2019)Volume 10, Issue 1
Background: Psoriasis is a chronic, immune-mediated, inflammatory systemic skin disorder that predominantly involves the skin, nails, and joints. It affects approximately 2% of the population and poses a lifelong burden on those, affected.
Objective: To evaluate the efficacy of topical application of Novel Botanical Formulation PS-35 on psoriasis affected areas of the body and to evaluate whether PS-35 can be recommended as safe and effective topical dosage form for treatment of plaque psoriasis.
Methods: Totally 185 patients were selected for the study on the basis of PASI score (<10%). Patients were given the topical application of PS-35 twice a day. The duration of therapy was at least 12 weeks and effect of withdrawal of therapy was observed for 15 weeks.
Results: The results showed the reduction in itchiness (90%), scaliness (91.5%), hard plaque (88%) and hyperpigmentation (78%). No relapse of psoriatic symptoms was observed in 56.6% patients.
Conclusion: The topical application of PS-35 is found to be an acceptable and effective method to treat symptoms of chronic plaque psoriasis.
Plaque psoriasis; Novel botanical formulation; PS-35; PASI score
Psoriasis is a chronic, immune-mediated, inflammatory relapsing systemic skin disorder that predominantly involves the skin, nails, and joints with intermittent remissions. The disease is resulting from a polygenic predisposition, combined with environmental triggering factors. The worldwide prevalence of psoriasis is estimated to be approximately 2-3% [1]. Prevalence varies according to race, geographic location and environmental factors [2]. About two-thirds of affected individuals suffer from mild psoriasis, whereas about onethird have a more severe involvement.
The disease has wide clinical spectra that range from plaque, palmar and palmopustular, generalized pustular, guttate and erythrodermic psoriasis. The commonest type of psoriasis, accounting for 90% of all cases, is psoriasis vulgaris, in which papulosquamous plaques are welldelineated from surrounding normal skin. They are most active at the edge; rapidly progressing lesions may be annular, with normal skin in the centre. Plaques are usually distributed symmetrically, and occur most commonly on the extensor aspects of elbows and knees; scalp, lumbosacral region, and umbilicus [3]. Psoriasis can have profound effects on patients’ daily living and functioning [4,5].
Selection of treatment for psoriasis is based on several factors such as (1) age, (2) type of psoriasis, (3) site and extent of involvement based on Percentage Body Surface Area (%BSA) and Psoriasis Area and Severity Index (PASI) scores, (4) previous treatment undergone, (5) associated medical disorders (such as HIV disease, cardio vascular disease etc.).
The National Psoriasis Foundation found that more than half of the 5,600 people with psoriasis polled in a 2013 survey were dissatisfied with their disease management. This shows that new, more effective treatments are needed.
The current management for severe psoriasis is based on the principles of rotational therapy, which stress frequent alternation in treatment approach in order to reduce the cumulative risk of side effects [6]. An evidence based approach is essential in delineating difference between the many available treatments. Effect of novel botanical compounds in treatment of psoriasis can be evaluated. The effect of topical application of Novel Botanical Formulation PS-35 was studied in patients affected with chronic plaque psoriasis.
Study formulation
PS-35 is a Novel Botanical formulation developed for the effective management of psoriasis of the skin. The composition of the Novel Botanical Formulation PS-35 includes extracts of Wrightia tinctoria , Psoralea corylifolia , Nigella sativa , Rubia cordifolia and Cocus nucifera oil.
A clinical study was conducted to evaluate the efficacy of topical application of Novel Botanical Formulation PS-35 on psoriasis of the skin and to evaluate whether PS-35 can be recommended as safe and effective topical dosage form for treatment of chronic plaque psoriasis.
To conduct this single arm, open labelled, clinical study on evaluation of effect of topical application of Novel Botanical Formulation PS-35 on patients affected with chronic plaque psoriasis approval was obtained from the Institutional Ethical Committee at Madras Medical College, Chennai, India.
Patients of age above 18 years, both gender who were affected by chronic plaque psoriasis with PASI<10% were considered for the topical monotherapy with PS-35. Suitability of patients to topical therapy was ascertained by the clinicians. Exclusion criteria was, patients with PASI>10%.
Evaluation of the efficacy of topical monotherapy with PS-35
The total number of patients enrolled for the study was 315. Out of the total of 315, a total of 230 patients visited the psoriasis specialty clinic regularly. Remaining 85 patients were lost to follow-up because they were either unable to make it to the study centre, or unwilling to appear for fortnightly follow-ups. The total number of patients who were on treatment for at least 3 months with PS-35 was 185. The remaining 45 patients, who did not complete the minimum duration of treatment of 12 weeks, were excluded from the study.
The PASI score, affected areas of the body, symptoms present, and other required details were noted. A sample of PS-35 was provided to every patient with instructions for application. They were counselled to return for the next visit to collect PS-35. During the treatment sufficient quantity of PS-35 was applied topically as a layer on the lesion-affected area of the skin, twice a day in morning and night every day for at least 12 weeks, or until disappearance of the symptoms of psoriasis, as advised by the clinician. Fortnightly follow-up visits were carried out. Assessed parameters of therapy were reduction in symptoms, i.e., reduction in itchiness, scaliness, hard plaques, and/or hyperpigmentation. Improvement in psoriasis symptoms was noted, if any, on an improvised scale of 0 to 5 for each of the four parameters (parameter scores) for reduction in itchiness, scaliness, hard plaques and skin hyperpigmentation.
The duration of the study was 1 year and 4 months. For each patient, the expected period of therapy was minimum 3 months; the measured average period of therapy was 4.31 months. Therapy was extended in specific patients until hyperpigmentation reduced by 90% or greater.
Evaluation of effect of withdrawal of therapy
To evaluate the effect of withdrawal of therapy, PS-35 therapy was discontinued for a period of time as decided by the clinician for individual patients. After initial therapy and symptoms resolution, 106 patients were taken up for withdrawal of therapy (average 8 weeks up to 15 weeks). Out of 106, 91 patients were followed up for 90 days for relapse of symptoms. 15 patients were lost to follow up. Withdrawal continued till any relapse of symptoms (itchiness, scaliness, hard plaques, and/or hyperpigmentation) occurred and the number of days withdrawn were noted. For the patients with relapse of psoriatic symptoms in the area of application, PS-35 therapy was readministered.
Data collection
• Patient’s details such as name, age, clinical history, voluntary consent were documented.
• The Score on PASI scale for every patient was collected at the time of enrolment. Reduction in severity of psoriasis symptoms was obtained as ‘parameter scores’.
• Parameter scores include scores for ‘reduction in itchiness’ and ‘reduction in scaliness’, ascertained on a scale of 0 to 5 after 1 month of PS-35 application, and those for ‘reduction in hard plaques’ and ‘reduction in hyper pigmentation of skin’, that were ascertained after 3 months of PS-35 application (Table 1).
| S.No | Parameter score | Improvement in symptom | Parameters |
|---|---|---|---|
| 1. | Score 0 | 100% Reduction | (1) Reduction of itchiness (2) Reduction of scaliness, (3) Reduction of hard plaques (4) Reduction of skin hyperpigmentation |
| 2. | Score 1 | 90% Reduction | |
| 3. | Score 2 | 75% Reduction | |
| 4. | Score 3 | 50% Reduction | |
| 5. | Score 4 | 25% Reduction | |
| 6. | Score 5 | No Change in parameter level | |
| 7. | NA | Not Applicable |
Table 1: Parameter scores and the corresponding degree of improvement of psoriasis symptoms, as ascertained by the clinicians.
• Photographs of affected parts of the body were taken with the consent of the patient.
• Withdrawal data, including the number of days each patient actually did not apply the PS-35 was obtained.
The effects of withdrawal were assessed by the clinician. Based on clinician observations, a score of improvement (parameter score) was recorded for each parameter. The score was placed on an improvised scale of 0 to 5, where 0 generally denoted complete improvement, and 5, no change of condition from before commencement of therapy. The use of parameter score is as given in Table 2. Effect of therapy was determined by comparing parameter scores before and after therapy.
| No. of days therapy withdrawn |
Itchiness return |
Scaliness return |
Hard plaques return |
Hyperpigmentation return | Total |
|---|---|---|---|---|---|
| <30 | 0 | 1 | 0 | 0 | 1 |
| 30-60 | 12 | 2 | 0 | 1 | 15 |
| 60-90 | 12 | 3 | 2 | 0 | 17 |
| >90 | 3 | 1 | 0 | 0 | 4 |
| Total | 27 | 7 | 2 | 1 | 37 |
Table 2: Effect of withdrawal of PS-35: split-up into individual parameters.
Statistical analysis
Effect of therapy was determined by comparing parameter scores before and after therapy. Each individual patient acted as their own standard, making the need to normalize scores redundant. Effect of treatment was statistically analysed with ANOVA: two-factor without replication. Level of α was set to 0.01. F-test was applied, and the Fvalue, F-critical and p value were compared.
The scores before and after therapy were compared. If the comparison passed the F-test (F-value>F-critical; p value<α), the null hypothesis that assumes that any difference in scores is owing to randomness would be rejected. Therefore, the change being compared (=effect of therapy) would become significant.
Evaluation of the efficacy of topical monotherapy with PS-35
The results of evaluation of efficacy of topical application of Novel Botanical Formulation PS-35 on psoriasis affected areas of the body are stated here. Reduction in severity of psoriasis symptoms was represented as parameter scores ascertained on a scale of 0 to 5.
The total number of patients who were on treatment with PS-35 was 185. Improvement of psoriasis symptoms among patients on PS-35 therapy in terms of reduction in itchiness and scaliness in 1 month, and hard plaques and hyperpigmentation in 3 months are given below;
Reduction in itchiness: Almost 90% patients on average reported complete absence of itching after one month of treatment with PS-35 as represented in Figure 1.
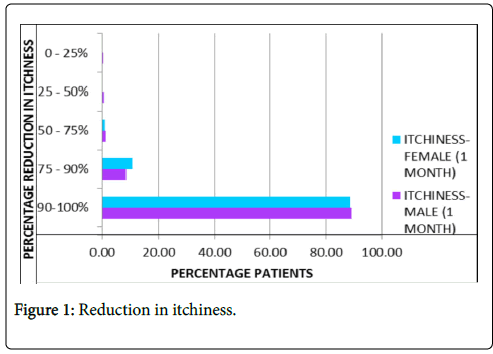
Figure 1: Reduction in itchiness.
Reduction in scaliness: About 91.5% patients on average reported complete absence of scaliness after one month of treatment as represented in Figure 2.
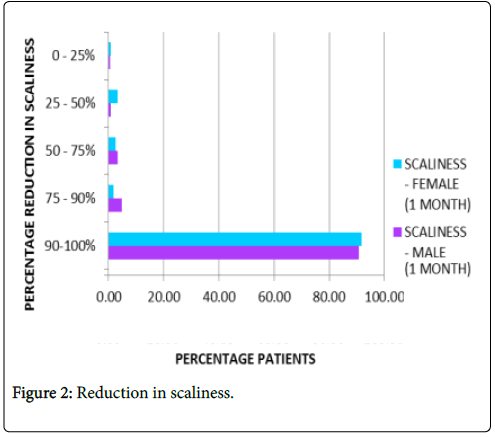
Figure 2: Reduction in scaliness.
Reduction in hard plaques: Nearly 88% patients on average reported complete absence of hard plaques after three months of treatment as represented in Figure 3.
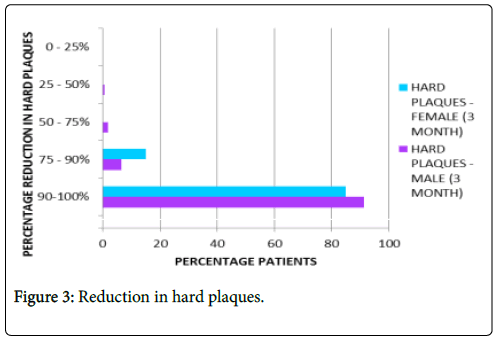
Figure 3: Reduction in hard plaques.
Reduction of skin hyper pigmentation: About 78% patients on average reported complete absence of hyper pigmentation after three months of treatment as represented in Figure 4.
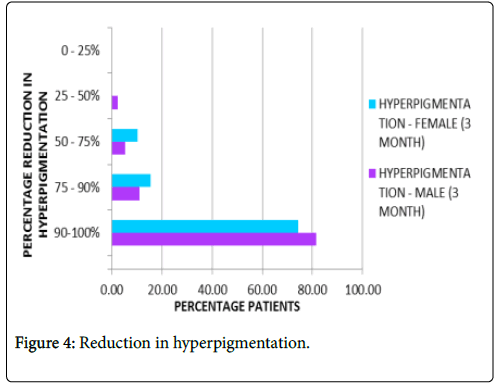
Figure 4: Reduction in hyperpigmentation.
As per the statistical analysis for all assessed parameters p-value is less than 0.01, this shows there is significant difference between before and after therapy groups.
Evaluation of effect of withdrawal of therapy
Total 91 patients were followed up for 90 days for relapse of symptoms in the area of application. The return of psoriatic symptoms was measured by the four parameters of assessment as; itchiness, scaliness, hard plaques and skin hyper pigmentation. For the patients with relapse of psoriatic symptoms the number of days withdrawn was noted.
Effect of withdrawal of therapy was observed in two groups of patients; namely no relapnse and relapse. The numbers of patients showing no relapse/relapse of symptoms are graphically represented in Figure 5. In the first group, total of 60 out of the 91 followed-up patients showed no relapse of psoriatic symptoms at the site of therapy after withdrawal of therapy up to 15 weeks.
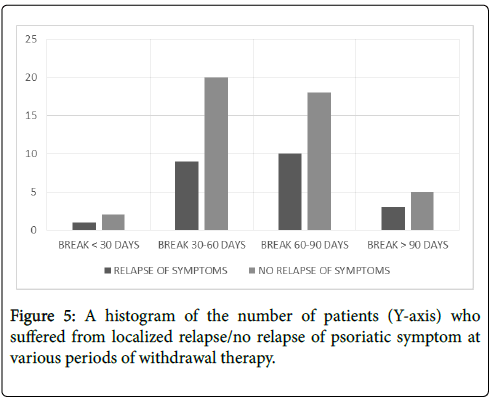
Figure 5: A histogram of the number of patients (Y-axis) who suffered from localized relapse/no relapse of psoriatic symptom at various periods of withdrawal therapy.
In the second group, 31 out of 91 followed-up patients showed relapse of at least one of the four parameters, namely return of itchiness, of scaliness, of hard plaques, and, of skin hyper pigmentation, in at least one part of the body that was subjected to PS-35 therapy. Effects of withdrawal of PS-35 as per individual parameters are given in Table 2.
Effective treatment with topical application of PS-35 is illustrated in Figures 6-11. Minimum duration of topical application was at least 12 weeks for PS-35 therapy.
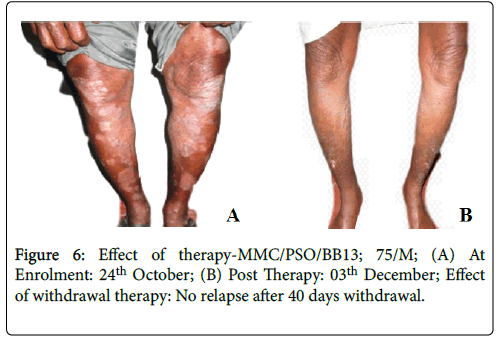
Figure 6: Effect of therapy-MMC/PSO/BB13; 75/M; (A) At Enrolment: 24th October; (B) Post Therapy: 03th December; Effect of withdrawal therapy: No relapse after 40 days withdrawal.
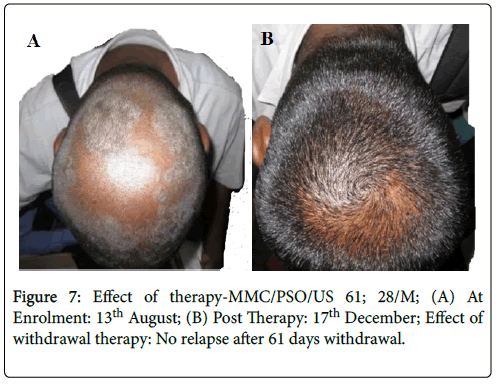
Figure 7: Effect of therapy-MMC/PSO/US 61; 28/M; (A) At Enrolment: 13th August; (B) Post Therapy: 17th December; Effect of withdrawal therapy: No relapse after 61 days withdrawal.
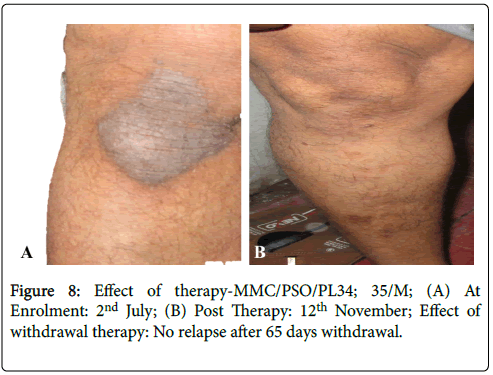
Figure 8: Effect of therapy-MMC/PSO/PL34; 35/M; (A) At Enrolment: 2nd July; (B) Post Therapy: 12th November; Effect of withdrawal therapy: No relapse after 65 days withdrawal.
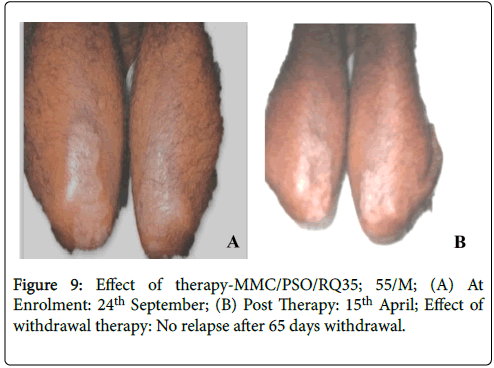
Figure 9: Effect of therapy-MMC/PSO/RQ35; 55/M; (A) At Enrolment: 24th September; (B) Post Therapy: 15th April; Effect of withdrawal therapy: No relapse after 65 days withdrawal.
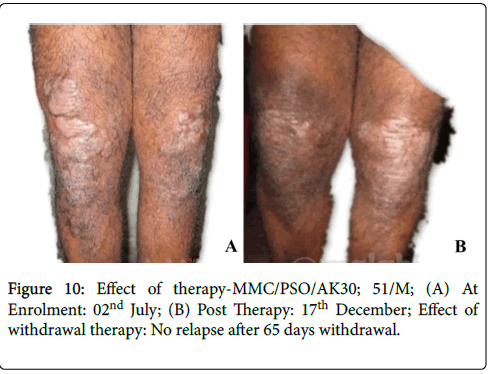
Figure 10: Effect of therapy-MMC/PSO/AK30; 51/M; (A) At Enrolment: 02nd July; (B) Post Therapy: 17th December; Effect of withdrawal therapy: No relapse after 65 days withdrawal.
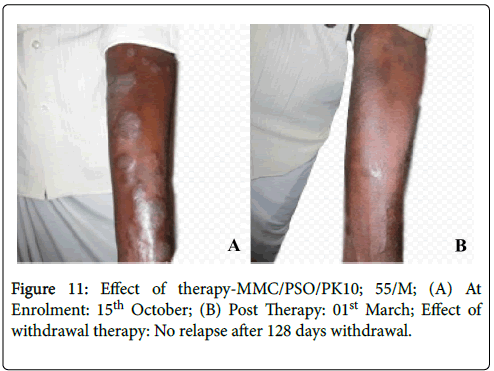
Figure 11: Effect of therapy-MMC/PSO/PK10; 55/M; (A) At Enrolment: 15th October; (B) Post Therapy: 01st March; Effect of withdrawal therapy: No relapse after 128 days withdrawal.
It was a single arm, open labelled clinical study. The study outcome was evaluated on four clinical parameters prospectively and on withdrawal of PS-35. For the patients who underwent PS-35 therapy, the improvement in psoriasis symptoms was determined on the basis of the four parameters of assessment as, reduction in itchiness, reduction in scaliness, reduction in hard plaques, and reduction in skin hyper pigmentation.
The objective of the study was to evaluate the efficacy of topical application of Novel Botanical Formulation PS-35 on psoriasis affected areas of the body and to evaluate whether PS-35 can be recommended as safe and effective topical dosage form for treatment of chronic plaque psoriasis. In the clinical study, PS-35 monotherapy was found to be effective for patients with PASI score <10%. No incidence of adverse event, exacerbation or allergy to the PS-35 was noted in this study, thus the botanical formulation was safe to use.
Psoriasis treatment is directed at decreasing signs and symptoms and modifying the natural progression of the disease. There are multiple effective treatment options with limited efficacy. Typically, topical agents are used for mild to moderate disease whereas topical phototherapy and systemic agents are used for moderate to severe disease. Biologics are prescribed to patients with moderate to severe psoriasis who were refractory to other treatments [7,8]. A large proportion of patients would benefit from an effective topical therapy, which can be initiated at the primary care level.
The adherence to topical therapy in patients with psoriasis is generally low; particularly in the long term for mild to moderate psoriasis the availability of effective topical therapy is limited [9]. The monotherapy with Novel Botanical formulation PS-35 has been found to be safe and effective topical dosage form for treatment of mild to moderate chronic plaque psoriasis.
The Novel Botanical Formulation PS-35 shows promise in being a standard, scientifically validated, easy-to-use, side-effect free, safe, lowcost, and effective topical therapy for chronic plaque psoriasis. Significant localized improvement was observed on the existing lesions of patients with chronic plaque psoriasis, who were on PS-35 topical therapy. The topical application of PS-35 persistently is found to be an acceptable and effective method to treat symptoms of chronic plaque psoriasis. Thus the single arm, open labelled clinical study results demonstrated that the Novel Botanical Formulation PS-35 is highly effective in treating chronic plaque psoriasis.
Citation:
Thomas J, Manoharan K, Dhanalakshmi A, Daniel SJ, Gowthaman AA, et al. (2019) Evaluation of Effect of Topical Application of Ps-35 on Patients Affected with Chronic Plaque Psoriasis: A Clinical Study. J Clin Exp Dermatol Res 10:478.
Received: 13-Aug-2018 Accepted: 18-Dec-2018 Published: 09-Jan-2019
Copyright:
© 2019 Thomas J, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.