Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2024)Volume 12, Issue 3
Three independent experiments have been performed for three human cancer cell lines: Adenocarcinomic Human Alveolar Basal Epithelial Cells, (A549), Hepatoblastoma Cell Line (HepG2) and Cancer Coli-2 (CaCo-2), where test samples were divided into six groups: Grape seed extract and licorice root extract as prebiotics, Lactobacillus acidophilus bacteria supernatant and cell sonicate as probiotic and combination of prebiotic and probiotic. Cell viability variations were evaluated, while further anticancer activity was assessed via flow cytometry for cell cycle arrest profile and caspase 3 expressions was monitored. Obtained results showed that IC50 was reduced by the combined administration of pre and probiotics more than indicted by probiotics individually. More prominently observed in HepG2 cell line compared to CaCo-2 and A549 cell lines respectively. Data revealed that the variation of the apoptotic profile was tested extract formulae dependent. Cells treatment induced a significantly elevated early apoptotic than in the case of control cells, while grape mix showed a significantly elevated late apoptotic than in the case of cells treated with grape extract and licorice mix. Also, there was an insignificant necrotic cell in the case of cell treatment with grape, grape mix and licorice mix as well. At the same time, it was noticed that the amount of active caspase-3 present in each culture sample was increased in cells treated with test samples more than in control cells by an obvious percentage.
Prebiotics; Probiotics; Synbiotics; Anticancer activities
Prebiotics, such as Fructo-Oligosaccharides (FOS) inulin and oligofructose, are non-digestible carbohydrates that beneficially impact the host's health by selectively stimulating the growth and/or activity of beneficial bacteria in the colon. These beneficial bacteria, mainly Bifidobacterium and Lactobacillus, reside in the human colon and exert their effects by modulating colon microflora, immunogenic responses and producing certain substances that improve host health. Probiotics have been shown to help prevent infections, reduce cholesterol levels, promote vitamin and cytokine synthesis and even exhibit anticancer effects. When prebiotics and probiotics are combined, they create a symbiotic activity that can provide even more significant benefits than when used alone. While FOS, inulin and oligofructose are the most studied prebiotics to date, other oligosaccharides like Xylo-Oligosaccharides (XOS), Pectic Oligosaccharides (POS), cyclodextrins, palatinose and oligosaccharides from pullulan are also important prebiotic candidates worthy of investigation. This diverse array of prebiotics offers potential benefits for gut health and overall well-being.
Commercially available prebiotics like FOS, XOS, cyclodextrins and palatinose are Generally Recognized as Safe (GRAS) food additives. These substances promote the growth and activity of beneficial microorganisms in the gut. The term "Probiotics" was first introduced in 1965 and refers to live microorganisms that, when consumed in adequate amounts, confer health benefits to the host. They can stimulate the growth of other beneficial microorganisms and have various positive effects on host health, including potential anticancer activities Guarner et al., Rafter et al., De LeBlanc et al., and Biradar et al., probiotic bacteria, particularly Lactic Acid Bacteria (LAB) and Acetic Acid Bacteria (AAB), have been studied for their potential anticancer effects, especially against colon cancer [1-4]. While there's substantial indirect evidence supporting the anticancer effects of probiotics, including LAB, information specifically regarding the anticancer activities of Acetobacter strains is limited. Acetobacter is a common genus within the AAB group, with species like A. aceti and A. syzygii showing industrial importance and potential as probiotics Aragon et al., Shukla et al., and Sengun et al., these bacteria can be isolated from various sources, particularly environments rich in ethanol, such as flower nectar, fruits, cocoa products and vinegar [5-7]. Acetobacter strains have industrial significance, particularly in the production of vinegar and other fermented products. They are also being explored for their potential health benefits, including as probiotics with anticancer properties [8,9].
The traditional dairy products prepared without the use of antibiotics and with higher acidity compared to commercial dairy products, may play a role in the lower cancer rates observed in rural areas of this region Najafi et al., this difference in cancer epidemiology could indeed be linked to dietary factors, including the consumption of these traditional dairy products [10].
One interesting aspect of these traditional dairy products is the presence of new Acetobacter strains with high probiotic capabilities. Probiotics offer various health benefits, including their ability to resist gastrointestinal conditions and potentially improve host health. However, it's essential to note that while probiotics have been attributed with several health benefits, they may not directly address all causes of gastroenteritis Untermann et al., Koopmans et al., Worm et al., and Emerson et al., gastroenteritis, the inflammation of the gastrointestinal tract, is commonly caused by enteric viruses such as caliciviruses, rotaviruses, hepatitis A and E viruses, and human cytomegalovirus [11-14]. While probiotics may provide a barrier against some food-borne pathogens, including viruses, their primary mode of action is through interactions with the gut microbiota and the mucosal immune system.
The gut barrier, once primarily seen as a physical blockade to pathogen entry, is now understood to play an active role in shaping immune responses to luminal pathogens Berin et al., and Acheson et al., understanding these interactions between probiotics, the gut microbiota and the mucosal immune system is important for elucidating their potential role in preventing or mitigating gastroenteritis and other gastrointestinal disorders [15,16].
The probiotic bacteria are widely used as functional foods which used for the maintenance of gastrointestinal microflora equilibrium and treatment of gastrointestinal disorders while prebiotics are dietary fibers with a well-established positive impact on the intestinal microflora, stimulating the proliferation and growth of non-pathogenic bacteria with health-promoting potential Cross et al., Clancy et al., Isolauri et al., Kidd et al., and Servin et al., in combination with prebiotics and probiotics, bacteria create symbiotic activity, which can provide even more benefits than probiotics and prebiotics alone Cunningham-Rundles et al., Rolfe et al., Rosenfeldt et al., Goossens et al., Saavedra et al., Kaila et al., Cross et al., and Kidd et al., this work aimed to make a screening study for the biological activity of probiotics bacteria in the sole phase and its combination with prebiotics, as synbiotics, and investigate the promotion and benefit of this combination [17-27]. The anticancer properties of these synbiotics were in vitro evaluated. The effects of probiotics, prebiotics and synbiotics on cancer cell viability, apoptosis induction and cell cycle modulation were examined and evaluated.
Materials, chemicals and equipment
Human liver carcinoma cells; HepG2 cells (ATCC HB-8065), Human colon cancer cells; CaCo-2 cells (ATCC HTB-37), Human Lung cancer cell line (A549) (ATCC CCL- 185) and African green monkey kidney cells; Vero cells (ATCC CCL-81) were supported by Cell culture department-VACSERA, EGYPT. Licorice roots (Glycyrrhiza glabra), grape seeds (Vitis vinifera), star anise seeds (Illicium verum), rosemary leaves (Salvia rosmarinus), green tea leaves (Camellia sinensis), green coffee seeds (Coffea arabica), ginger roots (Zingiber officinale), henna (Lawsonia inermis), a mixture of flax seeds (Linum usitatissimum) and cinnamon bark (Cinnamomum verum) were purchased from a local market in Giza, Egypt. The plant's taxonomic identities were confirmed by the Department of Pomology, Faculty of Agriculture, Cairo University. Lactobacillus acidophilus (ATCC4356), Lactobacillus plantarum (ATCC1491), Lactobacillus rhamnous (ATCC53103), Lactobacillus casei (ATCC39539), Lactobacillus reuteri (ATCC23272) were purchased Grace Company- Egypt. Staphylococcus aureus (ATCC 33591), Pseudomonas aeruginosa (ATCC 27853), Vascular Stomatitis Virus (VSV), Herpes Simplex Virus type 1 (Hsv1) were provided by Applied Research Sector, VACSERA-Egypt. RPMI 1640 and 199-E media with L-glutamine were purchased from Biowhittaker-Belgium. Fetal Bovine Serum (FBS) was purchased GIBCO-USA. Trypsin 0.25% (w/v) was supplied AMRESCO-USA. De Man–Rogosa–Sharpe agar (MRS) broth, MRS agar, media De Man et al., Mueller-Hinton broth medium (Mueller and Hinton 1941) were supplied Himedia Company-India [28]. Nutrient agar (American Public Health Association 1920) was supported by BD-USA. Trypan blue dye, 3-(4,5-dimethylthiazol-2-yl (MTT) 2,5 diphenyl tetrazolium bromide], Annexin V-FITC apoptosis detection kit, Propidium Iodide (PI), Annexin V binding buffer, Ethylene Diamine Tetraacetic Acid (EDTA), biuret kit of total protein, 0.9% sodium chloride solution, total carbohydrate kit, total cholesterol kit, triglyceride kit, antioxidant kit, oil-red-o dye, Formalin (10%), Isopropanol, 2,2'-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS) substrate, quartz cuvettes were supported from Sigma-Aldrich-USA. Di-Methyl Sulfoxide (DMSO), Ethanol 95% was supported from BDH-England. Normal and sterile saline 0.9% solution (w/v) were purchased from Adwic-Egypt. Phosphate Buffer Saline (PBS) was supplied from Biowhittaker-Belgium. McFarland standard 0.5 was purchased from Himedia Company-India. Phosphate Buffer saline pH 7.2 was provided by LUNZA-Swiss (Table 1).
| Cell lines | Sources |
|---|---|
| Human liver carcinoma cells; Hepg2 cells (ATCC HB-8065) | VACSERA, Egypt |
| Human colon cancer cells; Caco-2 cells (ATCC HTB-37) | |
| Human lung cancer cell line; (A549) (ATCC CCL-185) | |
| African green monkey kidney cells; Vero cell (ATCCCCL-81), | |
| Human breast carcinoma cells; MCF7 (ATCC HTB-22) | |
| Prebiotics | Sources |
| Licorice roots (Glycyrrhiza glabra), | Giza, Egypt. (The plant's taxonomic identities were confirmed by the department of pomology, faculty of agriculture, Cairo university) |
| Grape seeds (Vitis vinifera), | |
| Star anise seeds (Illicium verum), | |
| Rosemary leaves (Salvia rosmarinus), | |
| Green tea leaves (Camellia sinensis), | |
| Green coffee seeds (Coffea arabica), | |
| Ginger roots (Zingiber officinale), | |
| Henna (Lawsonia inermis), | |
| flax seeds (Linum usitatissimum), | |
| Cinnamon bark (Cinnamomum verum) | |
| Probiotics | Sources |
| Lactobacillus acidophilus ATCC 4356 | Grace company, Egypt |
| Lactobacillus plantarum ATCC 1491 | |
| Lactobacillus rhamnous ATCC 53103 | |
| Lactobacillus casei ATCC 39539 | |
| Lactobacillus reuteri ATCC 23272 | |
| Pathogenic strain | Sources |
| Staphylococcus aureus ATCC 33591 | Grace company, Egypt |
| Pseudomonas aeruginosa ATCC 27853 | |
| Cell culture media | Sources |
| RPMI 1640 medium with L-glutamine | Biowhittaker, Belgium |
| 199-E medium with L-glutamine | |
| Fetal bovine serum (FBS) | GIBCO-USA |
| Trypsin 0.25% (w/v) | AMRESCO-USA |
| Microbiological media | Sources |
| MRS broth medium (28) | Himedia company-India |
| MRS agar medium (28) | |
| Mueller-Hinton broth medium | |
| Nutrient agar | BD-USA |
| Chemical and reagents | Sources |
| Di-methyl sulfoxide | BDH-England |
| Ethanol 95% | |
| Normal saline 0.9% solution (w/v) | Adwic-Egypt |
| Phosphate buffer saline | Biowhittaker-Belgium |
| Deionized water | Sigma-Aldrich-USA |
| ABTS substrate | |
| Annexin V-FITC apoptosis detection kit | |
| Trypan blue dye | |
| MTT [3-(4,5-dimethylthiazol-2-yl) 2,5 diphenyl tetrazolium bromide] | |
| Propidium iodide | |
| Annexin V Binding Buffer | |
| EDTA | |
| Biuret kit of total protein | |
| 0.9% sodium chloride solution | |
| Total carbohydrate kit | |
| Total cholesterol kit | |
| Triglyceride kit | |
| Antioxidant kit | |
| Oil-RED-O dye | |
| Formalin (10%) | |
| Isopropanol | |
| McFarland standard 0.5 | Himedia company-India |
| Sterile normal saline 0.9% | Adwic-Egypt |
| Phosphate buffer saline pH 7.2 | LUNZA-Swiss |
| Experimental animals | Sources |
| 25-30 g weighted 20 Albino Swiss mice | VACSERA, Egypt. |
| Tools-disposable items | Sources |
| Cell culture Flasks 25 and 75-cm2 surface area | Griener-Germany |
| Eppendorf | |
| Bacterial loop Glassware | |
| Sterile syringe filters, 0.22 μm pore size | Millipore-USA |
| Sterile adhesive sheets | Nunc-USA |
| Sterile tips | BioPointe Scientific-USA |
| 96 well CELLSTARA plates | Greiner Bio-One-Austria |
| Falcon tubes | Corning Inc.-USA |
| 2 ml laboratory vials (biurets) | |
| Petri dishes | |
| Cotton swap sterile | Misr El-Mahla– Egypt |
| Syringe | AMECO-Egypt |
| Glass pipettes | Costar-USA |
| Equipments | Sources |
| CO2 incubator | Jouan-France |
| Deep freezer (-70°C) | RIFCO-USA |
| Refrigerator | Ideal-Italy |
| Cooling centrifuge | Jouan GR 412-France |
| Vertical laminar airflow | Nunclon-USA |
| Biological safety cabinet | Nuair-USA |
| Cell culture inverted microscope | Hund-Germany |
| ELISA plate reader | Dynatech-England |
| Plate shaker | Staurt-England |
| pH meter | Denver-USA |
| Vortex | Thermolyne-USA |
| Spectrophotometer | BiotechEngineering-UK |
| Microwave | JAK-China |
| Spin centrifuge | Firlabo-Belgium |
| UV transilluminator | BioDoc analyzer-Germany |
| Fluorescence-activated cell sorting flow cytometer. | FACSCAN |
| Multi-channel pipette (20 µl -200 µl) | Greiner – Germany |
| Greiner-Germany | |
| Single channel pipette (1-10 µl, 20-200 µl) | |
| Single-channel pipette (20-200 µl) | |
| Haemocytometer | New power-Germany |
| Quartz cuvettes | Sigma-Aldrich-USA |
| Cell Sonicator | Thermo Fisher-USA |
| Rotary evaporator | |
| Hot plate | Staurt-UK |
| Digital balance | BEL Engineering-Italy |
Note: UV transilluminator: ultra-violet transilluminator; ELISA plate reader: Enzyme-Linked Immunosorbent Assay; MTT: [3-(4,5-Dimethylthiazol-2-Yl) 2,5 Diphenyl Tetrazolium Bromide]; EDTA: Ethylenediamine Tetra acetic Acid; PI: Propidium Iodide; FITC: Fluorescein Isothiocyante; ABTS substrate: 2,2'-Azinobis [3-Ethylbenzothiazoline-6-Sulfonic Acid]-Diammonium Salt; PBS: Phosphate Buffer Saline; DMSO: Dimethyl Sulfoxide.
Table 1: Materials, chemicals and equipment’s of probiotics.
Preparation of prebiotic extracts
The process begins with the procurement of fresh plant materials from the local market. These materials are then cleaned to remove any dirt or impurities. The prebiotics within the plant materials are chopped and finely ground using a blender. This step increases the surface area, facilitating extraction. The ground samples are subjected to water extraction. This extraction process is carried out at room temperature (approximately 30°C) in a complete cover ambient for a duration of 4 h. It's mentioned that the extraction is performed only once. After the extraction process is completed, the extracts are filtered through a linen filter cloth. This step helps remove any solid particles or impurities from the extract. The filtrates obtained from the extraction process are concentrated using a rotary evaporator (specifically, a Thermo Fisher model from the USA). This step involves evaporating the solvent (water) from the extract to obtain a more concentrated solution. The concentrated samples are then stored in capped dark glass bottles at a temperature of -20°C until they are ready to be used. Storing the samples in dark glass bottles helps protect them from light-induced degradation and storing them at a low temperature helps preserve their stability over time [29].
Determination of total carbohydrate of prebiotic extracts
For determining the concentration of carbohydrates (specifically glucose) in a sample using a colorimetric assay based on the phenol-sulfuric acid reaction involves preparing glucose standards of varying concentrations and then treating them with phenol and concentrated sulfuric acid. The reaction results in the formation of a colored complex that can be measured using a spectrophotometer at 490 nm. Different concentrations of glucose solutions are prepared by serial dilution, starting from 0.1 mg/ml to 0.02 mg/ml. Each solution is adjusted to a final volume of 1 ml using distilled water. To each glucose solution, 1 ml of 5% phenol and 5 ml of 96% sulfuric acid are added sequentially. The tubes are mixed well to allow the phenol and sulfuric acid to react with the glucose. After the reagents are added, the tubes are incubated in a water bath for 15 min to allow the reaction to develop. The absorbance of each tube is measured at 490 nm using a spectrophotometer. The same procedure is repeated for 0.2 ml of different extract samples. The Optical Density (OD) values of these extract samples are measured at 490 nm. A standard curve is created by plotting the known glucose concentrations against the absorbance values measured at 490 nm. The standard curve is then used to determine the carbohydrate concentration in the test samples by comparing their O.D. values to the curve. Using the standard curve, the glucose (carbohydrate) concentration in the tested samples is determined. This method is sensitive and allows for the quantification of carbohydrate concentrations in samples, making it useful in a variety of biological and chemical analyses. When performing the assay, it is important to handle sulfuric acid with care as it is a highly corrosive substance. Proper safety precautions, including the use of gloves and eye protection, should be observed.
Preparation of probiotic (Lactobacillus) supernatants
Different concentrations of glucose solutions ranging from 0.1 mg/ml to 0.02 mg/ml are prepared through serial dilution. Each solution is made up to a final volume of 1 ml by adding distilled water. In each boiling tube containing the prepared glucose solutions, 1 ml of 5% phenol and 5 ml of 96% sulfuric acid are added sequentially. After each addition, the contents of the tube are shaken well to ensure thorough mixing of the phenol and sulfuric acid with the glucose solution. After the addition of phenol and sulfuric acid, all tubes are incubated for 10 min. Following the incubation period, all tubes are placed in a water bath for 15 min. The O.D. values of each tube are measured at a wavelength of 490 nm using a spectrophotometer. A blank containing only distilled water is used as a reference. After obtaining O.D. values for the glucose solutions, the same process is repeated with 0.2 ml of different extracts. The O.D. values of the extract solutions are recorded. A standard curve is constructed using the O.D. values obtained from the different concentrations (0.1 mg/ml-0.02 mg/ml) of glucose solutions. This standard curve is used to determine the carbohydrate concentration (mg/ml) of the tested samples, based on their respective O.D. values. By following the above procedure, we can quantitatively determine the carbohydrate concentration in the tested samples using spectrophotometry and the constructed standard curve. This method allows for the analysis of carbohydrate content in various samples, providing valuable information for research or analytical [28].
Preparation of probiotic Lactobacillus cell sonicate
Aliquots of bacterial suspensions from overnight cultures were prepared. These aliquots were sonicated using the intermediate sonication horn (Intermediate sonication horn (9.5 mm). The sonication was performed at 60% intensity. Each aliquot was placed in a 15 ml polypropylene tube containing 5-6 ml of cell suspension. Cooling was applied during sonication using an ice water bath (Sonic 300 Dismembrator, Fisher Scientific Co., New Jersey, USA). This cooling method helps prevent overheating of the samples during sonication [30].
Determination of total protein in Lactobacillus supernatant, cell sonicate and their mix
The total protein was estimated according to Lowry et al., 0.2 ml of 0.85% sodium chloride solution was added to the blank test tube and 0.2 ml of a test sample solutions were added to labelled test tubes [31]. 2.2 ml of the Biuret reagent was added. Vortex well and allow stand at room temperature for 10 min. 0.1 ml of the Folin and Ciocalteau's phenol reagent was added to each tube. Tubes were mixed well immediately and then allowed to stand at room temperature for 30 min. Each content was transferred to a micro plate and the absorbance was recorded at 550-750 nm within 30 min. The standard curve was achieved using different concentrations of bovine serum albumin solution and used to determine the protein concentration (mg/ml) of the test samples.
Maintenance of cell lines
The Human liver carcinoma cells (HepG2), Human colon cancer cells (CaCo-2), Lung cancer cell line (A549) and green monkey kidney cells (Vero) cell lines were cultured under the same conditions, except for the Vero cell line, which used RPMI medium instead of E-MEM medium James et al., the cells were maintained according to a protocol outlined by James et al., Griener flasks from Germany were used for cell culture [32]. The growth media were discarded from the cell culture flasks. The cell layer was then washed gently with sterile PBS. After washing with PBS, the cell monolayer was washed with 5 ml of pre-warmed trypsin solution (at 37°C). The trypsin solution was then decanted and the cell culture flasks were incubated with trace trypsin at 37°C until the cells detached from the surface. Once the cells detached from the surface, they were suspended in growth media to the desired concentration based on cell count. The cell suspension was then cultured in another cell culture flask or 96 well CELLSTAR plates from Greiner Bio-One, Austria. The cultured cells were then incubated at 37°C until they reached confluency.
Cell counting
The accurate cell number in the suspension was calculated using the hemocytometer according to James et al., as follows: Double fold dilution of the original cell suspension was prepared by adding 0.5 ml of the undiluted cell suspension to 0.5 ml of 0.4% trypan blue dye [32]. The mixture was mixed well with a fine pipette and immediately aspirated to fill the hemocytometer counting chambers. All viable (unstained) cells in the 8 squares of 2 hemocytometer chambers were counted omitting cells lying on the upper line and left line of each chamber. The volume of each chamber=0.1 mm3 (1.0 × 1.0 × 0.1). To perform an accurate cell count, 75% of the cells in the suspension should be viable and the difference between the cell counts in the 2 hemocytometer chambers should be minimal. If cell clumping (aggregation) was observed, the clumps were disaggregated by vigorous aspiration through a pipette. The mean count of the cells in each chamber was calculated. The total number of cells in the suspension was calculated using the following formula: N1=m × tb × V × 104 where: N1=number of cells in the cell suspension; m=mean of cell count per 0.1 mm3; tb=correction of the trypan blue dilution (2 in double fold dilution with trypan blue); V=volume of the original cell suspension in ml; 104=conversion factor for counting chamber volume; N (number of cells per ml)=N1/V. If a new suspension was required to be prepared with a new concentration (N2), the new volume (V2) could be calculated as follows: N × V=N2 × V2 and V2=N × V/N2. The new cell suspension can be prepared by adding a growth medium equal to the difference between the new volume (V2) and the original volume (V). Hemocytometer and coverslip were cleaned immediately after use with 70% ethanol.
Cytotoxicity test
The following methodology provides a quantitative assessment of cytotoxic effects on different cell lines and allows for the comparison of the efficacy of various test materials using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay, a common technique in cell biology research. The MTT assay measures cell viability based on the activity of mitochondrial enzymes in living cells. These enzymes convert MTT, a yellow tetrazolium dye, into purple formazan crystals. The purple formazan crystals are insoluble in water but can be solubilized using a solvent like DMSO, producing a colored solution whose absorbance can be measured. Prebiotic extracts (such as licorice roots, grape seeds, etc.), probiotics (e.g., L. acidophilus, L. plantarum) and combinations thereof are evaluated for cytotoxic effects. These materials are 2-fold serially diluted and tested on different cell lines (HepG2, CaCo-2, A549 and Vero) over a 48-h period. Cells are treated with the test materials for 48 h at 37°C after removing the growth medium. After treatment, cells are examined under a microscope for morphological changes and detachment. Dead cells are washed with PBS to remove debris. Residual live cells are incubated with MTT stain. Plates are further incubated for 3-4 h to allow the formation of formazan crystals. Formazan crystals are dissolved using DMSO on a plate shaker for 30 min. The absorbance of the dissolved formazan solution is measured using a spectrophotometer at a wavelength typically between 500 nm and 600 nm. Optical densities are recorded using an Enzyme Linked Immuno Sorbent Assay (ELISA) plate reader. The Half-Maximal Inhibitory Concentration (IC50) of treatments is determined using data analysis software. Results are reported for all independent experiments, with viability percentages calculated based on the optical densities of treated and untreated cells. Data were reported for all independent experiments Berridge et al., the viability percentage was calculated according to Chen et al., as follows: Cell viability percentage=(O.D of treated cells/O.D of untreated cells) X 100 [33,34].
Morphology structure determination
This procedure is common in cell biology and pharmacology research, particularly in studies aiming to understand the effects of different compounds or treatments on cell morphology, viability and proliferation [35]. It allows researchers to observe how cells respond to various stimuli, which can provide insights into the mechanisms of action of drugs or potential therapeutic agents. 96-well plates containing both cancer cells and normal cells. These cells have been pre-cultured, meaning they've been allowed to grow and stabilize in the wells prior to treatment. The cells in the plates are treated with specific test materials. These materials could be drugs, compounds or other substances being investigated for their effects on cell morphology or viability. After 24 h of treatment, the cells are observed for any morphological changes. This observation is done using an inverted phase-contrast microscope. Phase-contrast microscopy is a technique that enhances the contrast of transparent and colorless specimens, making it useful for observing living cells without the need for staining. It's mentioned that MTT staining will be performed after the morphological observation. MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) is a common assay used to assess cell viability and proliferation. It works by measuring the activity of mitochondrial enzymes in metabolically active cells, resulting in the formation of a purple formazan dye that can be quantified.
Flow cytometry analysis
Apoptosis is a normal physiological process that occurs during embryonic development as well as in the maintenance of tissue homeostasis. The apoptotic program is characterized by certain morphological features, including loss of plasma membrane asymmetry and attachment, condensation of the cytoplasm and nucleus and internucleosomal cleavage of Deoxy Ribo Nucleic Acid (DNA). Loss of plasma membrane is one of the earliest features. In apoptotic cells, the membrane phospholipid phosphatidylserine is translocated from the inner to the outer leaflet of the plasma membrane, thereby exposing PS to the external cellular environment Chen, et al., and Zhang et al., Annexin V is a 35-36 kDa Ca2+ dependent phospholipid-binding protein that has a high affinity for PS and binds to cells with exposed PS [34,35]. Annexin V may be conjugated to fluorochromes including Fluorescein Isothiocyanate (FITC). This format retains its high affinity for PS and thus serves as a sensitive probe for flow cytometric analysis of cells that are undergoing apoptosis. Since externalization of PS occurs in the earlier stages of apoptosis, FITC Annexin V staining can identify apoptosis at an earlier stage than assays based on nuclear changes such as DNA fragmentation. FITC Annexin V staining precedes the loss of membrane integrity which accompanies the latest stages of cell death resulting from either apoptotic or necrotic processes. Therefore, staining with FITC Annexin V is typically used in conjunction with a vital dye such as Propidium Iodide (PI) or 7-Amino-Actinomycin (7-AAD) to allow the investigator to identify early apoptotic cells (PI negative, FITC Annexin V positive).
The cells (HepG2, CaCo-2, A549) was washed twice with cold PBS to remove any residual media or serum and then suspended in Binding Buffer at a concentration of 1 × 10-6 cells/ml. 100 µl of the cell suspension (1 × 10^5 cells) was transferred to a 5 ml culture tube. 5 µl of FITC Annexin V and 5 µl of propidium iodide was added to the cell suspension. Then, gently vortex the cells to ensure proper mixing of the staining reagents. The cells were incubated for 15 minutes at room temperature (25°C) in the dark. During this time, FITC Annexin V will bind to phosphatidylserine residues exposed on the outer membrane of apoptotic cells, while PI will stain the nuclei of necrotic and late apoptotic cells with compromised membrane integrity. After the incubation period, 400 µl of binding buffer was added to each tube to dilute excess staining reagents and provide a suitable environment for flow cytometry analysis. The stained cells were analyzed by flow cytometry within 1 hour of staining. Flow cytometry will allow us to quantify the percentage of apoptotic (FITC Annexin V positive, PI negative), necrotic (FITC Annexin V positive, PI positive) and viable (FITC Annexin V negative, PI negative) cells in the population.
Caspase-3 colorimetric assay
Caspase-3 is indeed a critical enzyme in the process of apoptosis or programmed cell death. It plays a pivotal role in executing the biochemical events that lead to cell death. In non-apoptotic cells, caspase-3 exists in an inactive form called procaspase-3. Activation of caspase-3 occurs through proteolytic cleavage of procaspase-3 into its active form, which then initiates the cascade of events leading to apoptosis. The ubiquity of caspase-3 expression means that it is found in virtually all cell types across mammalian organisms. This widespread expression underscores its importance in regulating cell death processes across different tissues and cell types. The assay for caspase-3 activity, as described by Zhang et al., suggests that it can be performed using any mammalian cell line [36]. This flexibility in cell line choice is advantageous for researchers studying apoptosis, as it allows for the investigation of caspase-3 activity in various cellular contexts. Understanding the regulation and activity of caspase-3 is important in many fields of biology and medicine, including cancer research, neurobiology and developmental biology, as dysregulation of apoptosis can lead to various diseases and disorders.
Cell culture preparation
The cells (HepG2, CaCo-2, A549) were seeded in a 6-well plate and cultured under specific conditions. Each well received 2 ml of medium containing the appropriate nutrients and supplements to support cell growth. The cells were cultured in an environment with 5% CO2 to maintain physiological pH levels in the medium. After seeding, the cells were allowed to adhere and proliferate for at least 16 hours. During this time, they would attach to the culture surface and start to divide, establishing a monolayer. After the initial incubation period (16 h or more), the cell culture medium was replaced. This medium exchange ensures that the cells receive fresh nutrients and removes waste products, maintaining optimal growth conditions. Following the medium replacement, the cells were allowed to continue growing until they reached confluence. Confluence refers to the point at which the cells cover the entire surface area of the culture vessel and stop proliferating due to contact inhibition.
Cell lysate preparation
The procedure involves the removal of cell culture medium from wells followed by the addition of cell lysis buffer. Once the medium is removed, 1 ml of cell lysis buffer is added to each well. This buffer likely contains substances that aid in breaking down the cell membrane and releasing cellular contents. After adding the lysis buffer, the plates are gently shaken or tapped for 10 min. This shaking or tapping helps to facilitate cell lysis, ensuring that the cells are effectively broken open and their contents are released into the buffer.
Assay protocol
A biochemical assay to measure the activity of caspase-3, an enzyme involved in apoptosis (programmed cell death) was described as shown below: Each well of the reaction plate is filled with 100 µL of caspase-3 reaction buffer. Next, 100 µL of cell lysate is added to each well of the reaction plate. The cell lysate likely contains the cellular components, including caspase-3, that are necessary for the reaction to occur. The contents of each well are mixed thoroughly to ensure that the cell lysate is evenly distributed and mixed with the reaction buffer. Immediately after mixing, the absorbance of the contents in each well is measured at a wavelength of 405 nm. This measurement gives the initial reading, which serves as a baseline for the subsequent analysis. The reaction plate is then incubated for exactly 30 min. During this time, any active caspase-3 present in the cell lysate will catalyze a reaction that produces a measurable change in absorbance. After the 30 min incubation period, the absorbance of the contents in each well is measured again at 405 nm. This measurement gives the final reading, reflecting any change in absorbance that occurred during the incubation period. The difference between the final and initial absorbance readings is calculated. This difference in absorbance is directly proportional to the amount of active caspase-3 present in each culture sample. More active caspase-3 will lead to a greater increase in absorbance over the incubation period.
Evaluation of active caspase3
Caspase-3 enzyme activity was measured in six groups of treatments: Grape seed extract and licorice root extract as prebiotics, Lactobacillus acidophilus bacteria supernatant and cell sonicate as probiotic and a combination of pre and probiotic and control group (presumably untreated or treated with a placebo). Caspase-3 enzyme activity was measured using a colorimetric substrate called N-Acetyl-L-Α-Aspartyl-L-Α-Glutamyl-L-Valyl-N-(4-Nitrophenyl)-L-Α-Asparagine (Ac-DEVD-pNA)provided in the CaspACE assay system. The enzyme cleaves this substrate, releasing a yellow chromophore, para-Nitro Aniline (pNA), which can be quantified spectrophotometrically. Relative caspase-3 activities for each sample and sample plus inhibitor were calculated from the standard curve. This involves comparing the absorbance readings of the samples to a standard curve generated with known concentrations of the p-nitroaniline product. Caspase-3 activity values were normalized against sample protein content. Normalization helps to account for variations in sample concentration and ensures that any observed differences in caspase-3 activity are not due to differences in protein content. The normalized caspase-3 activity values were presented as a percentage of the control value. This allows for comparison between treatment groups relative to the control group.
Colorimetric assay
To quantify caspase-3 activity in lymphocytes under different treatment conditions. Lymphocytes were cultured using a previously described method. Cells were treated with either an anti-Fas antibody (at a concentration of 1 μg/ml) or an isotype-matched control antibody for varying periods. After treatment, the cells were lysed to release their contents, including caspases. The lysed cell samples were then incubated with specific substrates for caspase-8 (IETD- Peptide Nucleic Acid (pNA) and caspase-3 (DEVD-pNA) separately. Additionally, for caspase-3 activity, a specific peptide inhibitor (DEVD-CHO) was added to another set of samples along with the DEVD-pNA substrate. This step allows distinguishing the specific activity of caspase-3 from other proteases that might also cleave the DEVD-pNA substrate. After the incubation period, the samples were read using a spectrophotometer at a wavelength of 405 nm. This wavelength is appropriate for measuring the release of the chromophore p-nitroaniline from the substrates, which indicates caspase activity. The results were expressed as relative caspase activity, calculated as the ratio between the caspase activity measured in the sample and the activity measured in a control sample (without treatment). For caspase-3 activity, any background signal remaining after inhibition by DEVD-CHO was subtracted from all samples to ensure that only caspase-3 activity was measured.
Statistical analysis
Results were expressed as mean ± Standard Error (SE). One-way Analysis of Variance (ANOVA) was used to assess the significance of differences between groups. Tukey's test was employed as a post hoc test. This test is used to determine which specific group differences are significant after finding a significant result in ANOVA. Differences were considered statistically significant if the p-value was less than 0.05.
Total carbohydrate determination of each prebiotic extract
Table 2, outlines the total protein content in mg/ml for different preparations of Lactobacillus strains, including supernatants, cell sonicates and mixtures. For instance, the protein content of Lactobacillus acidophilus supernatant is 34.0 mg/ml, which is among the highest in the study. This data correlates with the observed antiviral effects, suggesting that higher protein content in probiotic preparations may enhance antiviral activity. We have summarized the findings of a study evaluating the total carbohydrate content of various prebiotic extracts. The results indicate that green tea extract had the highest concentration of total carbohydrate, followed by star anise and ginger extracts. To determine the total carbohydrate content, the optical density of each sample was compared to a standard curve of total carbohydrate. The values were reported in milligrams per millilitre (mg/ml) of extract. These results are significant as they provide insight into the composition of the prebiotic extracts tested as seen in Table 2. Furthermore, the information obtained will be utilized in further research to assess the cytotoxicity effects of these prebiotics on different cell lines. This suggests a potential application of these extracts in the context of cellular health and physiology.
| Sample | Total carbohydrate (mg/ml) |
|---|---|
| Grape seed | 2.6 |
| Licorice roots | 1.71 |
| Rosemary leaves+Licorice roots | 1.52 |
| Star anise | 5.64 |
| Green coffee | 1.37 |
| Green tea | 5.99 |
| Ginger | 5.53 |
| A mixture of flax seeds and Cinnamon bark | 3.49 |
| Lawsonia inermis (Henna) | 0.57 |
Table 2: The concentration of total carbohydrates in prebiotics.
Total protein content for Lactobacillus samples and their mixture with prebiotic
A scientific study where the total protein content of various Lactobacillus strains and their combinations was evaluated. This evaluation is important for determining the concentrations of probiotics required for further tests, particularly to assess their cytotoxic effects on different cell lines. To proceed with calculating the concentrations of probiotics needed for the subsequent tests, the total protein content of each test group was measured as represented in Table 3, of your study. Table 3, shows the virus titer reduction for group 1 across different treatments. Lactobacillus acidophilus treatments, including cell sonicate and supernatant, demonstrated effective reduction of HSV-1 and VSV titers. The data highlights that the most significant reduction in virus titer occurred with the mixture of L. acidophilus and licorice, reducing VSV titer by 44.44%. Once we have the total protein content for each group, we would convert this data into concentrations of probiotics.
| Sample | Total protein content (mg /ml) |
|---|---|
| L. acidophilus supernatant | 34 |
| L. acidophilus cell sonicate | 12.3 |
| Grape mix | 17.8 |
| Licorice mix | 19.3 |
Table 3: Total protein content for the anticancer group.
This conversion is based on the relationship between the total protein content and the concentration of probiotics. This relationship would need to be established through experimental calibration or referenced from previous studies if available. Depending on the concentration of probiotics needed for your further tests and the initial concentration obtained from the total protein evaluation, we dilute or concentrate the probiotic solutions accordingly. Ensure that the concentrations calculated align with the requirements of the experimental protocols and adhere to any standards or guidelines relevant to your research field.
With the appropriate concentrations prepared, we proceed with the cytotoxicity tests on different cell lines. This involves exposing the cells to varying concentrations of probiotics to assess their potential cytotoxic effects. After conducting the cytotoxicity tests, we analyze the results to determine the impact of the probiotics on the different cell lines and draw conclusions based on your findings.
Cytotoxicity assay of experimental samples
Table 4, showed moderate antiviral activity. The table indicates that while these treatments were less effective than those in group 1, they still managed to reduce viral titers, with the highest reduction observed for VSV at 22.22%. The study utilized the MTT assay to evaluate cell viability percentages and morphological changes between treated and untreated cells. The concentration-dependent nature of the treatments was observed, with increasing dilutions resulting in higher percentages of survived cells. The IC50 values for each sample on different cancer cell lines were calculated using Master-plex 2010 software after 48 hours of exposure to the treatment. The IC50 values represent the concentration of the treatment at which 50% of cell growth inhibition is achieved. These values are crucial for understanding the effectiveness of the treatments in inhibiting cancer cell proliferation. The specific IC50 values provided were shown in Figure 1 and Table 4, that give further analysis or discussion on the effectiveness of the treatments on the different cancer cell lines (A549, HepG2 and Caco-2) mentioned in the study.
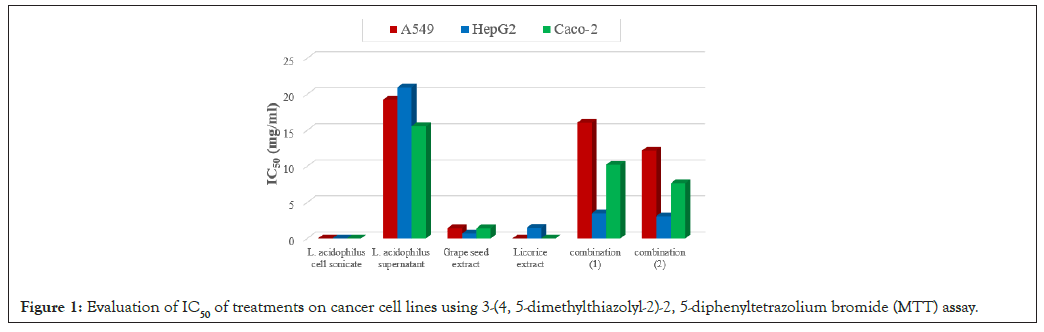
Figure 1: Evaluation of IC50 of treatments on cancer cell lines using 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT) assay.
| Samples | Cell lines IC50 (mg/ml) | ||
|---|---|---|---|
| A549 | HepG2 | Caco-2 | |
| L. acidophilus supernatant | 19.23 | 20.92 | 15.54 |
| L. acidophilus cell sonicate | NI | NI | NI |
| Grape seed extract | 1.42 | 0.66 | 1.41 |
| Licorice root extract | NI | 1.48 | NI |
| Combination (1) of grape seed+cell sonicate+supernatant | 16.03 | 3.47 | 10.2 |
| Combination (2) of licorice+cell sonicate+supernatant | 12.18 | 3.09 | 7.68 |
Table 4: The IC50 values for treatments on three cancer cell lines.
Figure 1, depicts the experimental design of the study, illustrating the various treatment groups used to assess antiviral activity. The groups are divided based on different combinations of Lactobacillus strains (e.g., Lactobacillus acidophilus, L. rhamnosus, L. plantarum) with prebiotics (such as licorice, anise and rosemary) and their combinations as synbiotics. The purpose of these groups is to explore the effects of these treatments on HSV-1 and VSV in Vero cells. Different treatments were applied to A549 cancer cells and their effects on cell growth inhibition (IC50) were measured. Here's a summary of the results: The lowest IC50 values were observed with licorice root extract and L. acidophilus cell sonicate, indicating the highest inhibition of cell growth. The IC50 for these treatments was so low that it resulted in no inhibition, meaning they were highly effective in inhibiting the growth of A549 cancer cells. The highest IC50 value was recorded with L. acidophilus supernatant, indicating the least inhibition of cell growth among the treatments tested. This means that L. acidophilus supernatant was the least effective in inhibiting the growth of A549 cancer cells. Following L. acidophilus supernatant, combination (1) showed the next highest IC50 value, indicating moderate inhibition of cell growth compared to other treatments. The specific IC50 values for each treatment were as follows: L. acidophilus supernatant: 19.23 mg/ml, Combination (1): 16.03 mg/ml.
The effects of various treatments on cell growth inhibition, particularly focusing on licorice root extract, Lactobacillus acidophilus cell sonicate and L. acidophilus supernatant, as well as their combinations, on different cell lines (HepG2, Caco-2 and A549). Licorice root extract and L. acidophilus cell sonicate exhibited the most significant inhibition of cell growth overall, while L. acidophilus supernatant showed the least inhibition among the tested treatments. Combination (1) showed moderate inhibition of cell growth. In the HepG2 cell line, the lowest IC50 values were recorded with L. acidophilus cell sonicate (no inhibition), grape seed extract (0.66 mg/ml), while the highest IC50 value was with L. acidophilus supernatant (20.92 mg/ml). In the Caco-2 cell line, L. acidophilus cell sonicate and licorice root extract did not inhibit cell growth, whereas L. acidophilus supernatant had the highest IC50 value (15.54 mg/ml).
Figure 2, presents the percentage reduction in viral titer for both HSV-1 and VSV in Group 1, which included treatments such as Lactobacillus acidophilus cell sonicate, supernatant and a mix with licorice. The results show that the combination treatments led to a significant reduction in viral infectivity, particularly against VSV, where the mixture of treatments (probiotics and prebiotics) reduced viral titer by 44.44%. The IC50 values were reduced when prebiotics and probiotics were administered in combination compared to when probiotics were administered individually. This indicates enhanced cytotoxicity in cells treated with combination (1) and combination (2), with a more prominent effect observed in the HepG2 cell line compared to Caco-2 and A549 cell lines. The cytotoxic effect of treatments on the cancer cell lines is illustrated in Figure 2, suggesting that combination therapy with prebiotics and probiotics enhances cytotoxicity in cancer cells, particularly in the HepG2 cell line. Overall, the findings suggest that licorice root extract, L. acidophilus cell sonicate and combination therapy with prebiotics and probiotics have potential as treatments for inhibiting cell growth and inducing cytotoxicity in cancer cells, with varying degrees of effectiveness depending on the specific cell line.
Figure 2: Cytotoxicity effect of samples (1): Grape seed Mix; (2): Licorice Mix; (3): Grape seed extract; (4): L. acidophillus supernatant; (5): root extract; (6): L. acidophillus cells on. (A): HepG2; (B): A549; (C): CaCo-2 cell lines.
Evaluation of anticancer activity
Flow cytometry assay: The experiment used Annexin V-FITC staining to differentiate between early and late apoptotic cells, as well as necrotic cells, via flow cytometry. Treatment with grape seed extract, licorice mix and grape seed mix resulted in a significant increase in both early and late apoptosis compared to untreated CaCo-2 cells (control group). The percentage of necrotic cells also increased, but the difference was not statistically significant. The variation in apoptotic profiles depended on the specific treatment formula used. Treatment with Licorice mix induced a significantly higher percentage of early apoptotic cells compared to treatment with grape seed extract and grape mix. Treatment with grape mix induced a significantly higher percentage of late apoptotic cells compared to treatment with grape seed extract and licorice mix.
These results suggest that different treatments have varying effects on apoptotic pathways in different cell lines. The specific composition of the treatment formulas appears to play a role in determining the observed effects on apoptosis. Additionally, the data imply that certain treatments may have differential effects on early versus late apoptotic processes within the same cell line. Table 5, presents the virus titer reduction results for group 3, which included treatments with Lactobacillus plantarum and anise. This group demonstrated the most potent antiviral activity, with reductions in both HSV-1 and VSV titers. Lactobacillus plantarum cell sonicate and supernatant achieved up to 41.66% reduction in HSV-1 titer and 38.88% in VSV titer, making this group the most effective in the study.
| Treatment | Apoptosis % | Debris | ||
|---|---|---|---|---|
| Normal | Early | Late | ||
| Grape seed extract | 85.23 | 13.32 | 1.45 | 0 |
| Grape mix | 82.51 | 16.82 | 0.67 | 0 |
| Licorice mix | 80.59 | 16.7 | 2.71 | 0 |
| Control | 99.41 | 0.57 | 0.02 | 0.01 |
| Grape seed extract | 84.22 | 13.44 | 2.34 | 0 |
| Licorice mix | 93 | 6.43 | 0.57 | 0 |
| Grape mix | 86.21 | 11.62 | 2.18 | 0 |
| Control | 99.64 | 0.31 | 0.04 | 0.01 |
| Grape seed extract | 87.22 | 11.33 | 1.45 | 0 |
| Grape mix | 89.61 | 9.57 | 0.81 | 0 |
| Licorice mix | 92.33 | 7.03 | 0.64 | 0 |
| Control | 99.35 | 0.59 | 0.05 | 0.01 |
Table 5: Evaluation of cell death nature on treated cell lines.
Based on the results provided: There was an insignificant (p>0.05) necrotic cell percentage in cells free of grape, as well as those treated with the grape mix and licorice mix. In treated A549 cells, both the grape mix and licorice mix showed a significantly (p<0.05) elevated early apoptotic percentage compared to the A549 cell control and those treated with the grape mix alone. Grape seed extract exhibited a mild significant (p<0.05) increase in late apoptotic percentage compared to its values in A549 cells treated with the grape mix and licorice mix.
The percentage of necrotic cells showed insignificant variation among treated groups but was significantly (p<0.05) elevated compared to A549 untreated cells (control). After 4 h of inoculation, all tested Lactobacillus cells increased caspase-3 activity compared to the control sample treated with DMSO. The highest expression of caspase-3 (33.7%) was observed under the effect of grape seed treatment, but this difference was not statistically significant (p>0.05) compared to the control (Figure 1 and Table 4).
Treatment with grape mix with probiotics and licorice mix with probiotics significantly elevated caspase-3 expression compared to treatment with sole licorice , L. acidophilus supernatant and the control (p<0.05). Caspase-3 expression did not significantly change under the effect of licorice mix with probiotics, L. acidophilus supernatant, and grape with probiotics (p>0.05). However, caspase-3 expression significantly increased under the effect of grape seed treatment (p<0.05).
Significantly elevated caspase-3 expression was observed after treatment with grape seed, licorice mix with probiotics, and grape mix with probiotics compared to treatment with L. acidophilus supernatant and the control. Moreover, grape mix with probiotics showed significantly higher caspase-3 expression compared to grape seed and licorice mix with probiotics. Overall, the results suggest that different formulations have varying effects on caspase-3 expression in different cell lines, with some formulations significantly increasing caspase-3 expression compared to others or the control group.
In Figure 3, group 2 results are depicted here, showing the antiviral effects of Lactobacillus rhamnosus and rosemary-licorice combinations. The virus reduction percentages indicate a moderate effect on both HSV-1 and VSV, with reductions in viral titer ranging from 4.17% to 22.22% across the different treatments. This suggests that while the group 2 treatments showed some antiviral activity, they were generally less effective compared to group 1, particularly against HSV-1.
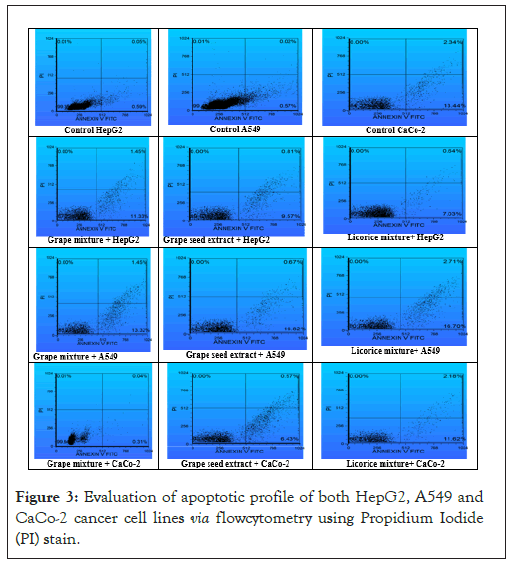
Figure 3: Evaluation of apoptotic profile of both HepG2, A549 and CaCo-2 cancer cell lines via flowcytometry using Propidium Iodide (PI) stain.
All treatments resulted in a significant increase in casp3 levels compared to the untreated control group. This suggests that the treatments exert some influence on casp3 levels, potentially indicating a physiological response. Probiotic bacteria are live microorganisms that, when consumed in sufficient quantities, provide a health benefit to the host.
This implies that these beneficial bacteria have a positive impact on the host's well-being, particularly concerning aspects of gastrointestinal health or beyond. Prebiotics are non-digestible oligosaccharides or ingredients that are selectively fermentable. These substances bring about specific changes in the composition and/or activity of the gastrointestinal microflora, thereby conferring health benefits.
Essentially, prebiotics serve as food for beneficial gut bacteria, promoting their growth and activity. Synbiotics are a combination of probiotic bacteria and prebiotic ingredients. This combination is proposed to exhibit synergistic effects, meaning that the presence of both probiotics and prebiotics together enhances their individual benefits. Synbiotics aim to optimize the health benefits associated with probiotics and prebiotics by leveraging their combined action. Raman et al., which further elaborates on these concepts and potentially provides experimental evidence or clinical observations supporting these definitions and their implications [37].
Table 6, provides a comparative summary of the antiviral activity observed across all groups. It highlights that Lactobacillus plantarum treatments (group 3) were the most effective, followed by Lactobacillus acidophilus (group 1). Lactobacillus rhamnosus (group 2) showed the least antiviral activity, particularly against HSV-1. The summary emphasizes the potential of synbiotic treatments (combinations of probiotics and prebiotics) in enhancing antiviral activity.
| Cell Line | Sample ID | Before | After | Difference | Difference % |
|---|---|---|---|---|---|
| HepG2 | Control | 0.424 | 0.43 | 0.006 | 1.4 |
| L. acidophilus sup. | 0.552 | 0.597 | 0.045 | 9 | |
| Licorice extract | 0.535 | 0.602 | 0.067 | 12 | |
| Grape seed extract | 0.523 | 0.699 | 0.176 | 33.7 | |
| Licorice+mix prob. | 0.541 | 0.68 | 0.139 | 25.7 | |
| Grape+mix prob. | 0.512 | 0.673 | 0.161 | 31.4 | |
| CaCo-2 | Control | 0.412 | 0.441 | 0.029 | 7 |
| L. acidophilus sup. | 0.421 | 0.5 | 0.079 | 18.8 | |
| grape seed extract | 0.442 | 0.616 | 0.174 | 39.4 | |
| Licorice+mix prob. | 0.439 | 0.502 | 0.063 | 14.4 | |
| Grape+mix prob. | 0.432 | 0.547 | 0.115 | 26.6 | |
| A549 | Control | 0.642 | 0.651 | 0.009 | 1.4 |
| L. acidophilus sup. | 0.66 | 0.705 | 0.045 | 6.8 | |
| Grape seed extract | 0.627 | 0.867 | 0.24 | 38.2 | |
| Licorice+mix prob. | 0.657 | 0.909 | 0.252 | 38.3 | |
| Grape+mix prob. | 0.654 | 0.984 | 0.33 | 50.4 |
Table 6: The amount of active caspase-3 present in each culture sample.
Results are intriguing and align with existing research on the potential health benefits of probiotics and synbiotics. Probiotics, which are live microorganisms that confer health benefits when consumed in adequate amounts, have been extensively studied for their various effects on human health. Probiotics are known to modulate the gut microbiota, enhancing antibacterial activity and reducing inflammation. This can have implications for gastrointestinal health as well as systemic inflammatory conditions. Certain probiotic strains can synthesize vitamins and other nutrients, enhancing their bioavailability.
This is particularly relevant for nutrients like vitamin K and certain B vitamins. Some probiotics exhibit anti-oxidative properties, which can help combat oxidative stress and reduce the risk of oxidative damage to cells and tissues. Probiotics have shown promise in alleviating symptoms associated with allergies, certain types of cancer, Acquired Immuno Deficiency Syndrome (AIDS) and various infections, including respiratory and urinary tract infections. These effects are attributed to their ability to modulate immune responses and enhance host defense mechanisms. There are emerging reports suggesting beneficial effects of probiotics on aging, fatigue, autism, osteoporosis, obesity and type 2 diabetes.
While more research is needed in these areas, preliminary evidence indicates a potential role for probiotics in mitigating these conditions. The ability of probiotics to modulate the gut microbiota and influence various physiological processes may play a role in their effects on cancer cells. It's important to note that while these findings are promising, further research, including clinical trials and is necessary to fully understand the mechanisms underlying the observed effects and to determine the potential therapeutic applications of probiotics and synbiotics in cancer prevention and treatment. Additionally, the safety and efficacy of probiotic interventions should be carefully evaluated, especially in clinical settings.
The evaluation of cell viability percentages of cell lines treated with prebiotics, probiotics and their combination, along with the respective concentrations needed for cancer cell growth inhibition, was conducted using the MTT assay. This assay relies on the conversion of MTT to formazan by metabolically active cells, resulting in a colorimetric change that can be quantified using an ELISA plate reader. Morphological changes were also observed to assess differences between treated and untreated cells on a morphological scale, providing additional insights into the effects of the treatments. The results indicated that the viability percentages of treated cells were concentration-dependent, with increasing dilutions leading to higher percentages of surviving cells in cultures. This suggests that the effectiveness of the treatments can be modulated by adjusting their concentrations. Regarding the toxicity effect of prebiotics, probiotics and synbiotics on cancer cell lines, significant effects were observed for each sample, with the combination of prebiotics with probiotics enhancing the probiotics' effect on cancer cell lines, particularly lung, liver and colon cancers.
Notably, the most pronounced effect was observed on liver cancer (HepG2), followed by colon and lung cancers. These findings are consistent with previous studies by Haghshenas et al., and Nami et al., which demonstrated the cytotoxic potential of certain probiotic strains, such as Lactobacillus plantarum, on cancer cell lines. Specifically, the antiproliferative effect of L. plantarum 17C secretion metabolites on human colorectal adenocarcinoma cell line (HT‐29) cancer cells differed significantly from that of other strains, highlighting strain-specific effects [38,39]. Additionally, screening results indicated that L. lactis subsp. lactis 44Lac exhibited significant anti-cancer activity against all examined cell lines.
Furthermore, the study referenced findings by El-Nezami et al. and Rafter et al., which highlighted the health-promoting and anti-carcinogenic properties of probiotics [40,41]. Probiotics were reported to exert apoptotic and antiproliferative effects on various cancer cell lines, including breast, bladder, colon, liver and gastric cancers. Flow cytometry analysis revealed apoptosis, particularly late apoptosis, as the main cytotoxic mechanism for probiotics, prebiotics and synbiotics, in agreement with previous research in conclusion, the study demonstrated the concentration-dependent effects of prebiotics, probiotics and their combination on cancer cell viability, with morphological changes and flow cytometry analysis providing further insights into their mechanisms of action, including apoptotic induction Ali et al., Su et al., these findings contribute to the growing understanding of the potential therapeutic applications of prebiotics and probiotics in cancer treatment [42,43].
In our current study, we observed that treatment of CaCo-2 cells with grape extract, liq. mix and grape-mix resulted in increased levels of early and late apoptosis compared to the untreated cell control. Additionally, our data revealed that the variation in the apoptotic profile was dependent on the type of extract used. Specifically, treatment of HepG2 cells with liq mix induced a significantly (p<0.05) higher percentage of Early apoptosis compared to treatment with grape extract and grape mix. Conversely, grape mix treatment led to a significantly elevated late apoptotic percentage compared to treatment with grape extract and liq mix in HepG2 cells. Furthermore, in A549 cells, both grape mix and liq mix treatments resulted in a significantly (p<0.05) higher percentage of early apoptosis compared to the control group and those treated with grape mix. Additionally, grape extract treatment showed a mild, yet significant (p<0.05) increase in late apoptotic percentage compared to treatment with grape mix and liq mix in A549 cells. Moreover, while there was no significant variation observed in the percentage of necrotic cells among the treated groups, it was significantly (p<0.05) higher than the untreated cell control in all tested cell lines.
The study by Kahouli et al., highlighted the potential of certain probiotic strains, specifically Lactobacillus fermentum NCIMB-5221 and -8829, in combating colorectal cancer cells and promoting the growth of normal epithelial colon cells [44]. This effect was attributed to their production of Short-Chain Fatty Acids (SCFAs), such as ferulic acids. In contrast, other probiotic strains like L. acidophilus ATCC 314 and L. rhamnosus ATCC 51303 were noted for their tumorigenic properties, suggesting a significant difference in the impact of various probiotics on colorectal health.
Moreover, the consumption of prebiotics, such as inulin and oligofructose found in fruits and vegetables, has been linked to protective effects against colon cancer. Studies, such as the one conducted by Mojka et al., have indicated that a diet rich in fruits and vegetables could suppress the development of colorectal carcinoma [45]. Additionally, the consumption of synbiotics, which combine probiotics and prebiotics, has shown promising results in inhibiting factors associated with colorectal cancer.
Eslamparast et al., found that synbiotic products could inhibit Nuclear Factor-kB (NF-kB) and reduce the production of Tumor Necrosis Factor α (TNF-α), both of which are implicated in the development and progression of colorectal cancer [46]. Overall, these findings emphasize the potential of probiotics, prebiotics and synbiotics in promoting colorectal health and preventing colorectal cancer through various mechanisms such as SCFA production, modulation of inflammatory pathways and promotion of normal epithelial cell growth. Incorporating these dietary components into a balanced diet rich in fruits and vegetables may contribute to the prevention and management of colorectal diseases.
Toll-Like Receptors (TLRs) activation in the intestinal mucosa triggers several signaling pathways including NF-kB, Mitogen-Activated Protein Kinase (MAPK) and caspase-dependent cascades these pathways lead to the production and release of protective peptides, cytokines, chemokines and phagocytes [47]. The outcome of these responses can vary, including protective responses to commensal bacteria, inflammatory responses to pathogenic organisms or initiation of apoptosis. High expression of caspase-9 and caspase-3 was observed in groups treated with Lactobacillus fermentum and SCFAs the pro-apoptotic protein Bax showed high expression, while the anti-apoptotic protein B-cell leukemia/lymphoma 2 protein (Bcl-2) exhibited low expression [48]. These findings suggest that Lactobacillus fermentum and SCFAs induce tumor apoptosis in mice. The milk-derived fusion peptide, anti-cancer fusion peptide (ACFP), promoted the expression of Bax and caspase-3 in a time- and dose-dependent manner only L. casei O13 significantly increased caspase-3 activity [49]. Overall, these studies highlight the complex interactions between gut microbiota, immune response and apoptosis regulation, suggesting potential therapeutic implications, particularly in the context of cancer treatment.
The tested Lactobacillus cells increased caspase-3 activity after 4 hours of inoculation compared to a DMSO control sample. This increase in caspase-3 activity could potentially indicate apoptosis (programmed cell death) induction, which may have implications for cancer treatment or prevention. This refers to strategies or interventions aimed at reducing the risk of cancer development. Caspase-3 activity suggests that the study may be exploring potential mechanisms through which Lactobacillus cells could contribute to cancer prevention. Polyphenols are natural compounds found in various foods, particularly in fruits, vegetables, tea and red wine. They have been studied for their potential health benefits, including cancer prevention. The human colonic microbiota plays a crucial role in breaking down complex polyphenols into smaller, more easily absorbable metabolites, which may have bioactive effects. Combining these points, it seems like the study may be investigating the potential of Lactobacillus cells, possibly in conjunction with dietary polyphenols, as a strategy for cancer chemoprevention. The observed increase in caspase-3 activity suggests a mechanism through which these interventions could exert their effects, potentially by promoting apoptosis in cancer cells.
Polyphenols are compounds found in plants that have been studied for their potential chemo preventive properties. These properties have been demonstrated in various in vitro studies. While the chemo preventive potential of polyphenols is known, there's limited research on how specific probiotic bacteria can aid in the bioconversion of polyphenols into metabolites with chemo preventive properties. Synbiotics refer to a combination of probiotics and prebiotics. This combination can alleviate interdependency and enhance the development of functional foods. By combining polyphenols with synbiotics, it's possible to overcome the low bioavailability of polyphenols and enhance their cancer chemo preventive properties. The use of polyphenol-based synbiotics represents a novel approach to cancer chemoprevention. This approach holds promise but requires further evaluation, particularly in vivo studies to assess effectiveness. More research is needed to identify microbial metabolites of complex polyphenols and evaluate the chemo preventive potential of these metabolites. Such studies are important for understanding the effectiveness of polyphenol-based synbiotics in cancer chemoprevention. Thilakarathna et al., providing evidence and insights into the potential of polyphenol-based synbiotics in cancer chemoprevention [50].
The data indicate that the combination of probiotics, prebiotics and synbiotics shows potential as antiviral agents, with varying degrees of effectiveness depending on the specific strains and combinations used. Group 3 (containing Lactobacillus plantarum and anise) demonstrated the most potent antiviral effects, particularly against HSV-1, highlighting the potential of this combination for therapeutic applications. The higher protein content in some preparations, such as L. acidophilus supernatant, also seems to correlate with increased antiviral activity, suggesting that certain components produced by these probiotic strains play a crucial role in inhibiting viral replication. In summary, our study shows promising results for the use of synbiotics in antiviral treatments, particularly against Ribo Nucleic Acid (RNA) viruses like VSV and DNA viruses like HSV-1. Further research could explore the mechanisms behind these antiviral effects, optimizing the combinations for therapeutic use.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Fazary A, Ismael E, Fathy H, Alfaifi M, Elbehairi SE, Badr D (2024). Evaluation of Potential Anticancer Activities of Prebiotics, Probiotics and Synbiotics: In Vitro Study. J Prob Health.12:365.
Received: 20-Aug-2024, Manuscript No. JPH-24-35112; Editor assigned: 22-Aug-2024, Pre QC No. JPH-24-35112 (PQ); Reviewed: 05-Sep-2024, QC No. JPH-24-35112; Revised: 12-Sep-2024, Manuscript No. JPH-24-35112 (R); Published: 20-Sep-2024 , DOI: 10.35248/2329-8901.24.12.365
Copyright: © 2024 Fazary A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.