Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2015) Volume 6, Issue 6
In the context of an epidemiological study, urinary concentrations of nine phthalic diester metabolites (monoethyl-, mono-(3-carboxypropyl)-, mono-n-butyl-, monoisobutyl-, monobenzyl-, mono-(2-ethylhexyl)-, mono-(5-hydroxy-2- ethylhexyl)-, mono-(5-oxo-2-ethylhexyl)- and mono-(5-carboxy-2-ethylpentyl)-phthalate) were quantified via LC-MS/ MS. As in the majority of epidemiological studies only single spot samples were available for urine analysis, the implicit assumption in this case is, that exposure data obtained from single spot samples are representative for a longer exposure period. To validate the relevance of single spot analyses we quantified the respective intra-individual variances of urine samples collected from ten volunteers once daily over a period of 30 days. Using the values for the daily variances, approximate values for the underlying population variances in the cohort samples representing the differences between the average individual metabolite levels were calculated. For most of the volunteers, daily metabolites variations were lower, than the variations observed in the epidemiological setup. The results showed that by accounting for the contribution of daily variance, the standard deviations of the log-transformed phthalate values of the cohort samples are reduced (14% to 28%) but still larger (3% to 66%) than daily standard deviation values, with the exception of MCPrP concentrations.
Keywords: Endocrine disruptors; Phthalates; Metabolites; Epidemiology; Variance; Individual
Phthalic diesters (phthalates) are a group of organic compounds that are produced at a scale of millions of tons per year. They have been in use for several decades now as plasticizers and additives in a large variety of consumer products. Since they are not covalently bound to other compounds within their respective formulations, significant amounts of these chemicals have accumulated in the environment and exposure is ubiquitous. For di-(2-ethylhexyl)-phthalate, diet is thought to be the main source of exposure, but non-dietary pathways can also be substantial, as has been shown for other phthalates [1,2].
Over the past 15 years, animal studies have shown the potential of several phthalates to act as endocrine disruptors in an estrogenic or anti-androgenic fashion, raising concerns over their health impacts [3-6]. However, compared with the large body of experimental evidence suggesting reproductive or developmental toxicity of phthalates, human data have been rather limited [7-9]. More recently, phthalates have been proposed to act as obesogens, endocrine disruptors which induce or contribute to adipogenesis and the development of obesity [10,11]. In this context, cell culture and animal studies have established the ability of mono-(2-ethylhexyl)-phthalate to selectively bind to peroxisome proliferator-activated receptors (PPARs) which belong to the nuclear receptor superfamily of ligand-activated transcription factors and to modulate their functions [12,13]. PPARα and PPARγ are expressed in adipocytes and play essential roles in the regulation of cellular differentiation, development, and metabolism.
Quantification of phthalate metabolites in urine is a good measure of exposure since urinary enzymatic activity is negligible and metabolites thus generally reflect internal exposure rather than contaminants introduced during sample collection and processing [14]. However, hydrolytic genesis of simple monoesters from the ubiquitous diesters seems to occur to a limited extent also via non-biogenic processes, and oxidatively transformed monoesters, if actually produced in significant amounts, represent the more reliable metabolites [15]. There have been several studies showing that phthalic diesters are metabolized and excreted fairly quickly with biologic half-lives of about ten hours and urinary phthalate levels have been shown to vary, probably in synchrony with the intermittent incorporation of their parent compounds during food uptake or through the use of personal care products [1,16-18].
Between 2006 and 2008, pregnant woman were recruited for the German Lifestyle and environmental factors and their Influence on Newborns Allergy risk (LINA) cohort study with the aim to investigate environmental influences during pregnancy on health risks of the child, in particular asthma and allergic diseases [19]. Since urinary phthalate metabolites are among the exposure parameters to be quantified, the question arose to what extent values obtained from individual spot samples at the 36th week of pregnancy constitutes a representative measure for exposure during the whole pregnancy. Several studies focused on the variability of phthalate and metabolites in human urine even for gestation periods [20-26]. However still less is known about the common approach to use a single spot urine analysis for correlation to health outcomes in the context of epidemiological studies. In order to obtain more information on this, phthalate metabolite levels in urine samples from more than 600 women in the 36th week of pregnancy were quantified and compared to day-to-day variability observed in the urine samples of ten volunteers over a period of 30 days. Aim of this study was I. to describe the interday variability and II. To evaluate the possibility to use single spot analysis of those phthalate metabolites for epidemiological studies.
Materials and reagents
N-(2-acetamido)-iminodiacetic acid (ADA) and formic acid (analytical grade / mass spec grade) were obtained from Fluka (Taufkirchen, Germany), acetic acid from Biosolve (Valkenswaard, The Netherlands). Acetonitrile and methanol (picograde) were obtained from LGC Promochem (Wesel, Gemany), methanol (LCMS grade) from Merck (Darmstadt, Germany). Ultrapure water was produced by a MiliporeQ (MerckMillipore, Darmstadt, Germany) water purification system. Isolute C18 SPE 200 mg/3 ml columns were obtained from Biotage (Düsseldorf, Germany), autosampler vials from Wicom (Heppenheim, Germany) and deglucuronidase / arylsulfatase (helix pomatiae) from Roche (Mannheim, Germany). 13C4-labeled and unlabeled standards for monoethyl-, mono-(3-carboxypropyl)-, monon- butyl-, monobenzyl-, mono-(2-ethylhexyl)-, mono-(5-hydroxy-2- ethylhexyl)-, mono-(5-oxo-2-ethylhexyl)- and mono-(5-carboxy-2- ethylpentyl)-phthalate (MEP, MCPrP, MnBP, MBzP, MEHP, MEHHP, MEOHP and MECPP, respectively), 13C4-labeled methylumbelliferone (MeUmb) and unlabeled mono-isobutylphthalate (MiBP) were purchased from LGC (Wesel, Germany). Unlabeled MeUmb was obtained from Aldrich (Steinheim, Germany), methylumbelliferone glucuronide (MeUmb-gluc) from Sigma-Aldrich (Seelze, Germany). Lyophilised ClinChek® control urine samples (level I and II) were purchased from Recipe Chemicals+Instruments (Munich, Germany).
Description of the study population
Six-hundred and twenty-two pregnant woman were recruited within the prospective mother-child-study LINA (Lifestyle and environmental factors and their Influence on Newborns Allergy risk) from March 2006 until December 2008 in Leipzig, Germany [27,28]. Participation in this study was voluntary and informed consent was given. This study was approved by the Ethics Committee of the University of Leipzig, Germany (file reference 046-2006, 160-2008). All participants agreed to the usage of the results in the context of the LINA study.
Urine samples and sample preparation
Within the LINA study maternal urine samples were collected during the 36th week of pregnancy. 300 additional longitudinal urine samples were collected from ten volunteers (25 to 49 years old; four male, six female) who gave informed consent. Samples and sample aliquots were stored separately at -80°C until further analysis.s
Phthalate quantification was carried out for 610 samples of the LINA cohort and all 300 samples of the longitudinal study. For this, 100 μl aliquots were buffered with 150 mM Na·ADA, pH 6.6, spiked with 25 μl 50% methanol containing 2 ng each of 13C4-labelled MEP, MCPrP, MnBP, MBzP, MEHP, MEHHP, MEOHP, MECPP, MeUmb and unlabeled MeUmb-gluc, and incubated for 5 hr at 37°C with 3.5 μl deglucuronidase/arylsulfatase in a total volume of 210 μl to achieve deglucuronation of phthalic monoesters. Reactions were stopped by addition of 510 μl 700 mM formic acid (FA) with 47 mM acetic acid. Purification of samples was performed via solid phase extraction (SPE) on Isolute C18 columns via gravity flow. After equilibration of the columns using 3 ml 100% methanol, followed by 3 ml of 2% FA in 5% methanol, samples were applied, followed by two washing steps of 3 ml 2% FA in 10% methanol. Analytes were eluted in 1.5 ml of 2% FA in 90% methanol, vacuum-dried at 45°C, resuspended in 100 μl 35% acetonitrile and transferred to insert-containing auto sampler vials, which were crimp-sealed.
With the preparation of each batch of about 40 samples, aliquots of three quality control urine samples (two commercially obtained, one self-prepared by spiking of unlabeled standards into an arbitrarily chosen urine sample at 20 ng/ml) were included in order to ensure correctness of the results obtained for each batch.
LC-MS/MS
10 μl aliquots were separated on a Dionex™ UltiMate™ 3000 UPLC System (Thermo Scientific, MA, USA) using a sigmoidal 21 min gradient from 5% to 100% methanol (eluent B) with 0.01% FA (Supplementary Table 1). Separation was achieved via reversed phase chromatography on an Acquity UPLC BEH C18, 1.7 μm, 2.1 mm × 100 mm column (Waters, Eschborn, Germany) at 50°C and 200 μl/min. Detection and quantification of analytes after elution and post-column infusion (50 μl/min in 0.2% NH3) was achieved on a Q-Trap 5500 triple quadrupole mass spectrometer (AB Sciex, Framingham, MA, USA) with electrospray ionisation at 350°C and 4500 V in negative mode via previously established, scheduled SRMs (Supplementary Table 2). Between samples, the column was washed for 5.5 min with 100% methanol, 0.01% FA and an injection of 100 μl isopropanol/acetonitrile/ acetone (45:45:10), followed by re-equilibration in 5% methanol, 0.01% FA. Absolute concentrations of phthalate analytes and the deglucuronation standard MeUmb were calculated with respect to the known, spiked-in concentrations of the isotopically labelled standards and previously obtained calibration curves, using dedicated software (Analyst, AB Sciex). Values for samples with concentrations below LOQ (23 and 43 instances for MCPrP and MEP, respectively) were set to LOQ/2 for data treatment. An overview of the covered analytes, the used internal standards, and calibration range including LOQ data is given in Table 1.
| Analyte | Internal Standard | Calibration Range [µg/l] | LOQ [µg/l] |
|---|---|---|---|
| MeUmb | 13C4-MeUmb | 10-100 | 10 |
| MEP | 13C4-MEP | 0.315-1000 | 0.315 |
| MiBP | 13C4-MnBP | 3.15-1000 | 3.15 |
| MnBP | 13C4-MnBP | 3.15-1000 | 3.15 |
| MCPrP | 13C4-MCPrP | 0.315-1000 | 0.315 |
| MBzP | 13C4-MBzP | 0.315-1000 | 0.315 |
| MEHP | 13C4-MEHP | 0.315-315 | 0.315 |
| MEHHP | 13C4-MEHHP | 1.0-1000 | 1.0 |
| MEOHP | 13C4-MEOHP | 0.315-315 | 0.315 |
| MECPP | 13C4-MECPP | 0.315-315 | 0.315 |
| BPA | 13C12-BPA | 3.15-1000 | 3.15 |
Table 1: Overview covered analytes, used internal standard, calibration range [μg/l] and limit of quantification (LOQ) [μg/l].
Finally, all metabolite concentrations were normalized against urinary creatinine concentrations. All samples were analyzed once with the described LC-MS/MS procedure.
Data analysis
Data analyses including numerical simulations was done using Excel, Past (version 3.0.1) or R (version 3.1.1) using RStudio (version 0.98.977) with packages xlsx, reshape2 and ggplot2. Graphics were prepared using Statistica (version 12), Excel or R. Before pooling the values of the ten volunteers from the longitudinal study into a single dataset, for each metabolite the concentration values of an individual were normalized by multiplication with k (k=mediantotal/medianindividual). Concentration values of zero were excluded from analyses based on logarithmically transformed data. Means and variances for the empirical cumulative distributions of the log-transformed concentration values were calculated using the iterative nonlinear least-squares (nls) curve fitting function for nonlinear models in R. From the obtained values for each metabolite, the mean and standard deviation (μPop and sdPop) of the "true" distribution (i.e., a distribution being based on average individual values) underlying the observed LINA cohort distribution was calculated as 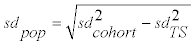 (Formula 1) and
(Formula 1) and 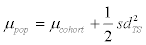 (Formula 2), with sdcohort and μcohort being the standard deviation and mean from the cohort study and sdTS the standard deviation of the longitudinal study.
(Formula 2), with sdcohort and μcohort being the standard deviation and mean from the cohort study and sdTS the standard deviation of the longitudinal study.
Good chromatographic separation was obtained for all the metabolites as shown in Figure 1(A) for a methanolic calibration sample as well in an authentic urine matrix as given in Figure 1(B). In addition low LOQ and good linearity were achieved as given in Table 1. High selectivity of the MS method was observed by the comparison of a methanolic standard (Figure 1(A)) and an authentic urine sample (Figure 1(B)).
In order to decide whether urinary creatinine concentrations should be used to normalize urinary phthalate metabolite levels, Spearman correlation analyses were carried out for datasets of the cohort and the longitudinal study. For both datasets strong and highly significant correlations (0.30 to 0.67; puncorr.<1.6 x 10-7) were found between creatinine and phthalate concentrations. However, when phthalate concentrations were normalized against their respective creatinine concentrations, correlations were drastically reduced for all metabolites (Supplementary Figure 1). For most metabolites, remaining correlations were weak and statistically not significant (puncorr.<0.05). For metabolites MiBP, MnBP, MEHP and, in the case of the longitudinal study, MCPrP, however, normalizing against creatinine led to "overcompensation", resulting in statistically significant negative correlations. According to these results, all analyses of the datasets were based on creatinine-normalized concentrations.
As has been reported for other cohort studies, concentrations of phthalate metabolites in urine samples collected for both studies showed considerable variation with an approximately log-normal distribution pattern (Figure 2, Supplementary Figure 1) [29-32].
Similarly, values documenting daily fluctuations in the urinary metabolite concentrations of individuals showed log-norm distributions as well. Trends in the rise and fall of concentrations over time courses of 2 days or more were not evident upon visual inspection of the respective plots (Supplementary Figure 2). Spearman correlation analyses of concentrations on consecutive days, however, yielded coefficients that in some cases (14 out of 90) were significant with puncorr.<0.05. Calculating Fisher-weighted means of Spearman correlation coefficients from all ten individuals for a given metabolite showed weak (0.14 to 0.24) but significant (puncorr. ≤ 0.018) correlations for all metabolites except for MEHP and MiBP (puncorr.>0.05).
Median metabolite concentrations obtained in the longitudinal study were generally lower than the respective median concentrations from the cohort samples (Figure 2). Specifically, median creatininenormalized concentration for MEP was 48.6 μg/g for cohort samples while averaged median creatinine-normalized concentration was 32.6 μg/g for the longitudinal study. For MiBP 65.6 μg/g and 27.0 μg/g, for MnBP 105.4 μg/g and 50.7 μg/g, for MCPrP 1.5 μg/g and 1.2 μg/g, for MBZP 6.7 μg/g and 5.3 μg/g, for MEHP 7.8 μg/g and 4.3 μg/g, for MEHHP 13.4 μg/g and 8.4 μg/g, for MEOHP 9.7 μg/g and 4.4 μg/g, and for MECPP 12.1 μg/g and 5.5 μg/g were determined as creatininenormalized concentrations for cohort samples and as average creatinine-normalized concentrations for the longitudinal study, respectively. For most of the volunteers, daily metabolites variations were lower, than the variations observed in the epidemiological setup.
In order to obtain an estimate to what extent the variability of metabolite concentrations seen in the cohort can be accounted for by day-to-day variance and which proportion is due to actual differences in (average) exposure of the individual participants (i.e., population variance), the standard deviation values of the respective log-scaled metabolite concentrations were determined. When the cumulative distribution plots of the data points were overlaid with the respective cumulative distribution functions (CDFs) using means and standard deviations calculated from log-scaled values, it was found that the resulting CDF curves did not accurately match the course of the data points (not shown). Using median values instead of means led to only slight improvements (Figure 3). The reason for this was a number of exceedingly high concentration values (outliers), which led to standard deviation values not adequately capturing the spread of the majority of data points. In order to obtain a better representation of the standard deviations without removing or arbitrarily rescaling outliers, a nonlinear least squares regression algorithm was used, which led to more accurate results (Figure 3) with standard deviations that were smaller on average by 13% for the cohort and 31% for the longitudinal cohort study (Table 2).
Figure 3: Exemplary cumulative distribution plots showing the concentration distributions (ln-values) of two phthalate metabolites for the cohort (cohort) and the longitudinal study (TS). Red curves represent cumulative distribution functions (CDFs) with median and variance values using the standard calculation procedure, green curves the respective functions with values obtained via nls curve-fitting.
| measure | MEP | MiBP | MnBP | MCPrP | MBzP | MEHP | MEHHP | MEOHP | MECPP | |
|---|---|---|---|---|---|---|---|---|---|---|
| Cohort | Median | 3.88 | 4.18 | 4.66 | 0.37 | 1.90 | 2.05 | 2.60 | 2.27 | 2.49 |
| µcohort | 3.91 | 4.21 | 4.64 | 0.38 | 1.89 | 2.09 | 2.60 | 2.27 | 2.49 | |
| Std. Dev. | 1.12 | 0.65 | 0.65 | 0.77 | 0.84 | 0.72 | 0.82 | 0.80 | 0.71 | |
| sdcohort | 1.10 | 0.54 | 0.57 | 0.52 | 0.81 | 0.64 | 0.74 | 0.71 | 0.62 | |
| Longitudinal study | Median | 3.20 | 3.25 | 3.89 | 0.18 | 1.63 | 1.36 | 2.03 | 1.43 | 1.71 |
| µTS | 3.22 | 3.24 | 3.91 | 0.21 | 1.64 | 1.36 | 2.06 | 1.46 | 1.73 | |
| Std. Dev. | 0.88 | 0.34 | 0.36 | 0.68 | 0.73 | 0.64 | 0.74 | 0.72 | 0.67 | |
| sdTS | 0.61 | 0.28 | 0.31 | 0.42 | 0.46 | 0.42 | 0.48 | 0.48 | 0.43 | |
| CohortPopulation | µPop | 4.09 | 4.25 | 4.69 | 0.47 | 2.00 | 2.18 | 2.72 | 2.39 | 2.58 |
| sdPop | 0.91 | 0.46 | 0.47 | 0.31 | 0.66 | 0.48 | 0.56 | 0.53 | 0.45 |
Table 2: Medians, means (μ) and standard deviations (sd) of phthalate metabolite concentration (ln-values) distributions. Means and standard deviations designated as μcohort, μTS, sdcohort and sdTS were obtained via iterative nonlinear least-squares (nls) regression. Means and standard deviations for the cohort (μPop and sdPop) accounting for the daily variation (representing population variance only) were calculated from μ and sd-values obtained of the cohort (representing population plus daily variance) and the sd-value of the longitudinal study cohort (sdTS, representing daily variance only) as described in Materials and Methods.
For each metabolite, the standard deviations obtained from the log-transformed concentration values of the cohort (representing population plus daily variance) and the longitudinal study cohort (representing daily variance only) were used to calculate standard deviation values representative for the average individual phthalate concentrations (i.e., population variance, based on log-transformed values) within the cohort (Formula 1). The results showed that by accounting for the contribution of daily variance, the standard deviations of the log-transformed phthalate values of the cohort samples are reduced (14% to 28%) but still larger (3% to 66%) than daily standard deviation values, with the exception of MCPrP concentrations (standard deviation reduced by 40%; day-to-day standard deviation 33% larger than the standard deviation of the cohort population; Table 2). Using formula 2 and the standard deviations observed in the longitudinal study, the means observed in the cohort increased by an average of 5.8% (untransformed concentrations; range 0.9% to 5.6%, except for MCPrP with an increase of 23%), yielding means representing average concentration values in the absence of daily fluctuations (Table 2).
One major problem by the analysis of phthalate is the ubiquitous presence of such compounds in the environment including laboratories. This background may be reduced e.g., by the usage of ultra-pure solvents and glass ware. However, high variations for phthalate background levels can never be excluded. This is especially true for this study, as the samples from the epidemiological study were obtained by different hospitals. By analysis of phthalate metabolites this problem can be overcome. This applies in particular for analysis of phase II metabolites. However, as phase I and phase II metabolites can be detected simultaneously in human urine, analytical techniques can either detect phase I and II metabolites independently or as a sum parameter of phase I and II metabolism after deconjugation. While the first option provides detailed information about the phase I and II ratio, the second one leads to lower detection limits and thus to higher detection rates. In this study the sum of phase I and II metabolites were detected as an enzymatic deconjugation step was carried out during samples preparation.
For urinary phthalate metabolite concentrations in the literature volume-based and creatinine normalized concentrations are given [2,32-39]. A correlation analysis of samples from our cohort and longitudinal study revealed that without normalizing against creatinine, there is a strong correlation between creatinine concentration and all phthalate metabolite values. As metabolite concentrations are generally used as a surrogate measure for exposure to the parent compound in epidemiological studies, volume-based urinary concentrations ideally would have to be multiplied by urine volume to provide the total amount of metabolites that have been excreted within a given time interval (the time between two voidings). However, since total volume is usually not available, creatinine concentration is a commonly used surrogate. Under the premise that this endogenous compound is produced and excreted at a constant rate which is the same for all people, it will behave in a reciprocal fashion to the volume of urine produced in the same time interval. However, this assumption is not generally correct as described by Wyss and Kaddurah-Daouk [40]. We have also seen individual differences in the results of correlation analyses performed for each of the ten participants in the longitudinal study, implying that this analyte is not perfectly suited in all cases (data not shown). Nevertheless, our results strongly support a normalization of phthalate concentrations against creatinine as opposed to no normalization at all (Supplementary Figure 3).
Negative correlations seen for some of the metabolites may indicate a problem with a more or less constant background contamination that is not dependent on the overall concentration of the sample. The parent compounds of MiBP, MnBP and MEHP are known ubiquitous environmental contaminants whose monoesters can also be generated under abiotic conditions [16,41,42]. Even if constituting a minor fraction of environmental phthalates, their presence may influence results obtained by sensitive detection methods. Addition of a constant background level will not influence the results of the Spearman correlation analysis on volume-based concentrations, since it is rankbased. However, relative differences between the samples will be reduced. When relatively similar values are divided by more dissimilar creatinine concentrations, creatinine values become the dominant determinant for the ranking, resulting in a negative correlation.
The fact that urinary concentrations taken 24 hr apart were only slightly correlated shows that metabolism and elimination of phthalates is fast and that even peak metabolite levels observed on one day will almost have returned to background levels on the following day. Such a result was to be expected, as experiments with human volunteers ingesting a dose of isotopically labeled phthalates with shorter (di-nbutyl phthalate, DnBP and di-(isobutyl)-phthalate, DiBP) or longer side chains (DEHP and di-(isononyl)-phthalate, DiNP) have shown these compounds to be quickly resorbed, metabolized and eliminated, with urinary peak concentrations occurring after 2-4 hr and half-lives of elimination - dependent on the type of metabolite - in the range of 2.6 to 4.2 hr or 2 to 5 hr, respectively, during the first 8 to 24 hr [43-45].
By comparing the obtained phthalate metabolite concentrations for cohort samples with other values from the literature (Table 3), it must be kept in mind, that those studies were either performed in different countries/continents, at different time points or with nonpregnant females or females and males. The study described by Zeman et al. represents in the authors opinion the most similar setup in comparison to our mother-child cohort [35]. Creatinine normalized phthalate concentrations were similar for MEP (48.6 μg/g and 34.3 μg/g) and MiBP (65.6 μg/g and 68.7 μg/g) by comparison of LiNA cohort values with those of Zeman et al. [35]. In comparison a ~ 2 fold higher median MnBP concentration was found in the cohort sample (105.4 μg/g vs. 45.5 μg/g) [35]. This difference may be explained by the different countries in which the studies were conducted, since an even higher median MnBP concentration had been observed in the German study described by Koch et al. in 2002 (157 μg/g) [37]. The same is true for median MEHP but interestingly not for the MEHHP, MEOHP, and MECPP concentrations. Concerning the metabolite ratio of DEHP and its oxidized metabolites, different ratios were observed in contrast to large scale studies [30,46,47]. This difference may be explained by the usage of helix pomatiae glucuronidase, which also provides low lipase activity and thus may convert DEHP to MEHP artificially. This may also affects the other monoester concentrations in this study. However, as this is a observational error, data and comparisons, results and conclusion obtained within this study should be still valid. The observed differences between the median metabolite concentrations from the cohort and the average median metabolite concentrations of the longitudinal study cohorts most probably reflect declining exposure levels within the six year interval in which the two sets of samples were collected, since the concentration ratios are close to the values that can be calculated from estimations of approximately exponential decay rates documented for the years 1988-2003 [39].
| Study | cohort study | 32 instead of 2 | 33 instead of | 34 instead of 4 | 35 instead of 1 | 31 instead of 5 | 37 instead of 6 | |
|---|---|---|---|---|---|---|---|---|
| N | 610 | 108 | 83 | 74 | 100 | 279/ | 1310 | 85 |
| 139 | ||||||||
| Sex | f (p) | f | f | m | f (p) | f (p) | f | f/m |
| Time | 2006-7 | 2007 | 2012 | 2001 | 2007 | 2007-8 | 2002 | |
| Country | GER | MEX | ITA | TWN | FRA | USA | GER | |
| Value | Med. | GM | GM | Med. | Med. | GM | Med. | |
| Conc. | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | µg/g | |
| PhthalateMetabolite | ||||||||
| MEP | 48.6[3.1-1944] | 83.2 | 77.1 | 56.4 | / | 34.3 | 106 | 73.3 |
| MiBP | 65.6 [15.9-1928] | 8.4 | / | / | 15.2 | 68.7 | 8.18 | / |
| MnBP | 105.4 [21.4-6294] | 72.4 | 20.3 | 17.6 | 87.5 | 45.5 | 23.1 | 157 |
| MCPrP | 1.45 [0.24-241.30] | 3.91 | / | / | / | / | / | / |
| MBzP | 6.70 [0.45-124] | 4.37 | 14.7 | 16.4 | 2.07 | 13.6 | 8.07 | 17.2 |
| MEHP | 7.8 [1.4-360.4] | 5.2 | 3.4 | 2.8 10.8 | 16.4 | 17.9 | 3.02 | 9.2 |
| MEHHP | 13.41 [1.42-1416] | 45.84 | 12.7/ | / | 32.2 | 44.4 | 25.2 | 40.2 |
| MEOHP | 9.7[ 1.0-1007] | 31.8 | / | / | 29.5 | 32.9 | 14.2 | 30.4 |
| MECPP | 12.1 [2.1-661] | 71.9 | / | / | 44.7 | 58.1 | 38.7 | / |
Table 3: Comparison of median (Med.) or geometric mean (GM) of phthalate concentrations from the cohort study with respective data from other publications. Values indicating concentration ranges are shown in brackets; values shown in gray (study [1]) refer to a sub-sample of the cohort. N: number of samples in cohort; Sex: female (f) or male (m) study participants, (p): pregnant; Time: year of sampling; Conc.: concentrations referring to creatinine mass (μg/g). Metabolites are grouped with respect to their different parent compounds (DEP, DnBP, DiBP, BBzP and DEHP).
Apart from MEP and MCPrP, for which Wittassek and coworkers did not supply any values, the only exception is MiBP, where the observed reduction by a factor of 2.4 is not forecast by a corresponding trend, as the values for this metabolite, after an initial slight increase between 1988 and 1996, had remained more or less constant in the following 7 years. According to data available from the German Federal Environment Agency, urinary MiBP values have been declining, albeit at a fairly low and seemingly linear rate of ~ 1.7 μg/l per year, between 1998 and 2008 [48]. However, following a study of the German Federal Institute for Risk Assessment (BfR) reporting high concentrations of the parent compound di-(isobutyl)phthalate (DiBP) in packaging materials for food, commercial use of this compound had been withdrawn voluntarily by the industry in 2009, possibly contributing to an increased reduction in exposure in the following years and thus to decreased urinary MiBP concentrations [49].
Another aspect accounting for the metabolite concentration differences observed between the cohort samples and those of the longitudinal study may be sampling bias. The longitudinal study consisted of people with a higher education level, whereas the participants of the cohort study represented a cross section of different educational backgrounds. However, to our knowledge, there has been no study to date showing phthalate exposure to be correlated with socio-economic factors. Furthermore, apart from the fact that pregnant women (cohort study) were compared to a population of mixed genders (longitudinal study), it has also to be kept in mind that the number of individuals in the latter sample set is only ten and thus might not be sufficiently representative for the general population
Comparing location and extent of the 10-90 percentile ranges of phthalate metabolite concentrations in the longitudinal study, differences between individual participants are apparent (Figure 2). However, no influence of gender on phthalate metabolite concentrations was found via unpaired two-tailed student t-test (data not shown). For MEP, the strong differences in concentration medians with respect to interday variability suggests a meaningful stratification of participants into groups of low exposed (p 2, 4, 5, 6 and 7) and high exposed persons (p 1, 3, 8, 9 and 10). With regard to daily variations, also differences between the ten volunteers were observed. Comparing minimum and maximum spread values, p 6 showed an ~25 fold higher spread of MEP concentration values than p 10 (based on 10-90% percentiles after normalization to their respective medians). A factor of~ 12 was observed for MBzP concentrations by comparing p 6 and p 9. For the DEHP metabolites MEHP, MEHHP, MEOHP and MECPP, p 5 showed daily variations larger by ~ 10, ~21, ~18 and ~12 times in comparison to p 9 or p 8
In order to get a measure for the day-to-day variance and to correct for variance between different individuals, concentration values of the ten participants of the longitudinal study were normalized by a correction factor k (k=mediantotal/medianindividual), thereby adjusting individual medians to the total median. As this scaling results in unchanged relative standard deviations or, with respect to using logarithmic values for data analysis, unchanged absolute standard deviations for each individual, the resulting values could be collated into a single dataset representative for the daily variance of a single person.
The distributions of urinary metabolite concentrations observed in the cohort study do not solely represent the different average exposure levels of the study participants (i.e., population heterogeneity), but time-dependent variation as well, since short-term fluctuations in exposure have a strong influence on the results obtained from spot samples. On the assumption that average individual phthalate exposure levels are log-normally distributed within a population (e.g., the levels of several phthalate diesters have been shown to be lognormally distributed in house dust samples) and that daily variations in the metabolite concentrations follow a log-normal distribution as well (as suggested by the data from our longitudinal study), it can be shown that population ( 2 pop sd ) and day-to-day variances ( 2 TS sd ) of the log-transformed concentrations behave in an additive manner 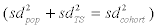 [50]. Using log-transformed concentration values, it is thus possible to use the standard deviations from the cohort and longitudinal study to obtain the standard deviation for the logtransformed concentrations of a given metabolite for the population (Formula 1; Table 2).
[50]. Using log-transformed concentration values, it is thus possible to use the standard deviations from the cohort and longitudinal study to obtain the standard deviation for the logtransformed concentrations of a given metabolite for the population (Formula 1; Table 2).
With respect to log-transformed concentration values, knowing day-to-day variance ( 2 TS sd ) and the mean observed for the cohort study (μcohort), the mean value of the respective underlying population (μPop) can be derived as well. Whereas the expected value EPop for a given metabolite concentration in the population is (approximately) represented by the mean of the observed concentration values in the LINA cohort study (Ecohort), the same is not true for the expected values of the log-transformed values (i.e., μPop, μcohort), since for log-normal distributions the respective relations are defined by in 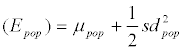 and in
and in 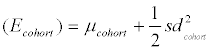 . It follows that
. It follows that 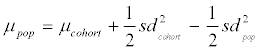 and, with formula 1 rearranged to
and, with formula 1 rearranged to 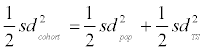 , it follows that
, it follows that  (Formula 2; Table 2). Comparing the values obtained for standard deviations of the log-transformed concentration values (Table 2), it is evident that after accounting for the contribution of daily variance, the standard deviations representing the spread of average concentrations (and thus exposure) within the population (sdPop) are of a similar magnitude for MCPrP, MEHP, MEHHP; MEOHP and MECPP, as the standard deviations representing daily variance (sdTS). For MEP, MiBP, MnBP and MBzP, lower standard deviations were observed for the longitudinal study in comparison to the cohort (approx. -30 to -40% based on log values). Together with the observation that urinary concentrations of samples taken only 24 hr apart show only marginal correlation, these results call into question the suitability of phthalate metabolite concentration values obtained from singular samples to represent average individual exposures. In a more general vein, if exposure to a given compound is subjected to strong daily variations and the respective compound does not bioaccumulate, but instead is excreted within a relatively short time frame, concentration values obtained from single samples may be of limited or even negligible value in the context of epidemiological studies, as the presence of actually existing correlations with other parameters will be obscured and not be detected by statistical evaluation, leading to false negative results [16,51-53]. This is true for all investigated phthalate metabolites except of MEP, MiBP and MnBP, as intra individual fluctuations were smaller in the longitudinal in contrast to the cohort study. However, in epidemiological studies with sufficient statistical power, correlations to certain outcomes may be detected even with analytes showing a strong day-to-day variability. Our results were in harmony with the results obtained by Frederiksen et al. showing the risk of single spot urine analysis for phthalate metabolites and the corresponding correlation to certain health outcomes [23].
(Formula 2; Table 2). Comparing the values obtained for standard deviations of the log-transformed concentration values (Table 2), it is evident that after accounting for the contribution of daily variance, the standard deviations representing the spread of average concentrations (and thus exposure) within the population (sdPop) are of a similar magnitude for MCPrP, MEHP, MEHHP; MEOHP and MECPP, as the standard deviations representing daily variance (sdTS). For MEP, MiBP, MnBP and MBzP, lower standard deviations were observed for the longitudinal study in comparison to the cohort (approx. -30 to -40% based on log values). Together with the observation that urinary concentrations of samples taken only 24 hr apart show only marginal correlation, these results call into question the suitability of phthalate metabolite concentration values obtained from singular samples to represent average individual exposures. In a more general vein, if exposure to a given compound is subjected to strong daily variations and the respective compound does not bioaccumulate, but instead is excreted within a relatively short time frame, concentration values obtained from single samples may be of limited or even negligible value in the context of epidemiological studies, as the presence of actually existing correlations with other parameters will be obscured and not be detected by statistical evaluation, leading to false negative results [16,51-53]. This is true for all investigated phthalate metabolites except of MEP, MiBP and MnBP, as intra individual fluctuations were smaller in the longitudinal in contrast to the cohort study. However, in epidemiological studies with sufficient statistical power, correlations to certain outcomes may be detected even with analytes showing a strong day-to-day variability. Our results were in harmony with the results obtained by Frederiksen et al. showing the risk of single spot urine analysis for phthalate metabolites and the corresponding correlation to certain health outcomes [23].
With new and ever more sensitive analytical methods becoming available, the amounts of quantitative analytical data from samples obtained in the context of epidemiological studies continues to increase. Whereas reproducibility and reliability of the methods themselves, if sufficiently validated, do not constitute a matter of concern, it is generally less clear how reliable the sample and the data gained from it are with respect to representing average or long-term measures of the respective study participant. In contrast to persistent organic pollutants with a high bioaccumulation potential, xenobiotic compounds with a large range of exposure levels and short metabolization and excretion rates are especially problematic in this regard. In such cases, instead of trying to increase cohort size, increasing the number of samples per study participant may produce a larger benefit as already stated by Fisher et al. since averaging individual concentration values will yield much more reliable estimates of a given person's general exposure and thereby increase the chance of discovering correlations with parameters likely to result from long-term effects such as medical outcomes [21].
The authors thank their colleagues Sandra Albrecht, Sven Baumann, Scarlett Gebauer, Carolin Graebsch, Joerg Hackermueller, Gabriele Heimpold, Markus Langhammer, Melanie Nowak, Susanne Pfeiffer, Ulrike Tzschoppe, Stefan Wicht, Brigitte Winkler, and Marleen Zurek for scientific discussion and technical assistance. This work was funded by the European Union and the Free State of Saxony via the European Regional Development Fund (EFRE) program No. 2007 DE 16 1 PO 004.
Supplementary information accompanies the paper on website.