Medicinal & Aromatic Plants
Open Access
ISSN: 2167-0412
ISSN: 2167-0412
Research Article - (2016) Volume 5, Issue 2
Background: Ocimum gratissimum Linn (Lamiaceae) is an aromatic plant popular for it culinary uses. The aqueous leaf extract is used in ethnomedicine for the treatment of various diseases including asthma and cough. The purpose of this study was to evaluate the anti-asthmatic, antitussive and muco-suppressant effects of the aqueous leaf extract (OGE) in rodent species.
Methods: Ovalbumin-sensitized guinea pigs were exposed to 0.2% histamine aerosol in a glass chamber. Latency to preconvulsive dyspnea (PCD), tracheal fluid volume and viscosity were measured. For the antitussive screening, guinea pigs were exposed to 7.5% citric acid aerosol in a glass chamber and the bouts of cough pre and post-acute exposure were recorded. Mucus expectoration was estimated in mice after seven-day treatment with OGE.
Results: Latency to preconvulsive dyspnea was not significantly increased by doses of 100, 200 and 400 mg/ kg of OGE when compared with distilled water-treated sensitized guinea pigs. Tracheal fluid volume but not viscosity was significantly (p<0.0001) reduced by all the doses of OGE when compared with distilled water-treated sensitized guinea pigs. Also, the doses of OGE significantly reduced (p<0.0001) the number of cough bouts when compared with distilled water control. All the doses of extract significantly (p<0.003) reduced phenol red dye expectoration from mice tracheae.
Conclusion: The aqueous leaf extract of O. gratissimum does not protect against acute bronchospasms but possesses antitussive and muco-suppressant effects that may be helpful in asthmatics. Central mechanism may be contributory to the antitussive effect. These results lend credence to its ethnomedicinal use in the treatment of these diseases.
Keywords: Ocimum gratissimum; Aqueous extract; Asthma; Cough; Muco-suppression
Herbal medicine remains relevant in meeting the healthcare needs of many people. In Nigeria, about 80 percent of the population uses herbal medicine almost exclusively while about 95 per cent use it concurrently with Western medicine [1]. The interest in herbal medicine is growing in the Western world as well [2]. Asthma and cough are some of the diseases for which herbal medicines are often sought [3-5].
Ocimum gratissimum Linn (Lamiaceae) is a shrub found in Africa, South Asia, and South America [6]. It is variously called clove basil, African basil or wild basil [6]. It is commonly planted around homes because the leaves are very popular as food spice and condiment. Herbalists use the leaf extract for the treatment of gastrointestinal problems such stomach aches, diarrhea, and dysentery [7,8]. Some of its ethnomedicinal uses have been reviewed [9]. In Benin City, Nigeria, the extract is used for the treatment of airway diseases such as cough and asthma. The medicinal properties that have been investigated include antibacterial [10-12], antifungal [13], anti-trypanosomal [14] and larviscidal effects [15]. Others include smooth muscle contractile effect [16], anti-diabetic effect [17-19], anti-hepatotoxic effect [20], analgesic effect [21], and anticonvulsant and anxiolytic effects [22].
Phytoconstituents found in O. gratissimum leaves include tannins, steroids, terpenoids, flavonoids, cardiac glycosides, anthraquinones and essential oil [23]. The essential oil contains different compounds but eugenol has been identified as the chief constituent [24-27]. Other constituents in the oil include the monoterpene-1, 8-cineole [26], camphor and methyl eugenol [27,28], and thymol [29].
Our laboratory has in recent times been evaluating the scientific basis for the ethnomedicinal uses of some plants in the treatment of cough and asthma [30-32]. In the present study, we evaluated the anti-asthmatic and anti-tussive effects of the aqueous leaf extract of O. gratissimum in rodent species.
Plant materials and extraction
Fresh leaves of Ocimum gratissimum which had never been exposed to herbicides were collected from Ekosodin community in Benin City, Nigeria, in August 2014. Adulterants were carefully picked out of the leaves before rinsing them thoroughly in tap water. The leaves (764.28 g) were chopped into small pieces and blended with 4 l of tap water, allowed to stand for 12 h and then filtered twice with a clean white cloth. The final filtrate which was free from particles was dried in an oven at 40°C over 24 h (yield=1.2% w/w) and then stored in amber-colored bottle in a refrigerator. The extract (OGE) was reconstituted in distilled water and administered according to the experimental protocols.
Animals
Antitussive and anti-asthmatic experiments were performed using 55 adult guinea pigs of either sex weighing 460-600 g. Muco suppressant effect was evaluated using 30 mice of either sex weighing 20-30 g. The guinea pigs were obtained from the animal facility of the Department of Physiology, Ambrose Alli University, Ekpoma, Nigeria. The mice were obtained from a private animal farm in Ibadan, Oyo State, Nigeria. All animals were allowed two weeks acclimatization in the animal facility of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City. They were all allowed free access to pellets and tap water and were exposed to natural light-dark cycle and room temperature. All animals were handled according to standard protocols for the use of laboratory animals [33] and the experiments were overseen by members of Ethics Committee of the Faculty of Pharmacy, University of Benin, Nigeria.
Anti-asthma screening protocols
Guinea pigs were randomly allotted into six groups (n=5 per group) comprising of: (1) non-sensitized control (given 2 ml/kg of distilled water); (2) ovalbumin (OA)-sensitized+2 ml/kg of distilled wate rtreated; (3) OA-sensitized+100 mg/kg OGE; (4) OA-sensitized+200 mg/kg OGE; (5) OA-sensitized+400 mg/kg OGE; and (6) OA sensitized+ 0.5 mg/kg salbutamol.
The animals were sensitized by modifying the Bramley et al. method [34]. They were given intraperitoneal injections of 100 mg OA, a booster dose of 50 mg OA (i.m.) 24 h later and a final dose of 50 mg (i.p) 24 h before exposure to histamine aerosol. The administration of extract and distilled water was done daily per os by use of an orogastric tube (CU.FNC-16-3) for 14 days.
On the 14th day, the extract, distilled water and salbutamol were administered as appropriate. One hour later, the animals were exposed to 0.2% histamine dihydrochloride (dissolved in normal saline) aerosol in glass chamber (60 × 56 × 60 cm) using Omron® (Omron Healthcare Ltd, Japan) compressor nebulizer (rate of 0.4 ml/min and particle size of 5 μm) until preconvulsive dyspnea (PCD) was observed. The animals were then removed from the chamber and placed in fresh air to recover [30,34].
Evaluation of trachea fluid volume and viscosity
Guinea pigs used in the experiments above were euthanized under chloroform anesthesia and 2 cm lengths of tracheae up to the point of thoracic bifurcation were isolated. The isolated tracheae were held in place with clamps and then flushed with 2 ml of 37°C deionized water. The effective tracheal fluid volume was taken as the difference between the flushing volume and the final volume [30].
Tracheal fluid viscosity was estimated by an indirect method first described by Reid and Ugwu [35]. The animals were sacrificed and using a graduated 1 ml syringe fitted with a hypodermic needle (21G, 0.8 × 40 mm, Nr2), 1 ml of tracheal fluid was withdrawn and the plunger of the syringe was carefully removed after turning the needle point-down and holding it in place with a retort stand so that the fluid could drop under the influence of gravity. Using stop watch, the time taken for the content of the syringe to be emptied was recorded. The flow rate was calculated as volume/second and was used as an index of viscosity.
Antitussive screening protocol
This was based on the guinea pig cough model of Nadig [36]. A day before the test, guinea pigs were placed individually in a glass chamber (60 × 36 × 60 cm) for 5 min before cough was induced by exposure to citric acid aerosol (7.5% w/v) using an Omron® (Omron Health Care Ltd, Japan) compressor nebulizer (rate of 0.4 ml/min and particle size 5 μm) for 10 min. The animals exhibiting 10 - 20 bouts of cough were selected for the study and fasted overnight but with access to water. The selected animals were randomly allotted to 5 groups (n=5 per group). The animals were treated orally thus: (1) control group given 2 ml/ kg distilled; (2) 100 mg/kg OGE; (3) 200 mg/kg OGE; (4) 400 mg/kg OGE; and (5) 25 mg/kg dihydrocodeine. An hour after administration, they were re-exposed to citric acid aerosol (as earlier described) and the numbers of cough bouts were recorded. Percentage suppression of cough was calculated using the formula:
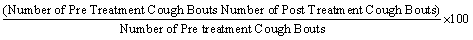
Mucus expectoration (phenol red) experiment
This was based on the method first described by Engler and Szelenyi [37]. Six groups of mice were used treated as follows: (1) 2 ml/kg/day distilled; (2) 100 mg/kg/day OGE; (3) 200 mg/kg/day OGE; (4) 400 mg/ kg/day OGE; (5) 15 mg/kg/day bromhexine hydrochloride; and (6) 50 mg/kg of sodium cromoglycate. All treatment was per os except for sodium cromoglycate that was administered intraperitoneally.
On the 8th day, after an overnight fast, treatment was done as usual and the animals in group 6 were given 50 mg/kg (i.p.) sodium cromoglycate 30 min prior to the administration of the secretagogue, ammonium chloride (5 mg/kg p.o.). Thirty minutes later, each mouse was injected with phenol red (500 mg/kg i.p.). All the mice were sacrificed by cervical dislocation 30 min after phenol red injection. The 2 cm of trachea was removed from the thyroid cartilage to the main stem bronchi. Each trachea was kept for 30 min in 2 ml normal saline. Sodium hydroxide (0.1 ml, 1 M) was added to the fluid to stabilize the pH. The absorbance of phenol red released from the trachea was read at 460 nm using a T80+UV/VIS spectrophotometer (PG Instruments Ltd, Beijing, China). A standard curve (graph of absorbance against concentration, R2=0.947) was plotted from which the concentrations of phenol red were extrapolated.
Drugs and chemicals
Chemicals and reagents were of analytical grade. They were obtained from internationally known suppliers such as Sigma (UK) and BDH (UK). Histamine, ovalbumin and citric acid crystals were manufactured by Sigma (UK). Salbutamol was manufactured by GlaxoSmithKline Nigeria Plc. Sodium cromoglycate was a kind gift by Dr S.O. Okpo of the Department of Pharmacology, University of Benin, Benin City. Dihydrocodeine phosphate was purchased from University of Benin Teaching Hospital, Benin City. Bromhexine hydrochloride was manufactured by Nigeria German Chemicals Plc (Nigeria). Drug and extract solutions were freshly prepared before administration.
Statistics and data presentation
Data are presented as mean ± SEM (Standard Error of Mean) and “n” represents number of guinea pigs or mice per group. Data were compared by use of one-way ANOVA with Tukey post hoc. All data were analyzed using GraphPad Prism version 6 software (GraphPad Software Inc. UK). P<0.05 indicates statistically significant difference.
Effect of extract on latency to precovulsive dyspnea
Figure 1 shows that the administration of 100, 200 and 400 mg/kg did not significantly inhibit the bronchospasm induced by histamine aerosol as indicated by the latency to preconvulsive dyspnea. Salbutamol (0.5 mg/kg) significantly (p<0.001) increased the latency when compared to other groups.
Effect of extract on tracheal fluid volume and viscosity
The tracheal fluid volumes of the various groups are shown in Figure 2. All doses of the extract and salbutamol significantly (p<0.0001) reduced tracheal fluid volume when compared with sensitized but distilled water-treated group. Tracheal fluid volume was reduced to levels comparable with that of non-sensitized group.
The extract and salbutamol did not significantly alter the viscosity of the tracheal fluid of guinea pigs (Figure 3). For example while the flow rate was 44.4 ± 1.3 × 10-3 ml/s in the non-sensitized group, it was 40.0 ± 0.5 × 10-3 ml/s and 41.4 ± 0.6 × 10-3 ml/s in the sensitized but distilled water-treated, 400 mg/kg OGE and salbutamol groups respectively.
Effect of extract on citric acid induced cough
Figure 4 shows that all doses (100, 200 and 400 mg/kg) of the extract significantly (p<0.0001) suppressed the number of cough bouts by 78.6 ± 10.3%, 79.6 ± 2.9% and 80.4 ± 4.6% respectively when compared with control (27.4 ± 9.5%). The standard drug, dihydrocodeine, significantly suppressed cough bouts by 83.9 ± 2.5% when compared to control.
Effect of extract on phenol red dye expectoration
In Figure 5, all the doses of OGE significantly reduced phenol red dye secretion in mice compared with control. The highest reduction was obtained with 200 mg/kg (p<0.0001) of the extract. Bromhexine and sodium cromoglycate also significantly (p<0.001, p<0.05) reduced dye secretion in mice but sodium cromoglycate was less effective than bromhexine.
Results from this study show that the aqueous leaf extract of O. gratissimum did not significantly increase the latency to preconvulsive dyspnea in guinea pigs exposed to histamine aerosol. Increase in the latency to preconvulsive dyspnea is an indication of the inhibitory action of substances on bronchospasm induced by spasmogens such as histamine and acetylcholine [30,34]. In the absence of protection against bronchoconstriction, the guinea pigs suffer dyspnea which may result in death. Previous studies have demonstrated that O. gratissimum has immunomodulatory effect, hence, preventing inflammatory response to allergens in an asthmatic lung [38]. This means that its usefulness in asthma is not likely due to bronchodilatation. Eugenol and thymol constituents of the essential oil of the plant have been reported to possess anti-inflammatory properties [39-41]. This glucocorticoid-like effect may be useful for prophylaxis and long term treatment of asthma [42].
Symptoms of asthma often include bronchosecretion and mucus plugging of the airways [43,44]. Sensitization with ovalbumin is a model of obstructive airway disease such that the airways become hyperresponsive and inflamed [45]. Therefore, the therapy of asthma also aims at reducing tracheal fluid viscosity and volume. Increased tracheal fluid volume was seen in the sensitized guinea pigs that were not given the extract but this parameter was significantly reduced in the extract treated groups. The extract contains tannins [23]. These secondary plant metabolites are known to have astringent/antisecretory effects [46,47] which may have been responsible for the decrease in tracheal fluid volume as well as the reduction in the secretion of phenol red dye in mice. Such reduction in dye secretion indicates lack of expectorant property by the extract. Plants containing tannins have been shown to possess antitussive effects [48,49]. The presence of secretions in the airway is often a trigger of the cough reflex [50]. Thus, although the extract may lack a bronchodilator property, the antisecretory effect may be helpful in cough and asthma.
The extract significantly reduced the number of cough bouts in guinea pigs. Although reduction of tracheal fluid volume may be contributory, it is possible that the extract possesses central nervous system effect similar to that of dihydrocodeine (DF118). Cough induction by citric acid is possibly by activating C-fibers in the airways or through activation of rapidly adapting receptors by tachykinins released from activated C-fibres [51,52]. The mechanisms by which opiods such as codeine and dextromethorphan act as antitussive are not well understood but they act by stimulating mu and kappa receptors in the cough centre [53]. In addition, nasal administration of thymol (constituent of the essential oil of O. gratissimum) has been associated with reduction in the urge to cough by olfactory mechanism [54].
The results from this study have shown that the aqueous leaf extract of O. gratissimum suppresses coughing by reducing tracheal fluid secretion in addition to a possible central effect of inhibiting the cough centre. Although acute doses of the extract did not protect guinea pigs from histamine induced bronchospasm, the antisecretory effect may be helpful in chronic asthma. The results lend credence to the ethnomedicinal use of the extract in the treatment of cough and asthma.
The authors have no competing interests in the conduct of the study.
The study was conceptualized by JMO. It was designed and supervised by RIO who also drafted the manuscript. DOU, AAM and SOO were all actively involved in the experiments. All authors read and approved the manuscript before its submission.
The authors wish to thank the laboratory staff of the Departments of Pharmacology and Toxicology, University of Benin, Benin City, Nigeria. The study was the outcome of final year project dissertation of DIU for the Pharm D degree of the University of Benin, Benin City, Nigeria.