Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2022)Volume 12, Issue 3
COVID-19 pandemic has claimed millions of lives and resulted in an economic slowdown worldwide. The mortality rate is approximately 2% in the United States, with many individuals requiring prolonged hospitalization. At least 10% of patients with severe COVID-19 will eventually have respiratory failure. The recent deployment of a vaccine will hopefully ease and ultimately deter the spread of this highly contagious virus. However, newer virulent strains continue to emerge since most people in different parts of the world have little or no access to vaccines. The virus is associated with the cytokine storm in its severe form. This manifestation is highly lethal, resulting in acute respiratory distress syndrome and multi-organ failure. Since only a few therapeutic drugs can treat severe COVID-19, it is essential to focus on therapeutic strategies to increase the COVID-19 survival rate and preventive vaccination. This review describes microparticulate cytokine inhibiting agents such as antisense oligonucleotides to Nuclear Factor kappa B (NF-kB), antioxidant, catalase, Tumor necrosis factor (TNF) and Interleukin-1 (IL-1) neutralizing antibodies, CNI-1493 and dexamethasone. These microparticulate cytokine inhibiting agents have demonstrated remarkable effectiveness in cytokine suppression intracellularly compared to equivalent drug doses in solution. Much of this work was published before the emergence of COVID-19 and its subsequent variants. Moreover, these microparticles might effectively moderate cytokine storms and requires additional review and investigation.
COVID-19; Cytokine; Inflammation; Cytokine inhibitors
The SARS-CoV-2 virus causes the infectious disease COVID-19. It causes mild to moderate respiratory illness in most people. However, the COVID-19 disease causes a severe overreaction of the body's immune response in some people due to cytokine storms. Different cells of the body's immune system release small proteins called cytokines to coordinate the immune systems' response to an infection and trigger the defense mechanisms. During COVID-19, in some patients, when the virus enters the lungs, it uncontrollably triggers the cytokine release and causes hyper inflammation, which can be fatal. Cytokine storm results when the pro-inflammatory cytokines no longer have regulatory feedback and can cause progressive tissue inflammation and death. Cytokine storm is also associated with diseases like other viruses of the SARS family, influenza, sepsis, Ebola, and also during Chimeric Antigen Receptor T-cell therapy (CAR-T) [1,2].
The transcription factor Nuclear Factor kappa light-chain enhancer of activated B cells (NF-kB) induces over 150 genes involved in inflammation and immune responses [3]. NF-kB resides in the cytoplasm and stimulates synthesis bound to an Inhibitor of kB (IkB), an inactive complex. However, when presented by bacteria or viruses, IkB is ubiquitinated. It then stimulates the synthesis of many pro-inflammatory cytokines within minutes. The inflammatory response is initiated and perpetuated. NF-kB plays an active role in the cytokine activation seen in cytokine storms [4,5].
Different therapies have been only moderately effective in improving mortality of the COVID-19 [6]. We have developed a microparticulate formulation containing cytokine inhibiting agents such as neutralizing antibodies to TNF/IL1, antisense oligonucleotides to NF-kB, dexamethasone, or antioxidant catalase [7]. The microparticles help in targeting a phagocytic cell whose function is to engulf the particulate matter and deliver a drug dispersed in a non-toxic substance (species-specific albumin) directly into the cell [8]. A higher concentration of an intracellular drug may result in a more significant physiological effect. Phagocytic cells include macrophage/monocytes, polymorphonuclear cells, endothelial cells, and dendritic cells. These cells have overlapping biologic functions, including cytokine synthesis, immunologic regulation, cell barrier function, and elimination of infectious organisms. In addition to these functions, endothelial cells play a vital role as a selective transport barrier. The pro-inflammatory cytokine TNF is a major contributing factor to the inflammatory response. TNF neutralizing antibodies are now clinically available for treating rheumatoid arthritis, inflammatory bowel disease, and dermatologic conditions such as psoriasis. TNF plays a vital role in the inflammation of these conditions [9]. Despite extensive experimental evidence implicating TNF in the pathogenesis of septic shock, clinical trials using TNF neutralizing antibodies have not been successful in altering changing mortality in clinical sepsis trials [10]. Other conditions, including Ebola syndrome [11], CAR-T cell-induced cytokine release syndrome in cancer chemotherapy [12], acute respiratory distress syndrome [13], and most recently COVID-19 respiratory involvement, have demonstrated "cytokine storm" [1]. Over activity of cytokines in these conditions can produce multiorgan failure with a high mortality rate.
NF-kB is activated in septic shock, rheumatoid arthritis, inflammatory bowel disease, as well as other conditions [14-16]. An antisense oligonucleotide to the p65 subgroup of NF-kB has been synthesized as a phosphonothioate oligonucleotide. We have used the sequence 5' GGA AAC ACA TCC TCC ATG 3' in rats to combine with the p65 component of the NF-kB complex. The specificity of the antisense sequence has been verified by western blot analysis [17]. Antisense compounds have several advantages, including stability, low toxicity incidence, and high specificity of protein inhibition. A significant disadvantage of antisense oligonucleotides is the difficulty of penetrating a large polar compound intracellularly for interaction with specific mRNA to inhibit protein synthesis [18].
We have developed a microparticulate drug delivery system that targets phagocytic cells, such as macrophages, neutrophils, dendritic cells, and endothelial cells. Macrophages play a pivotal role in synthesizing and releasing proinflammatory cytokines responsible for inflammation. However, neutrophils, endothelial cells, and dendritic cells have also been shown to synthesize TNF after activation of NF-kB. We have produced albumin microparticles 1-5 µm in size, which can contain water-soluble compounds, antisense oligonucleotides, or neutralizing antibodies. These microparticles are readily phagocytized by macrophages as well as other phagocytic cells. The microparticles are enzymatically degraded, and the drug is released intracellularly. The high intracellular concentration of the drug produces an enhanced physiological effect.
Our previous studies have demonstrated excellent results in the survival of experimental animals in the peritonitis model of septic shock. We have used a variety of compounds to inhibit pro-inflammatory cytokines, including neutralizing antibodies to TNF and IL1, water-soluble compounds (CNI-1493), antisense oligonucleotides to both TNF and NF-kB [19-22]. We observed a significant improvement in cytokine inhibition and survival of rats treated with these microencapsulated drugs compared to equivalent doses in the solution given intravenously. Studies in our laboratory have demonstrated a 10-fold increase in intracellular concentration of antisense to NF-kB compared to equivalent doses in solution in macrophages [20,22].
Recent studies have evaluated the effect of the administration of microencapsulated antisense oligonucleotides to NF-kB on TNF and IL1 after intratracheal administration of endotoxin in rats [23]. This resulted in prolonged pulmonary microsphere retention (48 hours) and inhibition of blood TNF and IL-1. These results suggest that the microparticles phagocytized lung produced prolonged cytokine suppression. NF-kB and macrophages are activated in inflammation induced by the influenza virus [24]. Administration of microcapsules containing antisense to NF-kB may benefit the treatment of pulmonary cytokine storm.
The previous use of antisense oligonucleotides has been well-tolerated in human trials [25]. The use of human serum albumin has also been found in several clinical reports [26,27]. Albumin aggregates 10-14 µm in size have been used diagnostically in lung scans for decades without complications in clinical medicine. Albumin has been utilized in inpatient care for over 30 years for volume expansion in hypotensive states. Human albumin has been used as an advanced delivery system to avoid problems with insolubility, instability in biological environments, rapid enzymatic degradation, poor uptake into cells, suboptimal selectivity for targets, and unwanted side effects [8].
Cytokine storm during COVID-19 infection
Significant problems associated with severe COVID-19 are mortality and poor prognostic characteristics [28]. It has been found that the hospitalized COVID-19 patients are afflicted with several severe problems such as pneumonia, serious damage to the airways, pulmonary edema, and Acute Respiratory Distress Syndrome (ARDS). ARDS is the most fatal among these syndromes and causes 70% of fatal COVID-19 cases. ARDS is associated with aggressive inflammatory responses induced by overproduction of cytokines or cytokine storm [29,30]. The cytokine storm occurs upon COVID-19 viral infection when the first layer cells, such as epithelial and macrophage cells and blood circulating monocytes, are activated via toll-like receptors as shown in Figure 2. These are Pattern Recognition Receptors (PRRs) produced by the virus and cause the production of an enormous number of inflammatory cytokines and chemokines. These cytokines and chemokines further attract T cells and monocytes, leading to widespread lung inflammation. In the lungs of COVID-19 patients, interstitial mononuclear inflammatory infiltrates dominated by lymphocytes were found. In addition, severe lymphopenia with hyperactivated T cells was found in their peripheral blood [31].
Furthermore, COVID-19 patients with severe disease have low levels of regulatory T cells [32]. Serum of COVID-19 patients with the severe illness has shown to contain high levels of TNF, IL-2,7,10, CXCL10, G-CSF, MCP1, and MIP1α [29], which indicates that severe COVID-19 is associated with Cytokine Release Syndrome (CRS), which is a disorder induced by cytokine storm [1,33,34]. Amongst the increased levels of inflammatory mediators in COVID-19 patients, the levels of IL-6 are noticeably more in the blood of non-survivors than in survivors [35,36].
However, the therapeutic use of Tocilizumab has proven to be marginally effective [37]. The cytokine storm causes intravascular coagulation leading to the failure of multiple organs. COVID-19 patients exhibit multiorgan loss with coagulation abnormalities indicated by lower platelet count and increased D-dimer, which are associated with poor prognosis and explain the microthrombi of the lungs, lower limbs, hands, brain, heart, liver, and kidneys [38-41]. Similar remarks have also been found with COVID-19 patients with renal failure [42,43]. Multiorgan failure can be caused by several other factors, such as endothelial cells causing cell death, leading to vascular leakage, and inducing a cytopathic effect on airway epithelial cells [44]. However, multiorgan failure caused by intravascular coagulation mediated by cytokine storm has also been detected as a significant cause of death among the COVID-19 patients [31,45,46]. These findings suggest that one of the leading causes of death in COVID-19 is ARDS with cytokine storm. Therefore, inhibiting cytokine production can be the lifesaving therapeutic approach to COVID-19 patients. Cytokine inhibiting agents such as cytokine neutralizing antibodies, antisense oligonucleotides, glucocorticoids can be used for medicinal purposes. However, delivering these cytokine inhibitors is involved with some challenges such as enzymatic degradation, poor bioavailability, etc. A particulate form of cytokines with biodegradable and biocompatible polymers can be applied to overcome these challenges. Cytokine inhibitors in microparticulate form can help improve the bioavailability by better uptake into the cells, preventing their rapid degradation and thereby leading to better treatment. Previously, in our lab, we have prepared formulations in microparticulate form of cytokine inhibitors [20,24]. Briefly, biocompatible and biodegradable polymeric solution with cytokine inhibitors were spray dried using Buchi Mini Spray dryer to form microparticles. The microparticles were then characterized for size uniformity, yield and content analysis. The cytokines were extracted from the microparticles by breaking the polymer matrix and quantified using cytokine specific assays. Different cytokine inhibitors are briefly described in the following section (Figures 1 and 2).
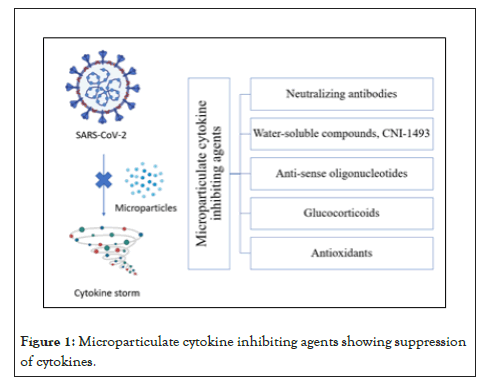
Figure 1: Microparticulate cytokine inhibiting agents showing suppression of cytokines.
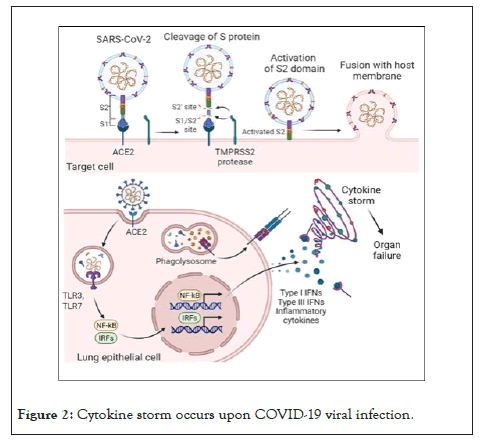
Figure 2: Cytokine storm occurs upon COVID-19 viral infection.
Cytokine inhibitors
Phagocytic cells are natural targets for microencapsulated drug delivery. Cells such as macrophages/monocytes, polymorphonuclear, and endothelial cells rapidly phagocytize albumin microcapsules both in vitro and in vivo as shown in Figure 3. In vitro studies have shown that within 1 h, 70% of a dose of Iodine-125 (125I) radio-labeled microencapsulated IL1 was taken up by peritoneal macrophages [47]. Other studies utilizing whole blood demonstrated that in 2 h, individual macrophages had phagocytized as many as five microcapsules (Figure 3) [21].
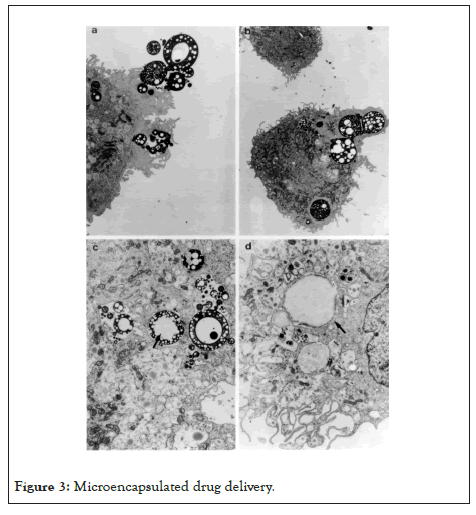
Figure 3: Microencapsulated drug delivery.
After injection of microcapsules into the bloodstream of an experimental animal, less than 2% of the injected dose was detected in the circulation in 5 min [48]. Macrophages are present in the liver (kupfer cells), lung (pneumocytes), spleen, kidney, Central Nervous System (CNS), and other organs. Macrophages play unique roles in each organ but have standard functions of pro-inflammatory cytokine release to initiate the inflammatory process, phagocytosis of micro-organisms and cellular debris, and communication with other immune cells. Rapid cytokine synthesis (TNF, IL1) by macrophages, endothelial cells, and white blood cells is a significant response in local and systemic inflammatory conditions. In addition, these cells produce hydrogen peroxide and other Reactive Oxygen Species (ROS) as part of the respiratory burst, an essential mechanism in bacterial removal. ROS such as hydrogen peroxide activates NF-B, the nuclear transcription factor initiating cytokine synthesis [49]. The inflammatory response resulting from cytokine synthesis leads to vascular changes in permeability and amplification of the inflammatory process. Endothelial cells are phagocytic and rapidly engulf albumin microspheres in vitro [50]; a total of 28% of endothelial cells phagocytize microcapsules in vitro in 5.5 h. By 24 h, 97% of endothelial cells had phagocytized microcapsules. After exposure to endotoxin, 47% of endothelial cells phagocytized microcapsules by 5.5 h. After phagocytosis of NF-kB microparticles, TNF synthesis by endothelial cells was inhibited by 75% after endotoxin stimulation. This resulted in the reversal of endotoxin-induced permeability using an in vitro model as shown in Figure 4. Endothelial cells comprise one of the largest cell types in the body. Thus, permeability regulation can be crucial in minimizing pulmonary interstitial fluid accumulation. The following study demonstrates the organ distribution of intravenously injected microparticles. Clodronate, a bisphosphonate, was encapsulated in albumin and injected intravenously into rats [47]. Microparticles cause the cellular death of macrophages after phagocytosis [51]. Macrophages were documented by histologic staining for macrophage antigen ED-1. Macrophages were decreased by greater than 90% in the liver, spleen, blood, and kidney. These findings demonstrate high organ penetration and uptake of microparticles (Figure 4).
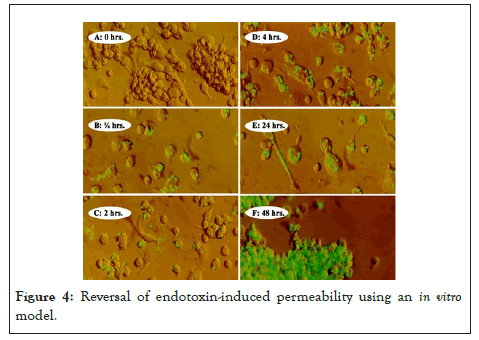
Figure 4: Reversal of endotoxin-induced permeability using an in vitro model.
Our research has been focused on compounds that affect inhibiting pro-inflammatory cytokines. Endotoxin, infectious organisms, and viruses are some of the most potent stimulators of the cytokine cascade. The microparticles we have evaluated contain neutralizing antibodies to TNF and IL1, CNI 1493, antisense oligonucleotides to TNF and NF-kB, the antioxidant enzyme catalase, and dexamethasone as shown in Table 1 [7].
| Cytokine inhibitors | Inhibition target | References |
|---|---|---|
| Neutralizing antibodies | Against TNF and IL1 | [47], [55] |
| Water-soluble compounds, CNI-1493 | Translational inhibitor of TNF synthesis and IL1 inhibitor | [58] |
| Antisense oligonucleotides | Antisense oligonucleotides to NF-kB, inhibiting TNF, IL1, and IL6 | [21,22,59] |
| Glucocorticoids | Dexamethasone potent inhibitor of NF-kB | [64] |
| Antioxidants | Catalase inhibits hydrogen peroxide production, nitrate synthesis, and TNF release | [19,71] |
Table 1: Summary of microparticulate cytokine inhibitors.
These compounds in albumin microparticles have proven very effective in TNF and IL1 inhibition with improved animal survival in a peritonitis model of septic shock (Table 1).
Neutralizing antibodies: Neutralizing antibodies to TNF have been used in experimental conditions and clinical disease states such as rheumatoid arthritis [52,53]. These antibodies combine with the specific cytokine to neutralize the inflammatory effect of the cytokine. The preparations are of high molecular weight and may not easily penetrate inflamed tissues. In previous animal models, these antibody preparations administered in solution form had no effect in experimental models of compartmentalized infection such as peritonitis [54]. Microparticles containing antibodies to TNF and IL1 significantly improved TNF and IL1 inhibition in vitro and survival in experimental peritonitis models using either S. aureus or E. coli [52-60]. The combination of both cytokines was most effective, producing 100% survival. Microparticles containing antibodies were effective even when administered 4 hours after the infectious organisms were administered. This time point is significant as it is the time peak of TNF release in this model. This demonstrates that the microparticles were effective even when TNF was at its highest concentration in response to the infectious organisms. The proposed mechanism for the effectiveness is a greater degree of cytokine inhibition by intracellular delivery of the antibody, high levels of tissue penetration to resident phagocytic cells, and phagocytes containing microparticles to inflamed areas as a result of chemoattractant protein MCP-1 [57].
Water-soluble compounds (CNI-1493): Low molecular weight water-soluble compounds are ideally suited to microparticles. CNI-1493 is a water-soluble guanyl hydrazone compound with a molecular weight of 890. CNI-1493 has shown to be a potent post-translational inhibitor of TNF synthesis [58]. CNI-1493 microparticles followed by E. coli endotoxin 15 mg/kg resulted in 100% survival. The peritonitis model had an 83% survival in an otherwise fatal infection. Both TNF and IL1 were markedly inhibited. The duration of action of microparticulate CNI-1493 was 24 hours [47].
Antisense oligonucleotides: Antisense oligonucleotides are large molecules composed of nucleotide bases that can combine with specific mRNA sequences in the nucleus to prevent the translation of protein synthesis by the ribosome. Antisense oligonucleotides to NF-kB (5’GGA AAC ACA TCC TCC ATC 3’) in the P65 subunit [21,22,59]. The antisense oligonucleotide's intracellular concentration was significantly greater in the microparticle formulation in macrophages and endothelial cells [50]. These microparticles containing antisense oligonucleotides to NF-kB produced significant cytokine suppression and 80% survival in the peritonitis model [22].
Microparticles containing antisense oligonucleotides to NF-kB have been used intravenously in the typical primate model [50]. Blood was sampled after a dose of antisense to NF-kB in a solution followed by an amount of microparticulate antisense to NF-kB. Ex-vivo whole blood endotoxin stimulation was performed for three days to measure TNF, IL1, IL6, and IL8 [50,56]. TNF, IL1, and IL6 were greater than 90% inhibited. IL-8 was not affected. These microparticles were found to be non-toxic and non-antigenic in the primate. Another study evaluating the toxicity of a phosphonothioate oligonucleotide in cynomolgus monkeys found no toxicities that were expected to be clinically significant [60]. Antisense oligonucleotides to NF-kB have also been used to inhibit the inflammatory response in a rodent model of rheumatoid arthritis [17]. After induction of arthritis, microparticles were administered intraperitoneally for two weeks. An 82% reduction in rat paw volume was observed 15 days after injections of 7.5 mg/kg. There was no evidence of toxicity and no observable allergic reactions noted in the rat. These studies demonstrate effectiveness in 2 animal models and prolonged suppression of TNF, IL1, and IL6 in the normal primate [61].
Glucocorticoids: Dexamethasone is a potent inhibitor of NF-kB and the inflammatory process [62,63]. However, the systemic administration of glucocorticoids is associated with many well-known side effects. Dexamethasone microparticles were shown to inhibit TNF by 84%, IL1 by 93%, IL6 by 83%, and IL8 by 81% after stimulation with endotoxin in a whole blood model [64]. This inhibition was significantly more effective in cytokine inhibition than the equivalent dose given in peritonitis of dexamethasone given in the solution. Microparticles in combination with antibiotics resulted in 90% survival in a peritonitis model compared to 30% in a group given an equivalent amount of dexamethasone given in solution with antibiotics [64]. Thus, microparticles containing dexamethasone are an excellent inhibitor of both in vitro and in vivo proinflammatory cytokines. Microparticulate dexamethasone may cause fewer systemic side effects in animals than dexamethasone in solution.
Antioxidants: Recent work has implicated Reactive Oxygen Species (ROS) in many disease processes [65]. The production of hydroxyl ion, hydrogen peroxide, and other by-products of anaerobic metabolism, when formed in excess, has been associated with tissue damage to nucleic acids, lipids, and other cellular components. Septic shock, ischemia/reperfusion situations, atherosclerosis, and many other clinical conditions are associated with ROS overproduction. Phagocytic cells produce ROS as a part of the respiratory burst [66]. Antioxidants have been postulated to be a potential form of therapy in severe COVID-19 infections [67,68]. Recent work from our laboratory has reported in the microparticles containing the antioxidant catalase [17,19,69]. Catalase is a naturally occurring large molecular weight enzyme present in the peroxisomes of nearly all cells. Catalase has an enormous capacity to degrade hydrogen peroxide into O2 and H2O. Catalase has been shown to protect endothelial cells against attack from H2O2 [70]. In cell culture models using endothelial cells, microparticulate catalase inhibited hydrogen peroxide production, nitrate synthesis and TNF release. The intracellular catalase was 7-fold higher using microparticles. In an in-vivo endotoxic shock model, TNF was significantly inhibited and there was a survival rate of 60% in an otherwise uniformly fatal experimental model [71]. The addition of microparticles to NF-kB improved the survival rate to 80% with synergistic inhibition of TNF. These results conclusively demonstrate that intracellular delivery of antioxidants can produce a favorable biologic effect.
Additive and synergetic effects in cytokine inhibition
The intracellular delivery of cytokine inhibiting drugs resulting in high intracellular concentrations makes it possible that drugs inhibiting cytokine synthesis by different mechanisms may be beneficial. The combination of neutralizing antibodies to TNF and IL1 produced survival and proinflammatory cytokine inhibition on the peritonitis model [55]. TNF and IL1 are similar molecular weight and function but have different time-release profiles in sepsis. Using the whole blood in vitro method, TNF reaches peak concentrations after endotoxin stimulation in 2 hr. IL1 synthesis begins after 1 hour and rises for 24 hours [72]. Thus, the combination of microparticles containing neutralizing antibodies to TNF and IL1 act at different stages of the inflammatory response. The combination of CNI-1493 and antisense oligonucleotides to NF-kB produced synergistic effects in survival and additive effects in TNF inhibition [73]. CNI-1493 acts by inhibition of post-translational synthesis of TNF, antisense oligonucleotides to NF-kB inhibits proinflammatory cytokines at the translational level. The combination of microparticulate catalase and antisense oligonucleotides to NF-kB was additive to animal survival and TNF inhibition in the septic rat model.
Therapeutic particulate form of cytokine inhibitors for COVID-19
Thus, by far, the preventive approach has been used as a significant tool to fight COVID-19 globally. Several vaccines in the market and vaccinations have reduced infection cases globally. However, the death rate caused by COVID-19 is still alarming. A robust therapeutic approach towards the fatal disease can reduce the death toll caused by COVID-19. Microparticulate form of cytokine inhibitor may reduce the significant cause of death, which is cytokine storm. There are many cytokines involved in a cytokine storm, such as high serum levels of TNF, IL-2, 7, 10, CXCL10, G-CSF, MCP1, and MIP1α [6]. The use of albumin is ideal for this purpose. The particulate form of cytokine inhibitors has several advantages. These can protect the cytokine inhibitors from acidic or enzymatic degradation and increase bioavailability. We have found the enhanced bioavailability of dexamethasone when administered in particulate form. Another advantage of particulate form is that more than one cytokine inhibitor can be incorporated. As cytokine inhibitors for COVID-19 cytokine storm, antisense oligonucleotides to NF-kB inhibit over 150 substances involved in the inflammatory process. The broad action of NF-kB antisense may be most effective. Alternatively, we can also use dexamethasone or catalase, and both have been found as effective inflammatory reducers in particulate form. We have conducted several animal studies of cytokine inhibitory experiments.
All the studies showed the enhanced effect of cytokine inhibitory agents against inflammation. These results in animal models warrant the further investigation of microparticles in the highly fatal cytokine storm of COVID-19 and other viral conditions causing similar syndromes. If successful, this therapy might significantly reduce the mortality seen in this COVID-19 pandemic. On the other hand, Molnupiravir, an antiviral pill against COVID-19, received emergency use authorization by the FDA in specific select populations in December 2021 [74]. It is a prodrug of N4-hydroxycytidine. The mechanism of this antiviral agent is that it introduces copying errors during viral RNA replication [75]. However, there are underlying concerns of the generation of newer variants due to the drug's mutagenic effects.
These studies utilizing microparticles containing compounds of differing classes (neutralizing antibodies, antioxidants, water-soluble drugs, and antisense oligonucleotides) demonstrate that intracellular delivery of cytokine inhibiting drugs to phagocytic cells is highly effective in cytokine inhibition by increasing intracellular concentrations of the compound. The uptake of microparticles by endothelial cells has great potential in reversing the increased vascular permeability present in the cytokine storm seen in recent COVID-19 and other diseases. Microparticles containing compounds of different classes may be additive or synergistic in inhibiting cytokine inhibition and further improve clinical outcomes. Treatment of endotoxic shock treated with microparticles containing the naturally occurring antioxidant catalase is the first experiment to demonstrate their effectiveness in such a model. Also, the use of microparticles containing antisense oligomers to NF-kB plus antibiotics was the first studies to demonstrate animal survival in a septic shock model using oligonucleotides to NF-kB. The use of microparticles containing antisense to NF-kB was also very effective in reversing the effects of arthritis induced by M. butyricum with no animal side effects. Other microparticulate dosage forms such as antioxidant, TNF and IL1 neutralizing antibodies, CNI-1493, and dexamethasone were also found as very effective cytokine inhibiting agents. These microparticulate cytokine inhibiting agents have demonstrated remarkable effectiveness in cytokine suppression intracellularly compared to equivalent drug doses in solution. These microparticulate cytokine inhibitors can be the most effective way to treat the cytokine storm caused by recent COVID-19 which is prevailing all over the world. Further investigation requires optimizing the best therapeutic dosage form among these microparticulate cytokine inhibiting agents.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google scholar] [PubMed]
Citation: Uddin MN, Bagwe P, Oettinger C, D’Souza MJ (2022) Evaluation of Microparticulate Cytokine Inhibitors for their Potential Use for the Treatment of COVID-19 Cytokine Storm. Trans Med. 12:258.
Received: 17-May-2022, Manuscript No. TMCR-22-17530; Editor assigned: 19-May-2022, Pre QC No. TMCR-22-17530 (PQ); Reviewed: 02-Jun-2022, QC No. TMCR-22-17530; Revised: 09-Jun-2022, Manuscript No. TMCR-22-17530 (R); Published: 20-Jun-2022 , DOI: 10.35248/2161- 1025.22.12.258
Copyright: © 2022 Uddin MN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.