Journal of Drug Metabolism & Toxicology
Open Access
ISSN: 2157-7609
ISSN: 2157-7609
Research Article - (2016) Volume 7, Issue 1
Keywords: DPPH; 1,1-Diphenyl-2-Picryl-Hydrazyl; Antioxidant assay; Free radical scavenger
In one of our field survey we found an herb with beautiful tender fruits by name Gardenia gummifera (Rubiaceae). The Gardenia gummifera is well known for its medicinal properties in indigenous medicine in India [1]. In Indian System of Medicine, the gum Dikamali is one of the important drugs, upon literature survey it was found that research has been carried out on the antibacterial [2], sedative, analgesic, antianxiety [3], hypocholesterolemic [4] Anticonvulsant [5] and anticancer activity [6] And flavones including gardenin, flavonoids, tannins, elagic acid, anthraquinones, phenolic acids, Dikamaliartane-A [6] and Triterpenoids [7] from stem bark of Gardenia gummifera Oleanoic aldehyde, sitosterol, D-mannitol, erythrodiol and 19 hydroxyerythrodiol have been reported from the plant. Except these studies, so far no other biological and phytochemical investigations have been reported on the whole plant. Based on the traditional uses as well as earlier work on other species, the Gardenia gummifera is selected for the hepatoprotective study with pharmacological screening of the different fractions using animal models. The toxicity of the extract was also assed in animal models.
Plant material
Fresh parts of G. gummifera were collected from the Sri Venkateswara University, and it is authenticated by Dr. K. Madhava chetty, Professor, Department of Botany, Sri Venkateswara University, Thirupathi, Andhra Pradesh, India. The specimen of herbarium is stored in S.V University, Thirupathi. (Reference no. 1052).
Preparation of extract
The whole plant were dried under shade and powdered and stored in an airtight container. For extraction, 250 g of dried powder was loosely packed in the thimble of soxlet apparatus and extracted with methanol for 18 h at 55°C. For oral administration, extract was dissolved with 2% gum acacia.
Fractionation: The methanolic extract of plant was dispersed in 1 L of distilled water separately and fractionated [8] with toluene, ethylacetate, 2-butanone, n-butyl alcohol and petroleum ether in succession.
Phytochemical screening
The methanolic extract of G. gummifera was evaluated for the prescence of flavanoids, tannins, alkaloids, saponins, glycosides, and sterols/terpens [9]
Animals
Healthy Wistar albino rats, weighing 150-200 g were selected for hepatoprotective activity and animals were procured from the Teena Biolabs Pvt. Ltd. (Reg, no. 177/99 CPCSEA), Hyderabad, Andhra Pradesh. Animals were housed at CPCSEA approved animal house of Vaagdevi Institute of Pharmaceutical Sciences, (1533/PO/a/11/ CPCSEA) Warangal. The study was approved by the Institutional Animal Ethical Committee of Vaagdevi Institute of Pharmaceutical Sciences, (VIPS/IAEC/02/2013/17, 12/10/2013) prior to the beginning of the project work. Ethical norms were strictly followed during all experimental procedure.
Acute toxicity studies
Male and female Swiss mice were randomly divided into groups (n=10) that orally received saline solution (10 ml/kg) with G. gummifera methanolic extract at the same dose of 100, 200, 400, 800, 1000, 1200, 1400, 1800 and 2000 mg/kg p.o. [10]. The same method carried out to the GGME fractions BTF, BAF, TLF. After oral administration, the acute toxicity and behavioural parameters were described according to the methods of Souza Brito [11]. The observations were performed at 30, 60, 120, 240 and 360 min after the oral treatments. For 14 days, the animals were weighed and the number of deaths noted.
Evaluation of hepatoprotective effect of GGME against paracetamol induced liver damage [12]
The protective effect of GGME treated against paracetamol-induced liver damage was carried out in healthy albino rats.
Group-I (Vehicle control) received the 2% w/v acacia 1 ml/kg body weight per oral for 8 days. Group-II (Diseased control) received the vehicle for 7 days followed by an acute oral dose of paracetamol (2 g/ kg.b.w.) on the 8th day alone. Group-III received with 50 mg/kg of Silymarin per oral for 7 days followed by an acute oral dose of paracetamol (2 g/kg.b.w.) on the 8th day. Group-IV and V test groups were administered with GGME 150 and 300 mg/kg of extract by oral route for 7 days followed by an acute oral dose of paracetamol (2 g/ kg.b.w.) on the 8th day. The blood was withdrawn 24 hrs after the administration of paracetamol. Then the withdrawal of blood by retro orbital, separation of serum, dissection of liver and calculation of percentage protection of the biochemical parameters were carried out.
Study of selected bioactive fractions
Protective effect of selected fractions against Paracetamol induced liver damage: Group-I (vehicle control 2% acacia), Group-II (diseased control paracetamol 2 g/kg), Group-III (standard-silymarin 50 mg/kg), Group IV and IX test groups were treated with 50 mg/kg and 100 mg/kg b.w.p.o of the selected fractions given 7 days once a day, on 8th day an acute oral dose of paracetamol (2 g/kg.b.w.) was administered to the group-II to group IX of animals. On 8th day blood was withdrawn under anesthesia using thiopental sodium (65 mg/kg b.w.i.p). The biochemical parameters, such as ALT, AST, ALP, TB, ALB, TP and CHL were determined using UV-visible spectrophotometer [13]. The animals were then dissected and the livers were carefully removed and washed with 0.9% saline solution and preserved in formalin solution (10% formaldehyde) for histopathological studies.
Determination of sleeping time: Pentobarbitone-induced sleeping time [14] was carried out in Swiss albino mice.
Group-I vehicle control, Group-II disease control and was given 2% gum acacia for seven days followed by paracetamol (2 g/kg.b.w.) only on the 7th day. Group-III (Standard) animals were administered with 50 mg/kg of Silymarin per oral for seven days followed by paracetamol (2 g/kg.b.w.) only on the 7th day. Groups IV-VI was treated with TLF, BTF, BAF (100 mg/kg b.w.p.o) fractions respectively. All the various groups of animals were given pentobarbitone 60 mg/kg i.p. 2 hrs after administration of paracetamol (2 g/kg.b.w.). The time between loss of righting reflex and its recovery was recorded.
Determination of wet liver weight: Animals were sacrificed and livers were isolated and washed with saline and weights determined by using an electronic balance. The liver weights were expressed with respect to its body weight, i.e., gm/100 gm.
Measurement of in vitro antioxidant activity with DPPH: The antioxidant activity was determined on the basis of a free radical scavenging activity of stable 1, 1-Di Phenyl-1, 2-picryl hydrazyl (DPPH). In 100 ml volumetric flask, added 2 ml of DPPH solution+0.5 ml of TRIS Buffer+2 ml of methanol+0.5 ml of prepared dilutions (methanolic extract dilutions/methanolic ascorbic acid dilutions). Also, the control absorbance of (DPPH) was taken by replacing 0.5 ml of prepared dilutions with methanol. The absorbance of all the further made dilutions in 100 ml volumetric flask was taken after 30 min. at 517 nm using methanol as a blank [15]. The percentage inhibition is calculated with the following formula:
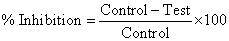
Statistical analysis
The data obtained were analyzed by one way analysis variance (ANOVA) followed by dunnett’s comparison test using computerized program. P value <0.05 was taken as the criterion of significance.
Preliminary phytochemical studies
Preliminary phytochemical studies of extracts and its fractions toluene, 2-Butanone and n-Butyl alcohol fractions shows desired phytochemicals, Flavonoids, Cardiac glycosides, Alkaloids, Tannins and phenolic compounds. Hence, further studies carried out with GGME and its Fractions Toluene (TLF), 2-Butanone (BNF) and n- Butyl Alcohol (BAF).
Acute toxicity study
Mild adverse effects and no mortality rate of the animals were observed during the period of 48 hrs study up to the dose 2000 mg/kg b.w. of the GGME. The two random doses of 150 mg/kg and 300 mg/ kg were selected. The GGME fractions TLF, BTF and BAF exhibited Mild adverse effects and no mortality upto a dose of 1000 mg/kg b.w. The two random doses of fractions 50 mg/kg and 100 mg/kg were selected for the investigation.
Hepatoprotective activity of GGME on Paracetamol induced liver damage in rats
Effect of GGME on serum biochemical parameters: In the Paracetamol treated diseased control group were significantly (P<0.001) increased the serum ALT, AST, ALP and TB levels with a significant decrease in TP and ALB levels, CHL levels mild increased when compared to the control. The levels of these parameters except TP at a dose of 300 mg/kg b.w. were significantly (P<0.001) reversed in GGME-150 and GGME-300 groups. In toxic group, a significant decrease in serum TP and ALB levels were observed and these levels were reversed in both the GGME treated groups (Table 1 and Figure 1).
| GROUP | TREATMENT | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | T.P(gm/dL) | T.B(mg/dL) | ALB (gm %) | CHL (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| I | Vehicle control | 94.28 ± 1.5 | 57.12 ± 8.5 | 66.71 ± 6.68 | 8.913 ± 0.66 | 3.122 ± 1.11 | 2.657 ± 0.16 | 104 ± 8.44 |
| II | Diseased control | 232.8 ± 23.8** | 247.5 ± 30.7** | 190.4 ± 30.0* | 3.537 ± 0.18** | 12.71 ± 0.85** | 1.391 ± 0.17** | 225 ± 16.1** |
| III | Standard | 97.18 ± 0.89 | 72.99 ± 4.91 | 85.45 ± 10.40 | 8.627 ± 0.23* | 4.790 ± 0.32* | 2.432 ± 0.12** | 111 ± 3.98* |
| IV | GGME 150 mg | 130.4 ± 0.90* | 135.9 ± 20.16* | 152.7 ± 10.74** | 5.597 ± 0.49** | 9.983 ± 1.14* | 1.715 ± 0.23** | 166 ± 22.6** |
| V | GGME 300 mg | 104.8 ± 1.5** | 92.03 ± 17.7** | 112.9 ± 16.05** | 7.593 ± 0.58** | 6.663 ± 0.85* | 2.257 ± 0.20** | 139 ± 20.0** |
Table 1: Biochemical parameters of GGME on paracetamol induced liver damage rats. Values are mean ± SEM (n=6) one way ANOVA. Where, * represents significant at p<0.05, ** represents highly significant at p< 0.01, and *** represents very significant at p<0.001. All values are compared with toxicant.
Histopathological studies: The histopathological studies (Figure 2) of the liver showed massive fatty changes, ballooning, gross centrilobular necrosis and loss of cellular boundaries in the toxic group. Both the standard (Silymarin 50 mg/kg b.w.p.o) and GGME (300 mg/kg b.w.p.o) group showed reduced lesser necrotic zones and ballooning degeneration when compared with the toxic group.
Hepatoprotective activity of fractions (TLF, BAF, BTF) of GGME on paracetamol induced liver damage in rats
| GROUP | TREATMENT | AST (IU/L) | ALT (IU/L) | ALP (IU/L) | T.P (gm/dL) | T.B (mg/dL) | ALB (gm%) | CHL (mg/dL) |
|---|---|---|---|---|---|---|---|---|
| I | Vehicle control | 94.28 ± 1.5 | 57.12 ± 8.5 | 66.71 ± 6.6 | 8.913 ± 0.66 | 3.12 ± 1.11 | 2.65 ± 0.16 | 104 ± 8.44 |
| II | Diseased control | 232.8 ± 23.8* | 247.5 ± 30.7* | 190.4 ± 30.0** | 3.537 ± 0.1* | 12.71 ± 0.8* | 1.39 ± 0.17** | 225 ± 16.1** |
| III | Standard | 97.18 ± 0.89* | 72.99 ± 4.9* | 85.45 ± 10.4** | 8.627 ± 0.2** | 4.79 ± 0.3* | 2.43 ± 0.12* | 111 ± 3.98*** |
| IV | TLF 50mg | 127.5 ± 2.7* | 180.9 ± 22.5* | 185.8 ± 5.8* | 7.137 ± 0.3* | 12.50 ± 1.4** | 1.62 ± 0.17* | 170.4 ± 13.9* |
| V | TLF 100mg | 115.2 ± 3.1* | 158.7 ± 25.9* | 170.3 ± 1.1* | 7.710 ± 0.1* | 10.83 ± 1.2* | 1.67 ± 0.17** | 148.1 ± 6.0* |
| VI | BAF 50mg | 110.6 ± 4.7** | 82.51 ± 4.9** | 144.4 ± 6.0** | 7.700 ± 0.3** | 10.41 ± 0.3* | 1.95 ± 0.20** | 125.9 ± 16.5** |
| VII | BAF 100mg | 105.9 ± 1.8** | 76.16 ± 8.5** | 103.5 ± 9.5** | 7.997 ± 0.4** | 8.31 ± 0.2** | 2.30 ± 0.20** | 123.0 ± 2.2** |
| VIII | BTF 50mg | 113.6 ± 5.4** | 104.7 ± 17.0* | 169.7 ± 4.9** | 6.457 ± 0.5** | 12.29 ± 0.8* | 1.73 ± 0.33* | 133.3 ± 10.5* |
| IX | BTF 100mg | 108.4 ± 4.6* | 88.85 ± 4.9* | 140.3 ± 9.8** | 7.083 ± 0.4** | 10.62 ± 0.5** | 2.28 ± 0.23* | 118.5 ± 6.0** |
Table 2: Biochemical parameters of GGME fractions on paracetamol induced liver damage in rats. Values are mean ± SEM (n=6) one way ANOVA. Where, * represents significant at p<0.05, ** represents highly significant at p<0.01, and *** represents very significant at p<0.001. All values are compared with toxicant. (Toluene Fraction- TLF, 2-Butanone Fraction (BTF) and n-Butanol Fraction (BAF).
Biochemical parameters: The hepatic injury induced by paracetamol in the diseased control group caused a significant (P<0.001) rise in serum marker enzymes (AST, ALT, ALP), bilirubin levels, significant decrease in TP and ALB levels. The administration of BAF and BTF at 100 mg/kg significantly (P<0.001) attenuated the increased levels of serum enzymes and bilirubin with an elevation in the decreased levels of serum TP and ALB, produced by paracetamol. The activity exhibited by these fractions was well comparable with that of the reference drug, Silymarin. Further, it is evident that BAF-GGME was similar to Silymarin (P<0.001) with respect to the parameters studied, on the contrary, the other fraction, viz. TLF shows less significant activity (Table 2 and Figure 3).
Histopathological studies
The BAF and BTF are shown (Figure 4).
A marked improvement in histopathological damage induced by paracetamol. The recovery with BAF was more than that of BTF comparable to that of Silymarin the reference drug.
Effect of fractions of GGME on paracetamol induced hepatotoxicity liver weight in rats: In diseased control group, there was increase in the liver weight, i.e., 4.6 ± 0.33 g (p<0.001), whereas the value of vehicle control group is 2.5 ± 0.22 g. The group treated with 100 mg/kg of BAF (100 mg/kg) fraction was significantly reduced the liver weight to 3.1 ± 0.16**g, which is near to the liver weight of standard control group, i.e., 2.6 ± 0.21 g (p<0.001) (Table 3).
| Group | Sleeping time (min) Mean ± SEM | Percentage reduction | Liver weight(gms) Mean ± sem | Percentage reduction |
|---|---|---|---|---|
| Vehicle control | 47.17 ± 0.94 | -- | 2.5 ± 0.22 | -- |
| Diseased control | 144.8 ± 1.22 | -- | 4.6 ± 0.33 | -- |
| Standard | 57.33 ± 1.45*** | 60.4 | 2.6 ± 0.21** | 43.4 |
| TLF 100 | 131.2 ± 2.35 | 9.3 | 3.8 ± 0.30* | 17.3 |
| BAF 100 | 78.67 ± 2.01** | 45.6 | 3.1 ± 0.16** | 32.6 |
| BTF 100 | 94.50 ± 1.66* | 34.7 | 3.3 ± 0.21** | 28.2 |
Table 3: Effect of fractions of GGME 100 mg/kg on Pentobarbitone induced sleeping time in Paracetamol induced toxicity in mice and liver weight in rats. Values are mean ± SEM (n=6) one way ANOVA. Where, * represents significant at p<0.05, ** represents highly significant at p<0.01, and *** represents very significant at p<0.001. All values are compared with toxicant. (Toluene Fraction-TLF, 2-Butanone Fraction (BTF) and n-Butanol Fraction (BAF
Effect of fractions of GGME on pentobarbitone induced sleeping time in mice: (P<0.001) than that of the control. Prior treatment of animals with TLF, BAF and BTF (100 mg/kg) and Silymarin (50 mg/kg) significantly (P<0.001) shortened the pentobarbitone sleeping time as compared to the toxic The pentobarbitone at a dose of 75 mg/kg (i.p) caused sedation in mice of control group for a period of 50 min, whereas treatment of animals with paracetamol (diseased group) prolonged the pentobarbitone sleeping time to 144.8 ± 1.22 min, the value that was significantly higher group. Though, the extract exhibited activity the maximal reduction in sleeping time was observed in BAF 100 mg/kg group, which was close to the sleeping time observed in the reference drug, Silymarin group (Table 3).
In-vitro free radical scavenging activity of selected bioactive fractions by DPPH method:The DPPH scavenging activity of the test fraction of BAF-GGME was well comparable to the standard ascorbic acid. The test fraction and ascorbic acid exhibited a concentration dependent DPPH radical scavenging activity. At the highest test concentration (100 μg/ml), the order of degree of scavenging activity of the fractions BAF and BTF (IC50 values) were found to 45.4 μg/ml and 57.4 μg/ml.
In the present investigation, methanolic extracts and its fractions of whole plant of Gardenia gummifera is screened for hepatoprotective activities in rodents. The GGME and its effective fractions were subjected to hepatoprotective studies with paracetamol induced hepatotoxicity rats [16].
Treatment with GGME at 150 and 300 mg/kg b.w.p.o. significantly reduced the level of these marker enzymes in paracetamol induced liver damage in rats. The decrease in the levels of these enzymes may be a consequence of the stabilization of plasma membrane as well as repair of hepatic tissue damage caused by paracetamol [17]. Elevated serum TB level is due to defective excretion of bile by the liver indicates the loss of integrity of the liver and necrosis. This leads to increase in the binding, conjugating and excretory capacity of hepatocytes, which is proportional to the erythrocyte degeneration rate [13]. At both the test doses GGME showed a significant depletion in the serum bilirubin level suggesting the possibility of the extracts ability to stabilize biliary dysfunction of rat liver during injury with paracetamol in prophylactic studies. A depression in total protein occurs due to the disruption and dissociation of polyribosome on endoplasmic reticulum leading to defective protein biosynthesis [18]. In GGME at 150 mg/kg and 300 mg/kg doses increased the serum TP and ALB levels with varying degree of significance. This may be due to the promotion of the assembly of ribosome on endoplasmic reticulum to facilitate uninterrupted protein biosynthesis.
The histopathological profile of the liver revealed drastic alterations in histoarchitecture showing centrilobular necrosis, fatty changes, broad infiltration of lymphocytes and vacuolization. The GGME at both test doses showed definite signs of protection. The effects of the extracts were proportionate with the doses.
It is evident from the results that the biochemical and histopathological observations of the studies were complementing each other. The remarkable results obtained in the prophylactic studies for the GGME lead to the further investigations to isolate the active part of the extracts by fractionation. The BTF-GGME and BAF-GGME at 50 and 100 mg/kg could significantly prevent the changes in the levels of serum biochemical parameters such as ALT, AST, ALP, TB, TP, CHL and ALB as well as the histological changes in the liver of rats, comparable to that of the reference drug, Silymarin (50 mg/kg). The TLF did not exhibit significant protection.
Although, the two fractions offer the effect, BAF is more effective than BTF. The histopathological studies of liver of rats treated with the fractions showed a significant recovery from the hepatic lesions produced by paracetamol. It is evident from the results that the degree of recovery shown by the fractions is in consistent with the results of the biochemical parameters.
The hepatoprotective effect of the BTF and BAF was substantiated by pentobarbital sleeping time experiment in mice. The duration of pentobarbital-induced sleep in intact animals is considered as a reliable index for the activity of hepatic Microsomal Drug Metabolizing Enzymes (MDME) [19]. The damage conferred by paracetamol on hepatocytes as well as on the hepatic MDME causes a loss of drug metabolizing capacity of the liver, resulting in prolongation of pentobarbital induced sleep time [20]. This indicates the hepatoprotective potential of the BTF and BAF against paracetamol - induced damage to hepatocytes.
In-vitro antioxidant studies were conducted with the fractions to confirm the antioxidant mechanism involved in their hepatoprotective activity. DPPH radical serves as the oxidizing substrate, which can be reduced by an antioxidant compound to its hydrazine donation and as the reaction indicator molecule [21]. In DPPH method, the BAF exhibited a concentration dependent DPPH radical scavenging activity. The maximal activity for BAF was found at the highest concentration 100 μg/ml used. The antioxidant properties of flavonoids are found to be effective mainly via the scavenging of superoxide anions. BAF enriched with flavonoids and in accordance with the above statement.
The GGME and their respective fractions, such as 2- butanone and n- butanol exhibited hepatoprotective activity.
The studies substantiate the use of Gardenia gummifera in folklore medicine for the treatment of liver disorders. The GGME at a dose of 300 mg/kg b.w. exhibited a significant hepatoprotective effect and BTF, BAF were found to have potential protective effects at a dose of 50 and 100 mg/kg. The hepatoprotective effect BAF was well comparable to that of Silymarin (50 mg/kg). The two selected bioactive fractions were found to possess an antioxidant property, which strongly supports their hepatoprotective effect. The hepatoprotective effects of the extracts were supported by their effect in shortening the sleeping time and decrease the liver weight in rats. The activity of the fractions was attributed to the different classes of compounds present in them such as steroids/triterpenoids, flavonoids and phenolic compounds. A bioactive guided fraction of the extract is needed to be isolate, identify and elucidate the structure of the bioactive compounds responsible for the observed hepatoprotective activity.