Forest Research: Open Access
Open Access
ISSN: 2168-9776
ISSN: 2168-9776
Research Article - (2021)Volume 10, Issue 1
A pot study was conducted to measure the establishment success of five forages under 0%, 30% or 60% shade levels. The forages evaluated were ‘Pensacola” Bahia grass (Paspalum notatum Fluegge), “Texas Tough” Bermuda grass (Cynodon dactylon L. Pers.), “Alamo” switch grass (Panicum virgatum L.), “San Marcos” Eastern Gama grass (Tripsacum dactyloides L.), and a native mix containing by weight 45% “Texas” little bluestem (Schizachyrium scoparium Michx Nash), 15% sand love grass (Eragrostis trichodes Nutt. L. Alph. Wood), 15% “Blackwell” switch grass (Panicum virgatum L.), 10% “Lometa” Indian grass (Sorgastrum nutans L. Nash), 10% “Haskell” sideoats grama (Bouteloua curtipendula Michx Torr) and 5% “Earl” big bluestem (Andropgon gerardii Vitman). Mean biomass under 60% shades for all forages was less than under the other shade treatments, but did not differ among shade treatments within forages. Mean nutrient tissue concentration showed significant differences among treatments and forages for several nutrients. Shade treatments had no effect on plant density, but low germination of several forages appears to have influenced plant density. Based on these results, Bahia grass, eastern Gama grass and Bermuda grass may be suitable species if maximum biomass production were the goal of a silvopasture management system in east Texas.
Silvopasture; Loblolly pine; Bluestem; Switch grass; Bahia grass
Silvopasture, which combines timber production with livestock and forage production on the same land, might benefit landowners in the Southern United States with income from producing multiple crops simultaneously from the same land. While tree crops may take 10 to 12 years before the first harvest, silvopasture systems seek to utilize the land for additional income with little impact on the tree crop [1]. Revenues generated by silvopastures depend on variables such as fertilizer programs and type of cattle used; additional revenue may be gained over by allowing fee hunting, which can comprise five to nine percent of the total land value over the lifetime of the silvopasture [2]. Grasses are one of the earliest examples of the evolution of C4 photosynthesis, having first developed in the Oligocene epoch between 24 and 35 million years ago. Roughly 7,500 C4 plants currently exist, with 4,500 species of grasses representing the largest group [3].
While considerable research has been accomplished on forage crops in open pasture settings, little has been reported under partial shade. Specifically, analysis of light quality under loblolly pine (Pinus taeda L.) has not been widely performed. Common forage species such as Bermuda grass (Cynodon dactylon L. Pers.) and fescues (Festuca spp.) have been previously researched, but data is lacking for many forage grasses. Under different combinations of species and shade levels, mean dry weight (MDW) of 30 species was found to vary with amount of shade, species and growing season. Warm season grasses (C4) were found to have low shade tolerance under 50% and 80% shade regardless of season due to the poor response of the C4 metabolic pathway to shade [4]. Comparing Bahia grass (Paspalum notatum Fluegge) under a canopy of Eucalyptus grandis to full sun found dry weight yield of Bahia grass leaf matter under full sun summer growth under the canopy to be 35% greater than full sun plots; winter growth was similar [5]. Soil moisture and nitrogen inputs from accumulated organic matter from tree leaf inputs were suggested as reasons for the increased growth under eucalypt canopies. As trees increase in age the canopy of the forest becomes denser, necessitating thinning for maintenance of forage quality and quantity, as in pine/wire grass (Pinus/Aristida) and pine/bluestem (Pinus/Andropogon) ecosystems in the Southern United States [6].
Spectral light range between 400 nm and 700 nm, known as photosynthetically active radiation (PAR), describes the range of light which is most active in inducing photosynthetic reactions in plants [7,8]. Spatial and temporal variations in light are further limited by the specific leaf area of a given tree species [9]. Shade cast by vegetation has also been shown to influence Red:Far Red (R:FR) light ratios, where vegetation absorbs or reflects high amounts of red wavelength, while far red wavelengths increased under the vegetation canopy. R:FR light is known to influence tiller production in grasses and induce photoperiod responses in reaction to R:FR absorption in plant cells [10-12].
Differences also exist between plant nutrient content under full sun and shade environments [4]; grasses under shade generally produce increased non-protein nitrogen and silica concentration, have increased average leaf area, and because leaves contain less fiber and more total protein than stems, the quality for foraging livestock may be greater. Internode length may increase, and increased lignification may occur under shade conditions.
Moisture content of surface soils has been found to be higher under forest canopy gaps than under a closed canopy due to the lack of overhead vegetation to intercept falling precipitation. However, the physical conditions under canopies and canopy gaps are variable and unpredictable [13], as greater tree density increases shade, and therefore lowers the transpiration of subcanopy forage species [5].
Some forages require specific physical conditions for germination and establishment, while others are adapted to a broader range of conditions, and Panciera M [14] suggests addressing limitations in forages with one or more of the following methods: breed out the problem, simply “overcome it”, and adjust management for it.
The objectives of this study were to measure plant density, assess changes in tissue nutrient content, and quantify biomass production of common forages after one year under three uniform shade densities in east Texas.
Five forages were evaluated: ‘Pensacola” Bahia grass (Paspalum notatum Fluegge), “Texas Tough” Bermuda grass, “Alamo” switch grass (Panicum virgatum L.), “San Marcos” Eastern Gama grass (Tripsacum dactyloides L.), and a native mix containing 45% “Texas” little bluestem (Schizachyrium scoparium Michx Nash), 15% sand love grass (Eragrostis trichodes Nutt. L. Alph. Wood), 15% “Blackwell” switch grass (Panicum virgatum L.), 10% “Lometa” Indian grass (Sorgastrum nutans L. Nash), 10% “Haskell” side oats grama (Bouteloua curtipendula Michx Torr) and 5% “Earl” big bluestem (Andropgon gerardii Vitman) by weight.
Bahia grass (Paspalum notatum Fluegge) is native to South America but is a frequently used forage in the southern Gulf Coast region of the United States [15,16]. Bahia grass has the ability to provide adequate forage on low fertility, dry sites, but has seed that is slow to germinate, called “hard” seed, that hinders the development of a pure stand. Bahia grass also produces large amounts of seed, further aiding in its rapid establishment. Bahia grass is seen as a weed species in some situations where less competitive grasses, such as Bermuda grass, can be rapidly crowded out.
Bermuda grass (Cynodon dactylon (L.) Pers.) is native to Africa but is a common forage species in the Southern United States due to its wide growth range and adaptability. Bermuda grass is inhibited by excessively wet soils, but is able to survive drought due to deeper rooting than most other warm season forages. Forage quality of common Bermuda grass is similar to the Coastal cultivar; however, common Bermuda grass has a generally lower yield than other cultivars [17].
Native grasses of the United States, once common across central United States, include little bluestem (Schizachyrium scoparium (Michx) Nash), Indian grass, “Haskell” sideoats grama (Bouteloua curtipendula (Michx) Torr), switch grass, sand love grass (Eragrostis trichodes (Nutt.) Alph. Wood) and big bluestem. Although adapted to a broad range of conditions these plants are notorious for their difficulty to establish from seed [14]. Native grasses often take 1 to 2 years to become well established and during that critical period weed species should be suppressed. One possible explanation for the prevalence of low germination and establishment rates may be the lack of selection pressures on the native species compared to naturalized forage species [18]. Switch grass (Panicum virgatum L.) is a native warm season perennial grass, and cultivars vary regarding germination rates, cold tolerance, and drought tolerance [19]. Cultivars such as the “Alamo” cultivar are better suited for high biomass production, while the “Blackwell” cultivar has been shown to be a suitable forage [20]. Eastern Gama grass, (Tripsacum dactyloides ( L.), a perennial bunch grass, grows naturally in North, Central and South America and parts of the Caribbean. The primary limitation of establishing E. Gama grass is low germination. Although seeds generally have high viability, overcoming dormancy often poses a problem [21].
Germination rates were determined for Bahia grass, Bermuda grass and switch grass using guidelines established by the Association of Official Seed Analysis (AOSA). Germination rate was assessed for E. Gama grass using methods described [22]. Native mix seed was 90% pure. All other seed was at least 98% pure.
Forages were seeded by hand on April 25, 2008 (Table 1). The 13.2L pots were 30cm in diameter and filled with a commercial bagged potting mix. Approximately 84 g of Osmocote standard 9-month release 13-13-13 fertilizer was incorporated using a cement mixer in each pot. One forage was randomly assigned to one of five pots in each plot. Plots were blocked and randomly assigned 0%, 30% or 60% shade treatments, achieved using 30% and 60% black knitted polyethylene shade cloth (DeWitt (Sikeston, MO)). Plots receiving 0% shade were left in full sun. Five blocks were created with each containing all species-treatment combinations. Plots were spaced at 1.5m intervals so shading from adjacent plots did not occur.
| Forages | kg PLS ha-1* | lbs PLS ac-1 | Germination (%) |
|---|---|---|---|
| Bahia grass | 33.6 | 30.0 | 50.5 |
| Bermuda grass | 4.5 | 4.0 | 61.3 |
| Native Mix | 7.2 | 6.8 | 17.3 |
| Switchgrass | 6.7 | 6.0 | 77.0 |
| Eastern Gama grass | 22.4 | 20.0 | 31.0 |
| *PLS=Pure Live Seed. |
Table 1: Seeding and germination rates of forages.
Pots were arranged equidistant from one center pot and were elevated on wooden pallets. Shade cloth was supported by one post at each of the four corners of each plot. Shade cloth formed a canopy over the pots and stretched between corner posts to form side walls around the pots. This design reduced light from all directions. Shade cloth was fastened to the corner posts and underlying wooden pallets with nails (Figure 1). The canopy of each enclosure was approximately 0.5m above the top of the pots. Pots were watered to saturation each morning using drip emitters regulated by a battery-powered automatic timer. Rates were 1.9 lph (liters per hour) for one hour min each morning at 6:30 am from seeding to June 6, regardless of weather pattern. Irrigation was increased to 90 min each morning after June 6 in response to higher summer temperatures. Undiluted Round-Up (2% Glyphosate isopromaline salt, 2% pelargonic acid and related fatty acids) was brushed on to weed species that appeared, and forage species were visually monitored for negative effects from transference of the herbicide.

Figure 1. Shade cloth enclosures in the foreground are 60%, middle-ground 0%, and background 30% shade treatments.
Plant density and biomass
Plants in each pot were counted before vegetation was cut for biomass sampling in August, 2008. Stoloniferous and rhizomatous species, such as Bahia grass and Bermuda grass, were tabulated by counting each plant crown as one plant and not counting runners which had rooted. Successful establishment (%) was calculated by dividing plant density by number of seeds sown then multiplying by 100. Mean weight (mg) of 10 seeds of each species was used to determine the number of seeds sown into each pot. Biomass production was assessed with the vegetation clipped and dried to a constant weight (grams) at 60°C in August, 2008.
Forage analysis
Forage analysis was conducted on above-ground biomass samples at the Stephen F. Austin State University Soil, Plant and Water Analysis Laboratory, Nacogdoches, Texas. Samples were collected in August 2008, dried at 60°C and ground with a Thomas-Wiley Laboratory Mill (Model 4) by Thomas Scientific with 0.5mm screen attached. Analysis was made for crude protein (CP), acid detergent fiber (ADF) and estimated total digestible nitrogen (TDN), as well as for P, K, Ca, Mg, S (mg kg-1). Except for N, nitric digestion was used to prepare samples. Nutrient analysis was performed using an inductively coupled plasma mass spectrometer unit. N (mg kg-1) was determined using a CN Analyzer.
Verification of shade cloth
Light interception and absorption by shade cloth was evaluated informally. One light reading was made beneath each shade cloth treatment between 10:00 AM and 2:00 PM. These readings were used to examine quality of light beneath the shade cloth and to quantify light intercepted and absorbed by the shade cloth.
Data analysis
This study was a randomized block design with five blocks and a 5 × 3 factorial within each block. Biomass data and plant density data were analyzed using two separate 3-ways mixed (Model III) ANOVAs, with forages and shade treatments fixed. The third factor was a random block effect. A 5 × 3 factorial existed within each block (five forages and three levels of shade). Total sample size was 75 (five forages × three shade treatments × five blocks). For each species, sample size was 15 (one individual from each forage under each shade treatment × three shade treatments × five blocks). Orthogonal contrast was used to compare each specific combination of forage and shade treatment for both biomass and plant density data, and adjusted for Tukey analysis and used the error term specified in the ANOVA table to create p values for each desired combination. Both 3-way ANOVAs and orthogonal contrasts were performed using SAS (SAS Institute, Inc.). Tissue nutrient content of above ground biomass was analyzed with 2-way ANOVA for each nutrient. Tukey test and orthogonal contrast was used to analyze species-treatment combinations.
Germination rates of the native mix and E. Gama grass were below 50% (Table 1), and switch grass was the highest of the five forages. Viability of ungerminated seed was not determined.
Forage analysis
Analysis of forage tissue macronutrients (Table 2) found significant differences among shade treatments (Figure 2). N concentration under 60% shade was significantly different from under 0% shade (p=0.013). Vegetation beneath 60% shade and 30% shade showed significantly different concentrations of P (p<0.001) and K (p<0.001), compared to vegetation grown beneath 0% shade. Mg concentration was found to be significantly different beneath 0% and 60% shade treatments (p=0.007). Calcium concentration was significantly different beneath 60% shade (p<0.001). Comparison of all forage species revealed significant (p<0.001) variation of macronutrient concentration under the shade treatments among forages (Figure 3). P concentration in E. Gama grass was found to be significantly lower than the other forages, while K concentration was significantly higher in Bahia grass, followed by Bermuda grass, native mix and switch grass, but significantly lower in E. Gama grass. Bermuda grass and Bahia grass had the highest Ca concentration followed by the native mix and switch grass; the lowest was in E. Gama grass. Total N was found to be statistically similar for the native mix, Bermuda grass and Bahia grass and significantly different for E. Gama grass. Crude protein concentration was statistically similar for the native mix, Bermuda grass and Bahia grass and significantly lower for E. Gama grass. ADF concentration was significantly less for E. Gama grass. TDN concentration was significantly less for E. Gama grass.
| % Shade | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| mg kg-1 | ||||||
| 0 | 9415b | 1345b | 17577b | 3135b | 1421b | 1900a |
| 30 | 12284ab | 1724a | 16067a | 3237b | 1721ab | 2100a |
| 60 | 16848a | 2059a | 13297a | 4158a | 1886a | 2255a |
| Same letters within a column not significantly different (α=0.05). | ||||||
Table 2: Above ground tissue macronutrient concentration by treatment.
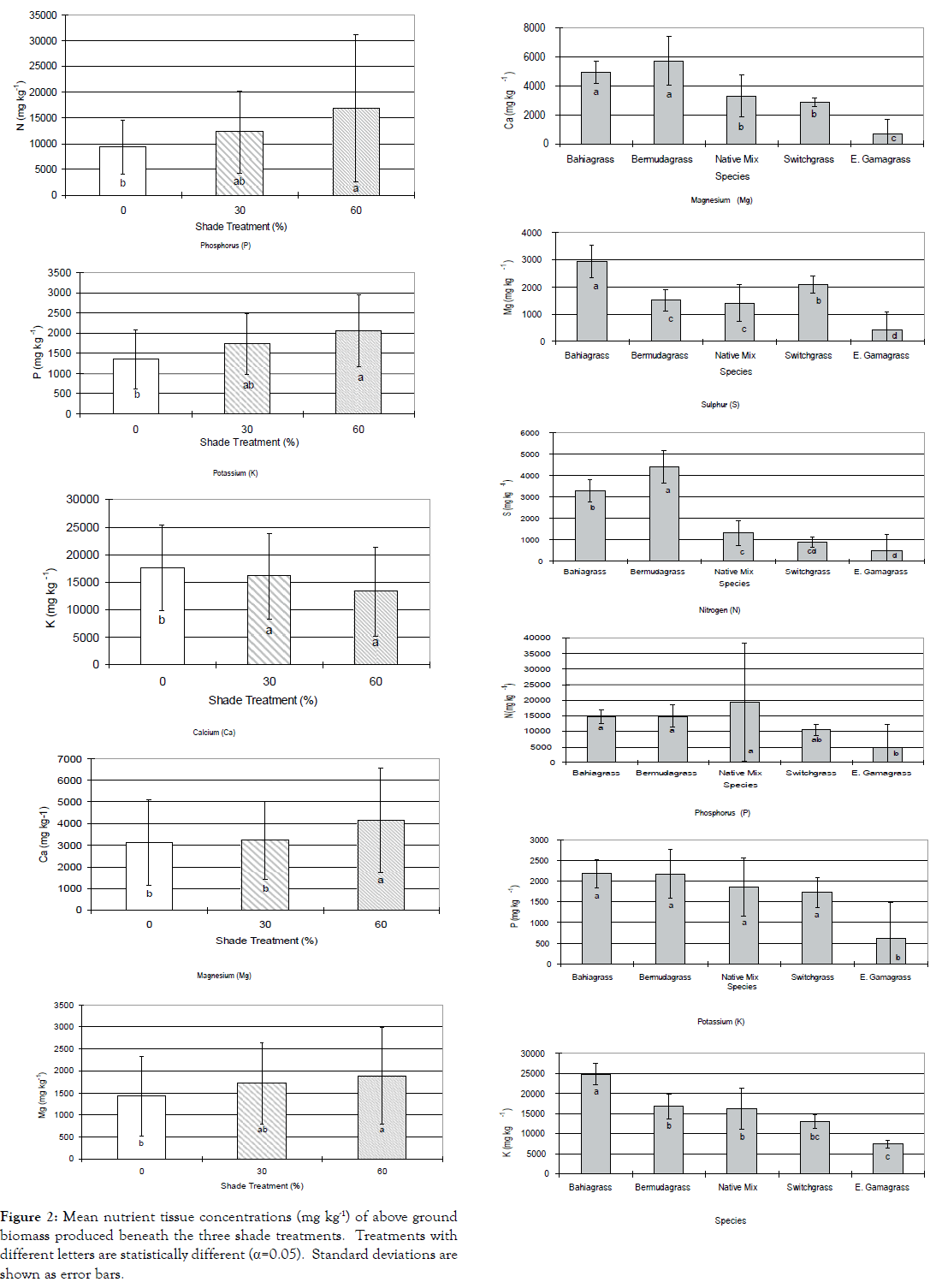
Figure 2. Mean nutrient tissue concentrations (mg kg-1) of above ground biomass produced beneath the three shade treatments. Treatments with different letters are statistically different (α=0.05). Standard deviations are shown as error bars.
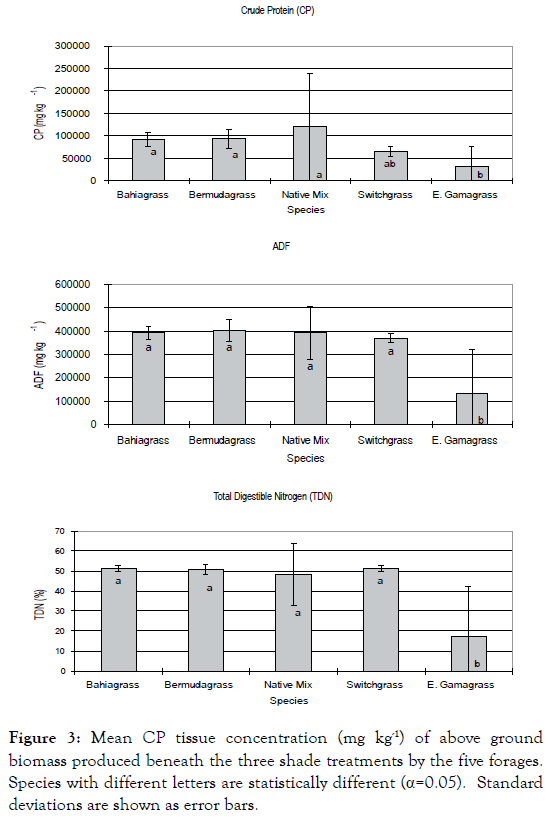
Figure 3. Mean CP tissue concentration (mg kg-1) of above ground biomass produced beneath the three shade treatments by the five forages. Species with different letters are statistically different (α=0.05). Standard deviations are shown as error bars.
Some forages exhibited differences in tissue nutrient concentration between shade treatments (Table 3). Bermuda grass was found to have significantly different concentration of Ca beneath 30% and 60% shade (p=0.003) and beneath 0% and 60% shade (p=0.032). Native mix concentration of P was significantly different beneath 0% and 30% shade treatments (p=0.016). Native mix was significantly different beneath 0% and 60% shade for tissue concentrations of K (p=0.004), Cu (p=0.004), N (p=0.017), and CP (p=0.006).
| Species | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|
| mg kg-1 | ||||||
| Bahia grass | 14719a | 2186a | 24815a | 4949a | 2939a | 3300b |
| Bermuda grass | 14837a | 2181a | 16801b | 5754a | 1508c | 4400a |
| Native Mix | 19300a | 1848a | 16227b | 3307b | 1405c | 1300c |
| Switch grass | 10489ab | 1728a | 12979bc | 2866bc | 2092b | 890cd |
| Eastern Gama grass | 4901b | 603b | 7413c | 669c | 434d | 500 |
| Same letters within a column not significantly different (α=0.05). | ||||||
Table 3: Above ground tissue macronutrient concentration by forage.
Plant density
The shade treatments had no effect on plant density (p=0.056), but density did differ significantly among forages (p=0.008). Switch grass, native mix and Bahia grass produced statistically similar plant densities (Table 4). Eastern Gama grass produced statistically lower plant densities and was found to be statistically different from the other species (p=0.008). Forages were not affected by Round-Up applied to weed species.
| Variables | Forage | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bahia grass | Bermuda grass | Native Mix | Switchgrass | E. Gama grass | ||||||
| Shade Treatment (% shade) | mean plants per pot | |||||||||
| 0 | 16.0 | (1.9) | 9.0 | (0.8) | 8.0 | (1.1) | 14.0 | (1.9) | 3.0 | (0.5) |
| Success Rate (%) | 12.8 | 6.5 | 15.7 | 35.3 | 150.0 | |||||
| 30 | 12.0 | (2.8) | 15.0 | (1.6) | 23.0 | (2.7) | 20.0 | (0.7) | 3.0 | (0.5) |
| Success Rate (%) | 9.6 | 10.9 | 45.2 | 50.4 | 150.0 | |||||
| 60 | 11.0 | (1.7) | 15.0 | (1.7) | 17.0 | (2.5) | 19.0 | (0.4) | 2.0 | (0.5) |
| Success Rate (%) | 8.8 | 10.9 | 33.4 | 47.9 | 100.0 | |||||
| Standard deviation is given in parentheses. | ||||||||||
Table 4: Mean plant density data and success rate of establishment for each forage and shade treatment combination.
Biomass
Biomass production differed among the three shade treatments (p=0.004). Mean biomass beneath 0% shade was 186.7 g and 195.3 g beneath 30% shade (σ=39.5, significantly different from the 60% shade treatment (132.4 g). Biomass also varied among species (p=0.001) (Table 5). Eastern Gama grass, Bahia grass and Bermuda grass all produced statistically similar amounts of biomass: 239.9 g for E. Gama grass, 177.4 g for Bahia grass, and 216.8 g for Bermuda grass. Native mix produced statistically less biomass (62.4 g). Switch grass biomass production was not significantly different among the shade treatments. Orthogonal contrasts indicated that no significant difference in biomass exists within forages between treatments at the 95% confidence level. Bahia grass, Bermuda grass and native mix produced the greatest biomass beneath 30% shade treatments. Switch grass and E. Gama grass produced the highest biomass beneath the 0% shade treatment.
| Forage | Treatment | N | P | K | Ca | Mg | S |
|---|---|---|---|---|---|---|---|
| % Shade | mg kg-1 | ||||||
| Bahia grass | 0 | 12900 | 1920 | 22550 | 4647 | 2400 | 3200 |
| 30 | 15500 | 2176 | 25760 | 5009 | 2900 | 3700 | |
| 60 | 16000 | 2447 | 26130 | 5192 | 3500 | 3200 | |
| Bermuda grass | 0 | 12000 | 1714 | 14720 | 5126 | 1400 | 4200 |
| 30 | 13700 | 2144 | 16360 | 4650 | 1400 | 4000 | |
| 60 | 18800 | 2699 | 19320 | 7480 | 1700 | 5100 | |
| Native Mix | 0 | 8200 | 1195 | 11990 | 2650 | 1100 | 900 |
| 30 | 18100 | 1803 | 17540 | 3030 | 1700 | 1300 | |
| 60 | 31600 | 2545 | 19140 | 4250 | 1400 | 1500 | |
| Switchgrass | 0 | 10800 | 1531 | 13110 | 2900 | 2000 | 1000 |
| 30 | 9200 | 1741 | 12060 | 2670 | 1900 | 900 | |
| 60 | 11400 | 1911 | 13750 | 3040 | 2300 | 800 | |
| E. Gama grass | 0 | 3100 | 362 | 360 | 360 | 200 | 300 |
| 30 | 5100 | 753 | 750 | 820 | 600 | 600 | |
| 60 | 6500 | 693 | 690 | 829 | 500 | 600 | |
Table 5: Above ground biomass tissue macronutrient concentration for each forage-shade treatment combination.
Light quality and quantity
Total light quantum intensity was reduced 14.3% beneath 30% shade cloth, and 37.8% beneath 60% shade cloth. Light quality did not appear to have been altered by the shade cloth (Table 6).
| Treatment | Proportion to PARFR | Total PARFR QI | Full Sun | |||
|---|---|---|---|---|---|---|
| Shade Cloth Density (%) | B | G | R | FR | μmol m-2 s-1 | % |
| 0 | 0.26 | 0.28 | 0.25 | 0.21 | 423.9 | 100.0 |
| 30 | 0.26 | 0.28 | 0.25 | 0.21 | 363.4 | 85.7 |
| 60 | 0.26 | 0.28 | 0.25 | 0.21 | 160.3 | 37.8 |
Table 6: Light quality proportion, quantum intensity and percent full sun beneath shade cloth treatments.
Germination verification results confirmed the need for high seed rates of forages for adequate establishment, as low germination rates of some forages resulted in the absence of growth in some pots. During biomass sampling, newly germinated E. Gama grass were included, suggesting that ungerminated, viable seed still remained after 7 months. Seed dormancy may have been a contributing factor to plant density. Establishment rates greater than 100% is explained by variability in weight and size of E. Gama grass seed; seeding rates were based on weight and variability of seed weight was high, so some pots received five seeds while others received only three. Due to low germination and high dormancy of many native species, evaluation of establishment success over multiple seasons may be a more effective assessment.
Several species were found to be pot bound during biomass sampling, and may have been an unmeasured factor, particularly affecting E. Gama grass and switch grass, which are known to extend roots up to 180cm into soil profiles. Crowding of large native mix species, such as switch grass, may also have affected results by intraspecific competition. Correlation of plant density and soil depth [23] supports conclusions regarding root restrictions and low plant density.
Nutrient concentration of the native mix was effected by the shade treatments. Mean tissue content of K and CP for all species was significantly higher under the 60% shade treatment. Increased crude protein content of forages beneath shade is significant for silvopasture management because it increases the value of the forage to grazing livestock and as cut hay. Interaction of species and shade treatment were found for several nutrients. The native mix also had increased tissue concentration of Cu, N, and P beneath the 60% shade treatment, and Ca concentration increased in Bermuda grass with shade. Increased tissue nutrient concentration in response to increased shade density suggests that the native mix and Bermuda grass may be nutritionally better forages under the partial shade of silvopasture systems.
Eastern Gama grass is generally considered a high quality forage species as Mashingo [24] found ADF in “Pete” E. Gama grass to range from 31.1% to 44.5%; this study found the greatest mean ADF for “San Marcos” E. Gama grass (20.3%) produced beneath 60% shade. Low ADF indicates that livestock can efficiently digest the forage material; therefore, E. Gama grass becomes a more easily digestible energy source under shade. Conversely, E. Gama grass had relatively low mean tissue concentrations of N and TDN. Compared to the other forages, the native mix had large variations for Cu, Zn, N, ADF and TDN, which may be due to variation in tissue nutrient concentration of the individuals of each species in the native mix. Pots seeded with the native mix contained different numbers of individual species which may have contributed to the large variations.
“Alamo” switch grass is often used for biofuel production due to high biomass production, but E. Gama grass, Bahia grass and Bermuda grass produced higher mean biomass. Biomass production of switch grass may be better studied in the field due to root development restrictions in pots. Biomass production for all species was similar under 0% and 30% shade treatments, indicating that forages are capable of producing comparable biomass when exposed to a 30% reduction in light. Similar to Richard M, et al. [23], a 28% reduction in total quantum intensity in August did not affect plant biomass.
The light compensation point is the point of irradiance where photosynthetic CO2 fixation matches photosynthetic CO2 respiration rate. When photosynthesis continues beyond the light compensation point, a net gain of NADPH and ATP occurs. Establishment and success of a forage species in a silvopasture system may depend on the ability of a species to photosynthesize under decreased irradiance. Estimated light saturation point for Bahia grass is greater than 2000 μmol photons m-2 s-1 [25]. Both switch grass and big bluestem are known to reach light saturation at quantum intensities greater than 2200 μmol photons m-2 s-1 [26].
There was no change in quality of light beneath the shade cloth. Shade density beneath the shade cloth was found to be considerably less than the density indicated for each grade cloth used. Stretching or overlapping of shade cloth would influence shade density. Black shade cloth was used in this study; however, other types of shade cloth exist. Some shade cloths are designed to absorb or reflect specific bands of light to create a desired light spectrum beneath. Shade cloths which obstruct specific wavelengths of light could be used in future research that alters light quality to more closely simulate a silvopasture canopy.
Shading as a variable is a surrogate for a number of site and physiological factors. Shading will have direct impact on soil temperature and air temperature, which in turn may influence initial germination success and also above ground plant growth.
Tissue nutrient concentration differences were found in some species grown under different shade treatments. Increased or decreased forage quality is important in silvopasture or grazing or hay harvesting. Biomass production beneath shade is also significant as an indirect measurement of biological productivity and may be used to evaluate the success of establishment. Results indicated that Bermuda grass had successfully established due to high biomass production. Eastern Gama grass and Bermuda grass may be a suitable species if maximum biomass production were the goal of a silvopasture management system due to their higher mean biomass production under 30% and 60% shade treatments. Environmental factors such as soil moisture were maintained at ideal levels for plant growth in the study, rendering direct comparison of these results to field data inappropriate. As an establishment study spanning one growing season, evaluations of the native mix and switch grass were limited because many native species show significantly increased growth during the second year.
Long term evaluations of establishment of forages under silvopasture conditions after multiple growing seasons are needed to investigate the sustainability of silvopasture management systems. Research regarding cultivars, other warm season forages and the use of cool season legumes as winter cover crops may benefit future research. Further examination of changes in plant nutrient content in response to shade may benefit silvopasture management, including fertilization. Plant density and establishment success suggest that most of the forages became well established. Changes in tissue nutrient concentration beneath varying shade densities may affect the quality of forages grown beneath the partial shade of a silvopasture system. Biomass production may not be affected by a reduction in irradiance up to 30%, suggesting that silvopasture management should include consideration of shade density within the silvopasture system. Additional research on the interaction of shading on soil and air temperatures, microscale relative humidly and the resulting impact of these on photosynthesis and plant growth should be performed.
We extend our appreciation to the USDA Natural Resource Conservation Service-Plant Material Centers, particularly the Knox City Plant Material Center for their donation of “San Marcos” Eastern Gama grass seed. Thanks are also due to Jim Stevens (retired) with the East Texas Plant Material Center for lending his expertise on several occasions. Funding was provided by Stephen F. Austin State University, Division of Environmental Science.
Citation: Richard M, Farrish KW, Oswald BP, Williams HM, Maurer M (2020) Evaluation of Five East Texas Forages under Differing Shade Levels. Fores Res. 9:243. doi: 10. 35248/2168-9776.20.9.243.
Received: 29-Sep-2020 Accepted: 20-Oct-2020 Published: 27-Oct-2020 , DOI: 10.35248/2168-9776.21.10.243
Copyright: © 2020 Richard M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.