Journal of Leukemia
Open Access
ISSN: 2329-6917
ISSN: 2329-6917
Research Article - (2023)Volume 11, Issue 6
Objectives: The Primary objective of this study is to compare the Overall Survival (OS), Event Free Survival (EFS), and Relapse Risk (RR) for Low Risk/Intermediate Risk (LR/IR) patients receiving four versus five chemotherapy courses as per MRC 15 chemotherapy protocol and to assess the therapy-associated toxicity and complications of therapy in both cohort arms. The secondary objective is to compere the cost and patients’ length of hospital stay in both cohorts, 4 versus 5 cycles patients.
Design: This is a retrospective descriptive study. It includes all patient’s less 16 year diagnosed with acute myeloid leukemia, non-high risk and treated with chemotherapy alone without allogeneic stem cell transplantation at King Fahad Specialist Hospital Dammam, Dammam (KFSHD), pediatric, hematology/oncology department between January 2008 and December 2021. Total number of cohorts is 32 patients.
Methods: After obtaining the Institutional Review Board (IRB) approval, all the data and information were
retrieved from the patients’ hard and electronic medical records and then computerized using a Microsoft Excel
sheet. Computerized data exported to Statistical Package for Social Sciences (SPSS) program updated version 24
(IBM Corp., Armonk, NY, USA) which was used for analysis of the data.
Results: A total of 32 patients diagnosed with LR and IR acute myeloid leukemia were included in this study, LR=14 and IR=18 patients. 9 patients received a total of 4 cycles chemotherapy, vs. 23 patients received 5 cycles. Comparison between the two groups showed fewer complications and better overall survival and disease-free survival in the four cycles group. The OS of 4 cycle group vs. 5 cycles is 100% and 90% respectively and the Disease- Free Survival (DFS) is 100% and 78% respectively.
Conclusion: Pediatric Acute Myeloid Leukemia (AML) is a rare heterogeneous disease with current survival rates around 70% despite all the advances in intensification of chemotherapy regimens and supportive care. International collaborative clinical trials should focus closely on understanding the molecular landscape and the biology and genetic background of AML to discover effective target therapies and immunotherapeutic agents, particularly when intensifying the currently used conventional chemotherapies may not be possible without significant treatment-related toxicities. Clinicians face challenges optimizing the ongoing therapeutic options that will provide maximum effectiveness with fewer toxicities. This study showed non-inferiority in survival parameters when giving four courses rather than five courses of chemotherapy in treating LR patients, especially for those who obtained negative Minimal Residual Disease (MRD) after the first cycle induction, with notably lower infection rates and serious toxic effects of chemotherapy.
Acute myeloid leukemia; Chemotherapy; Promyelocytic leukemia; Central nervous system
Nowadays, Leukemia is a significant concern for children worldwide. Most childhood leukemia cases comprise Acute Lymphoblastic Leukemia (ALL), whereas only 15%-20% are diagnosed with Acute Myeloid Leukemia (AML). So, Pediatric AML is a rare disease, with an incidence of seven cases per million children younger than 15 years, affecting children with a median age of 6 years [1].
The malignant blasts originate from early hematopoietic progenitors as an evolution from (pre-) leukemia stem cells. Environmental factors could explain only a tiny percentage. In addition, only less than 10% of pediatric AML are associated with predisposing syndromes or germline mutations [2].
Children have better outcomes than adults because of the more frequent presence of good prognostic genetic features and higher tolerance to intensive treatment. Complete Remission (CR) is now achieved in 90% of cases, whereas Event-Free Survival (EFS) and Overall Survival (OS) rates are commonly around 50% and 70%, respectively, due to the high rate of relapse [1].
Recent advances in the current knowledge of the genetic characterization of AML, emerging new biological concepts, and development of strategies for therapeutic intervention [3].
Unfortunately, managing pediatric AML remains a challenging clinical situation, with the outcome of pediatric acute myeloid leukemia still lagging behind that reported for patients with acute lymphoblastic leukemia, probably due to the heterogeneity nature of the disease, lack of multiple options of target therapies or immunotherapies, and scarcity of clinical trials in that field. On the other hand, the current treatment regimens, besides the wide recommendations of maximum supportive care, seem to reach the limit that unlikely any further intensification of conventional chemotherapy alone will impact the relapse rates [4].
Post-induction consolidation therapy has been shown to reduce relapse and prolong survival in AML; however, the optimal number of courses is uncertain [5]. The number of courses of chemotherapy needed to minimize the risk of relapse without increasing toxicity remains controversial. Based on the results of the Medical Research Council (MRC) AML trials, in which relapse and overall survival rates were the same for children who received 4 or 5 courses of chemotherapy, many other leukemia groups continue to tailor the therapy of AML to optimize the treatment for the best outcome with the minimum toxic effects [4].
The current study addressed the clinical outcome comparison between Low-risk AML patients who received four cycles of therapy vs. five cycles in a single institute that was included 32 patients.
Design
This is a retrospective descriptive study. It includes all the acute myeloid leukemia patients below sixteen years who were diagnosed as acute myeloid leukemia and risk stratified as nonhigh risk, so this patient was treated by chemotherapy alone without allogeneic stem cell transplantation.
All down syndrome patients, or cases with acute promyelocytic leukemia, patients who had disease progression or died during their therapy before completing the full course of treatments or those who was stratified as high-risk patients and underwent stem cell transplantation, or those with intermediate risk who had positive MRD after first induction cycle and shifted to high risk arm, in addition to those who had missing clinical data from their soft or hard electronic medical records, were all excluded from this study.
It is a study of a single institute at King Fahd specialist hospital in Dammam, pediatric hematology/oncology, and SCT department between January 2008 and December 2021.
Setting
It is a single-center study at King Fahad Specialist Hospital in Dammam, which is 400 beds tertiary referral hospital with 27 beds in the pediatric oncology Ward, four beds for stem cell transplantation, and 18 beds in the daycare unit. After obtaining the IRB approval, all the data and information were retrieved from the patients’ hard and electronic medical records and then computerized using a Microsoft Excel sheet.
Computerized data exported to (SPSS) program updated version 24 (IBM Corp., Armonk, NY, USA). Frequency tables were drawn to explore the findings (frequencies, percentages, grade/stage… etc). Overall survival and disease-free survival were illustrated by the Kaplan-Meier curves.
83 patients were diagnosed as case of AML at King Fahed Specialist Hospital in the period from 2008 to 2021. 51 cases were excluded (6 had Down syndrome with AML, 9 had APL, 19 patients were managed as high-risk AML and 17 missed follow up because either they passed away, had disease progression or they completed their treatment in other oncology center). 32 patients (38.5%) were eligible to be in this study. 16 cases (50%) of ours where male and mean age of our cohort is 9.6 years.
Fever is the most common presenting symptom (68.8%), the other initial presenting symptoms and signs (S/S) were listed in Table 1.
| Symptoms | Percent | N |
|---|---|---|
| Fever | 68.80% | 22 |
| Pallor | 31.30% | 10 |
| Bone pain | 18.80% | 6 |
| Bleeding | 18.80% | 6 |
| Wight loss | 25% | 8 |
| Limping | 6.3% | 2 |
Table 1: Initial presenting symptoms and signs (S/S).
The other clinical findings were, lymphadenopathy in 21.9% (n=7), 40.6% (n=13) had organomegaly. In addition to that, 12.5% (n=4) had chloroma and 6.3% had at least one or more CNS S/S. The initial white blood cell count assessment, showed the mean is 69 × 10^9/dL, and the mean hemoglobin level is 7.5 gram/dL; rest are shown in Table 2.
| Mean | |
|---|---|
| WBC | 69.16566 × 10^9/dL |
| HGB | 7.5925 gram/dL |
| PLT | 78.472 |
| Peripheral blast | 58.03% |
Table 2: The initial complete blood count.
Regarding the cytogenetics data, we had t (8;21) as the most common abnormality (31.3%) which is interpreted as favorable findings and normal cytogenetics in 34.4%. The normal cytogenetics categorized one-third of our patients as an intermediate risk group. The detailed cytogenetic/molecular abnormalities were shown in Table 3.
| Cytogenetics | Percent | n | |
|---|---|---|---|
| Favorable LR | t(8,21) | 31.30% | 10 |
| NPM | 3.10% | 1 | |
| Inv 16 | 9.40% | 3 | |
| IR (neutral) | Normal | 34.40% | 11 |
| Trisomy MLL gene | 3.10% | 1 | |
| t(9,11) | 6.30% | 2 | |
| Abnormal chromosome 7. | 3.10% | 1 | |
| ins(X;6) | 3.10% | 1 | |
| Complex | 3.10% | 1 | |
| compels partial deletion of p8 | 3.10% | 1 | |
| Total | 100 | 32 |
Table 3: The detailed cytogenetic/molecular abnormalities.
Minimal Residual Disease (MRD) status was illustrated well in this study. MRD for every case was done after first induction cycle and/or after second induction cycle. Based on MRD status illustrated in Figure 1, the subsequent consolidation therapy was planned. Also, MRD status reflected significantly on the OS and EFS.
Complications of therapy and major events during therapy were analyzed carefully in this study; Table 4 showed the most common complications.
| Complication | Overall Number | Percent (%) |
|---|---|---|
| Infection | 28 | 87.5 |
| Cardiac | 8 | 25 |
| PICU Admission | 13 | 40.6 |
| Relapse | 5 | 15.6 |
Table 4: Complications of therapy and major events during therapy.
The less common complications were: 12.5% (n=4) who got severe bleeding, 3.1% (n=1) had typhlitis, 3.1% (n= 1) had ovarian failure after end of therapy, followed up by gynecology and endocrinology services and 3.1% (n= 1) had sensory hearing loss, underwent cochlear implant Table 5.
| 4 cycles n=9 | 5 cycles n=23 | Total | ||||
|---|---|---|---|---|---|---|
| Number | Percent | Number | Percent | number | percent | |
| Infection | 7 | 25.00% | 21 | 75.00% | 28 | 100% |
| Cardiac | 2 | 25.00% | 6 | 75.00% | 8 | 100% |
| PICU | 2 | 15.40% | 11 | 84.60% | 13 | 100% |
| Admission | ||||||
| Relapse | 0 | 0 | 5 | 100% | 5 | 100% |
Table 5: Comparison of therapy complications in both study arms.
Infection is known as the most common associated complication in our patients. It was found that the 5 cycle’s cohort had higher infection than 4 cycle’s group. Data showed that the bacterial infection is the most common, followed by fungal, then viral. Types of bacterial organisms infection (Escherichia coli in 7 cases (22%), all received 5 cycles, Streptococcus mitis in 4 cases (14.2%), 3 of them received 5 cycle and one received 4, Streptococcus viridians in 3 cases (10.7%), all received 5 cycle). As far as regarding the fungal infection, (6 cases, 21.1%, 2 in 4 cycles arm and 4 in 5 cycles arm). For more compression between two groups, please, see Table 6. Some infection episodes were severe enough to get some patients admitted in Pediatric Intensive Care Unit (PICU) with septic shocks.
| Organism types | 4 cycles | Percent | 5 cycles | Percent | Total | |
|---|---|---|---|---|---|---|
| Bacterial | 4 | 30.8% | 9 | 69.2% | 13 | 100.0% |
| Bacterial +Fungal | 1 | 16.7% | 5 | 83.3% | 6 | 100% |
| Bacterial | 0 | 0 | 1 | 100.0% | 1 | 100% |
| +Fungal+Viral | ||||||
| Fungal | 1 | 33.3% | 2 | 66.70% | 3 | 100% |
| Viral | 0 | 0 | 1 | 100% | 1 | 100% |
| Viral +Bacterial | 0 | 0 | 1 | 100% | 1 | 100% |
| 6 | 24.0% | 19 | 76.0% | 25 | 100.0% |
Table 6: Isolated organism from infection episodes between two groups.
Cardiac complication were found in 8 cases. 2 cases (25%) from 4 cycle’s group developed cardiomyopathy, and the rest, 6 cases (75%) were from 5 cycles group; One case had cardiomyopathy, one case had infective endocarditis and 4 cases had decrease in cardiac function.
Finally, diseases relapse was compared and it was illustrated in The Kaplan-Meier curve as seen in Figures 2 and 3 and in Table 7. OS the Kaplan-Meier curve of all patients comparing 4 versus 5 cycles is showed in Figures 4 and 5, Tables 8 and 9. All relapsed patients as seen in Figure 1 were treated with HSCT and they are remission except two who died with multi organ failure in one and sudden home death in the other one with unexplained reason, see Table 10.
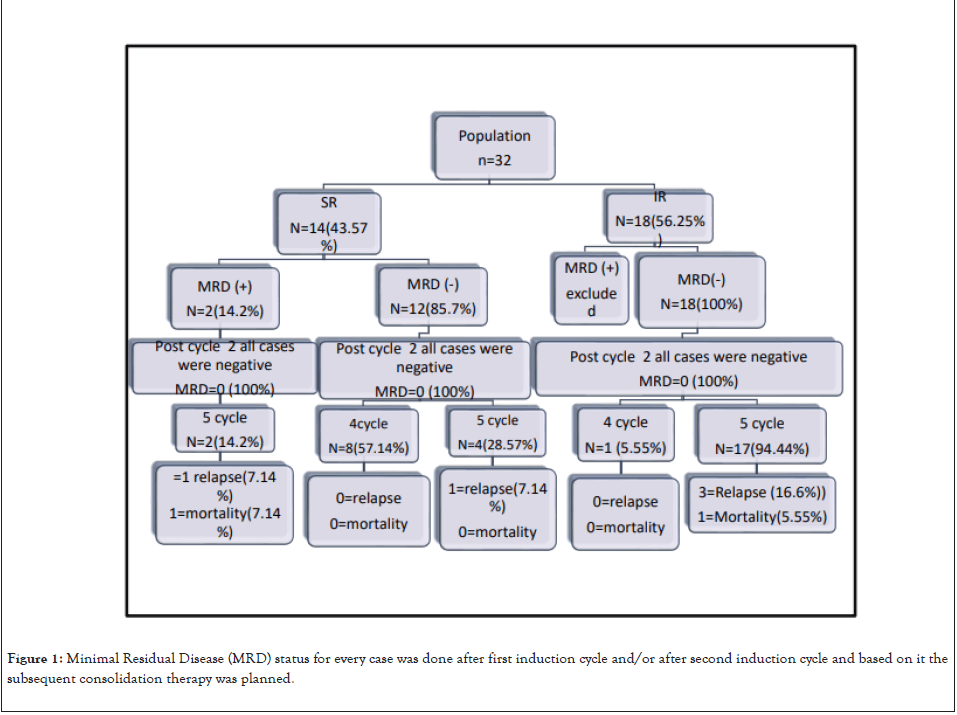
Figure 1: Minimal Residual Disease (MRD) status for every case was done after first induction cycle and/or after second induction cycle and based on it the subsequent consolidation therapy was planned.
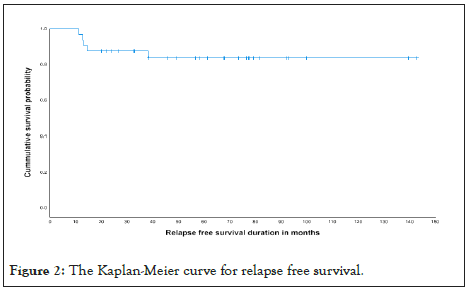
Figure 2: The Kaplan-Meier curve for relapse free survival.
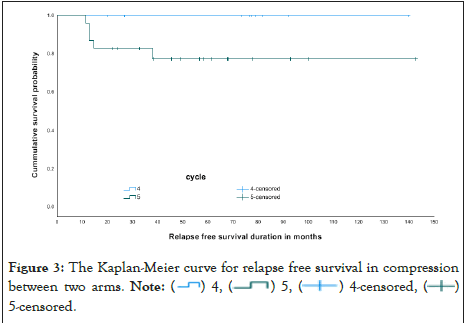
Figure 3: The Kaplan-Meier curve for relapse free survival in compression
between two arms. Note:  5-censored.
5-censored.
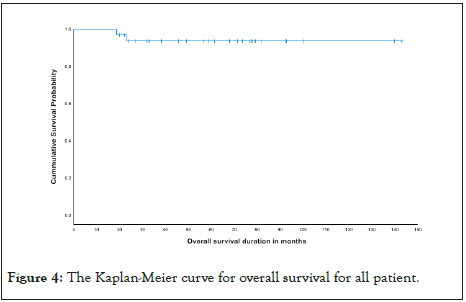
Figure 4: The Kaplan-Meier curve for overall survival for all patient.
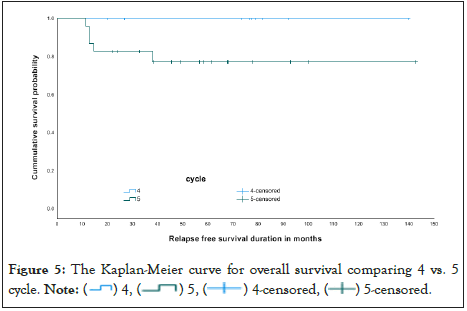
Figure 5: The Kaplan-Meier curve for overall survival comparing 4 vs. 5
cycle. Note:  5-censored.
5-censored.
| Cycle | Total N | N of events | N | Percent |
|---|---|---|---|---|
| 4 | 9 | 0 | 9 | 100% |
| 5 | 23 | 5 | 18 | 78.3% |
| overall | 32 | 5 | 27 | 84.4% |
Table 7: Relapse free survival in compression between two arms.
| cycle | Total N | N of Events | Censored | |
|---|---|---|---|---|
| N | Percent | |||
| 4 | 9 | 0 | 9 | 100.00% |
| 5 | 23 | 2 | 21 | 91.30% |
| Overall | 32 | 2 | 30 | 93.80% |
Table 8: Overall survival comparing 4 vs. 5 cycles.
| Overall Comparisons | |||
|---|---|---|---|
| Chi-Square | df | Sig. | |
| Log Rank (Mantel-Cox) | 0.772 | 100.00% | 0.38 |
| Test of equality of survival distributions for the different levels of cycle. | |||
Table 9: Test of equality of survival distributions for the different levels of cycle.
| Cause of death | Cycle 5 | Total | percent |
|---|---|---|---|
| unexplained death | 1 | 1 | 100% |
| sepsis and multiorgan failure | 1 | 1 | 100% |
| Total | 2 | 2 |
Table 10: The cause of death between mortality ceases.
Childhood Acute Myeloid Leukemia (AML) is a rare heterogeneous disease that requires a particular treatment approach. It represents 15%-20% of all pediatric acute leukemia’s.
Despite significant improvements in its outcome over the past few decades, Acute Myeloid Leukemia (AML) remains a life- threatening malignancy in children, with current survival rates of ∼70% [6].
AML tends to occur across the childhood years, but it's slightly more common during infancy, with another peaking during the teenage years. Its incidence in infants is 1.5 per 100,000 individuals per year, while that incidence decreases gradually later in children between 1 to 9 years (0.9 per 100,000 individuals aged 1-4 and 0.4 per 100,000 individuals aged 5-9 years,) but again it gradually increases toward adulthood, to reach 16.2 per 100,000 individuals aged over 65 years [7].
According to a study from Brazel 2019 regarding the age of AML patients at the time of diagnosis, 10.0% of their AML patients were less than one year old [8]. And around 15% were 1-9 years of age, 38.3% were 9-15 years of age, and 21.5% were >15 years of age. In contrast, our study data showed a median age of 9.59 years with no gender predominance, with 50% in each group between males and females.
Involvement of the Central Nervous System (CNS) in patients of Acute Myeloid Leukemia (AML) at diagnosis is not uncommon, with a published incidence of 6%-29% [9]. The current study showed an incidence of CNS 3 status disease at the time of diagnosis as 12.5% (4 cases).
A single institute study ran over 290 patients with pediatric AML; their findings showed that the presence of CNS involvement had no adverse prognostic significance, and patients with CNS2 status had similar outcomes to CNS1 patients on the other hand, another study that involves analysis of 1459 patients concluded that for patients with CNS disease at diagnosis despite their survival rate being similar to those without CNS disease yet they showed increased incidence of isolated CNS relapse [10,11].
Our data showed a 50% incidence of relapse (2 cases out of the 4 cases with CNS disease), with one disease-related mortality among those relapsed 2 cases, suggesting the adverse impact of CNS involvement in pediatric acute myeloid leukemia patients.
Similar to our findings, two consecutive COG trials found that CNS involvement, particularly CNS3 status, was associated with worse outcomes through complete remission rate, EFS, DFS, and an increased risk of relapse involving the CNS [12].
CNS disease in pediatric AML is found to be associated with elevated LDH levels, and WBC counts at diagnosis and is often associated with chromosome 16 inversion and chromosome 11 abnormalities. Patients with CNS3 status, in particular, were significantly at younger age and more likely to have the favorable cytogenetic features t (9;11), t (8;21), or inv D(16) (P<0.001) [10].
Risk stratification in pediatric AML depends on cytogenetic and molecular disease features and response to induction therapy detected by MRD. It helps to assign patients to treatments with sufficient intensity and avoid excess toxicities from extensive unnecessary therapy. And also it aids in identifying targetable lesions to incorporate targeted therapies.
Sequencing of AML genomes found that AML is genetically diverse and clonally heterogeneous, with multiple mutations acquired over time and complex patterns of clonal evolution shaping disease progression and response to therapy [13].
Analyses of clinical trials conducted from the late 1980s to the early 2000s revealed the prognostic significance of recurrent chromosomal aberrations in AML. In the United Kingdom (UK) studies MRC-AML10 and MRC-AML12, children with t(8;21)(q22;q22) (RUNX1::RUNX1T1) and inv(16)(p13q22) (CBFB::MYH11), the core-binding factor (CBF)-AML, had the best prognosis (80% OS rate), and the patients with chromosome 12 or 5q abnormalities, t (6;9)(p23;q34) (DEK::NUP214), monosomy 7, and t (9;22)(q34;q11) (BCR::ABL1) had the worst prognosis (36% OS rate). However, the majority (nearly 70%) of the patients were classified as intermediate risk (56% OS rate), which includes patients with normal karyotypes, chromosome 11q23 abnormalities, Acute Megakaryoblastic Leukemia (AMKL), and others [14].
Further analysis of chromosome 11q23 abnormalities identified more than 100 fusion gene partners, with each fusion having its unique prognosis. such as t (1;11)(q21;q23) (KMT2A::MLLT11) showed the best prognosis and t(6;11)(q27;q23) (KMT2A::AFDN), t (10;11)(p12;q23) (KMT2A::MLLT10), and t (10;11)(p11.2;q23)
(KMT2A::ABI1) showed unfavorable prognoses. Unbalanced abnormalities, such as monosomies of chromosomes 5 and 7, are less frequent in children, but they are associated with a poor outcome, as observed in adult [1]. Beyond cytogenetic markers, disease classification and, importantly, risk stratification remain challenging for about 50% of patients with AML who present with normal karyotypes [13].
Treatment decisions, namely the need for SCT, in pediatric AML are primarily driven by the risk stratification category in addition to the level of minimal residual disease and early response. For example Favorable-risk genetics such as inv(16), t(8;21), and CEBPA and NPM1 mutations may be cured with chemotherapy alone. In contrast, monosomy 7 and monosomy 5 or 5q deletions are High-Risk (HR) features that require SCT in the first remission for the best outcomes [15].
So from tha above discussion the LR was defined as the presence of inv (16)/t(16;16) or t(8;21) cytogenetic features, NPM1 or CEBPA mutations, or MRD<0.1% post-Induction I in those with no high risk features [16].
However, the outcomes for low-risk AML have improved significantly, yet the high-risk AML continues to be associated with an adverse prognosis [17].
The current study targets the LR- AML patients who are treated with chemotherapy only without the need for SCT. The study population involved the patient group with favorable cytogenetics (n=14, 43.8%) included those who have (t(8;21)31.3%, NPM 3.1%, inv16 9.4% ) and the Intermediate risk group (n=8% 56.2% ) who showed neither favorable nor adverse cytogenetics data according to Cytogenetic and molecular classification of the World Health Organization (WHO) 2016 [15].
Treatment of non-high-risk pediatric AML is generally managed with two courses of near-myeloablative therapy to induce remission, followed by two to three additional courses of chemotherapy. However, intensive induction therapy is associated with significant complications due to the effects of high leukemia burden and profound marrow suppression, with notable treatment-related deaths being exceptionally high in places with limited resources [18].
The clinical outcomes have been improved by identifying risk groups with variable prognosis and tailoring therapy to individual patients to minimize courses of chemotherapy without affecting the survival parameters or increasing the risk or relapse the concept of adjusting the total number of chemotherapy courses was addressed by the Medical Research Council (MRC) AML trials, in which they found that the relapse and the overall survival rates were the same for children who received 4 or 5 courses of chemotherapy [4].
Similarly, four therapy courses were given to patients enrolled in the St Jude AML08 and COG AAML1031 trials, except for those who underwent allo-HCT. On AAML1031, LR patients received a four-course chemotherapy backbone, including two induction courses of cytarabine/daunorubicin/etoposide and two consolidation courses: cytarabine/etoposide, followed by cytarabine/mitoxantrone.
On the other hand, current US-based trials (AML16 and AAML1831) give four courses only to patients with favorable genetics who are MRD negative at induction 1. In contrast, all other patients received five courses because of poor outcomes when given four courses in these previous, nonrandomized trials [4].
An important COG study done on 2017 and included a total of 921 LR patients (225 from AAML0531, 696 from AAML1031) to compare the outcome of the Four versus five chemotherapy courses, since the AAML0531, LR patients were scheduled to receive a five-course chemotherapy backbone that included the same four courses as AAML1031 plus an additional fifth course comprised of high-dose cytarabine/L- asparaginase that large study concluded that the removal of a fifth cytarabine-containing course appears to result in inferior outcome in form of OS, DFS, and RR in pediatric patients with LR AML [16].
Similarly, in Europe and Asia, many cooperative groups have continued to recommend five courses of chemotherapy, however the MyeChild 01 trial which is an international Randomised Phase III Clinical Trial in Children with Acute Myeloid Leukemia is designed to use total of four courses [19].
From the above discussion, it is evident that the optimal number of courses needed to minimize relapse without increasing toxicity remains controversial between different leukemia groups [4].
Our main current study objective is to compare the survival data and the major complications between patients treated in our center with 4 vs. 5 chemotherapy courses.
Among our study population, 43.5% (14 cases) were assigned to the SR group at diagnosis due to their favorable cytogenetics data; however, around 14% (2 cases) showed positive MRD after cycle one induction. Comparatively, 56% (18 cases) were assigned to the intermediate risk group, and all of these group patients had negative MRD after cycle one induction.
Our data also showed 28.1% (n=9 cases) received a total of 4 cycles; those patients included eight patients belonging to the SR group with negative MRD and one case from the IR group. On the other hand, 71.9% (n=23 ) were given a total of 5 cycles, this group involved ( 2 cases with SR and positive MRD after cycle one induction but negative MRD after cycle two induction and another 4 cases with SR and negative MRD after cycle one induction in addition to another 17 patients from the IR group)
The 4 cases belong to the SR group with negative MRD who were treated with five cycles of chemotherapy, and were given their therapy before the year 2015 when the five courses of therapy were the standard of care in contrast, we had one case from the IR group with negative MRD, managed by a total of 4 cycles due to severe complications and morbidities in the form of prolonged neutropenia, severe course of fungal infection, and frequent intensive care unit admission.
Minimal Residual Disease (MRD) that is measured at specific points during Induction therapy is now considered an independent, crucial predictive tool to estimate the risk of relapse in pediatric AML.
Flow cytometric assessment of MRD has been used to adapt further therapy. MRD levels >1% at the end of induction 1 were associated with a poor outcome. In contrast, the outcome of patients with MRD levels 0.1% to 1% was similar to that of patients with negative MRD. Conversely, any detectable MRD at the end of induction 2 was associated with an inferior prognosis and a high risk of relapse [20].
Similarly, the Nordic Society for Pediatric Hematology and Oncology (NOPHO) stated that the MRD at the end of induction 2 was the strongest predictor of outcome and was a significant prognostic factor even in analyses limited to patients without morphologically detectable blasts.
Furthermore, Investigators from the Children's Oncology Group (COG) recently reported that among patients treated on the AAML0531 trial, in which MRD was assessed by the "different from normal" flow cytometric method, detection of any MRD (>0.02%) at the end of induction 1 was associated with an increased risk of relapse and inferior overall survival. A metaanalysis confirmed the predictive value of MRD in AML across age groups and methods [20].
Although the MRD negativity does not exclude the possibility of relapse in pediatric AML, the early positivity during therapy does not exclude remission and cure possibility [21].
our data showed positive MRD post 1st cycle of induction of chemotherapy in 2 cases (6.25%) belonging to the SR group with favorable cytogenetics, both cases were assigned for 5 courses therapy and fortunately both of them got negative MRD in the reevaluation post cycle 2nd cycle induction. However, one of them (3.1%) experienced a relapse and died subsequently [22].
Over the past decade, despite the improvement of the clinical outcome in the management of pediatric acute myeloid leukemia, infectious complications remain very common and have an essential impact on the survival rates among those groups of children, the high rate of infections among them believed to be related to the prolonged severe neutropenia that was resulted from their intensified chemotherapy regimens and the profound disruption of their immune system.
Infections not only contribute to increased morbidities and mortalities, but also prolong hospitalization, delay the administration of chemotherapy, decrease quality of life, and require the administration of costly and often toxic antimicrobial medications.
Infection episodes are estimated to occur in 79% of SR group of pediatric acute myeloid leukemia patients the current study showed nearly similar incidence of documented infection of 87.5% among our study population, though it is far high than what is reported in another study that ran 341 patients with AML and median age of 7.1 years, this study showed sterile site microbiologically documented infection occurred in 24.5% [23]. Further analysis of our infectious events data showed 40.6% are bacterial in origin, 9.4% fungal, and 3.1% viral. Combined infectious events occurs as well with 18.8% are combined bacterial+fungal, 3.1% are combined bacterial+fungal+viral, 3.1% viral+bacterial.
However some studies showed that the incidence of Gram-positive and Gram-negative bacteremia did not differ significantly between the cycles of chemotherapy, whereas the incidence of sepsis due to Viridans Group Streptococci (VGS) was significantly higher in cycles of chemotherapy that included high-dose cytarabine and also high risk of fungal infectioTans, including candidiasis, aspergillosis, and mucormycosis [4,24].
In a study done in pediatric patients with acute myeloid leukemia in a tertiary hospital addressing the incidence of fungal infection, they reported an incidence of Invasive Fungal Disorders (IFD) of (11.6%) which is near to our findings of 9.4% considering that our study is only concerning the non-high risk patients and incidence may be far higher if all the risk group of AML are included in the study. That study also mentioned that the incidence of fungal infections is underestimated and some reports show that up to 75% of fungal infections go undiagnosed until autopsy [25].
As expected, the more Intensive chemotherapy directed against acute myeloid leukemia in childhood, the more likely it is to be followed by profound neutropenia and a high risk for bacterial and fungal infections. The current study aims to comparing the infection rate among those who were given total of 4 cycles vs. 5 cycles showed a significant difference (25% vs. 75%).
Our data also showed a significant difference in the severity of the infection and the need for admission to intensive care units between the two groups, as Severity of infection seemed to be worse in the group given 5 cycles with 84.6% needed PICU admission while only 15.4% of those who received four cycles admitted to PICU because of their infection events,
The rate of fungal infection among our patients is almost doubling in patients given 5 cycle’s chemotherapy vs. 4 cycles (66.6% to 33.3%). Anthracyclines are a vital component of AML therapy. They are often delivered at high doses to maximize the survival rates. Unfortunately, high-dose anthracyclines are associated with a major risk of cardiotoxicity, which may related to early and late cardiac complications, leading to significant morbidity and mortality in AML survivors.
In addition, early treatment-related cardiotoxicity has been reported to be associated with a worse outcome. Multiple strategies aim to maximize chemotherapy delivery while reducing the toxicity of intensive AML therapy took place as primary cardio protection and close serial cardiac monitoring by echocardiographic assessments for left ventricular function, incorporating new chemo agents as Liposomal anthracycline formulations, which thought to be associated with less cardiac toxicity though it still under investigation [26].
Our data showed a total incidence of declined cardiac functions as 25% (25.0% vs. 75.0%) 4 vs. 5 cycle of chemotherapy respectively. For patients with Acute Myeloid Leukemia (AML), supportive care is defined as a broad range of interventions that alleviate the symptoms of a disease or the side effects caused by treatment and that address psychological, cultural, social, and spiritual factors. Improved supportive care parameters have a major impact on increased cure rates.
Blood product transfusion support and infection management are vital examples of supportive care that have contributed significantly to the successes of more intensive chemotherapy, delivering improvements in outcomes despite, arguably, only modest improvements in chemotherapy regimens.
Despite this knowledge, there are no definite unified supportive care guidelines for the best approach to managing or preventing the known associated complications [27]. Our local center adopted the policy of prophylactic antimicrobial agents to minimize the risk of bacterial and fungal infections during their prolonged neutropenia, besides suitable blood product transfusion guidelines and consideration of prolonged admission during the intensified chemotherapy courses until evidence of peripheral blood count recovery.
Several different studies showed that this improvement in the outcomes of children with AML is multifactorial and attributed to recent policies of maximizing the supportive care, refinement of the risk stratification criteria to individualize the therapy to each patient accordingly, and then a further adjustment to the intensity of courses of treatment based on each patient's early response to chemotherapy Minimal Residual Disease (MRD).
Additionally, the introduction of new agents and target therapies, besides the selective use of Hematopoietic Stem- Cell Transplantation (HCT), and improved salvage therapy are essential factors that enhance pediatric AML outcomes.
Despite the remarkable improvements in overall survival in pediatric AML during the last few decades, the prevailing treatment challenges are preventing and managing relapsed disease, which is considered a significant cause of treatment failure. Unfortunately, approximately 30% of children and adolescents with AML suffer disease relapse and face a poor prognosis [28]. Some other studies reported an even higher rate of relapse up to 50% [29]. The disease recurrence rate is still significant in the low-risk genetic groups, as it remains common at up to 35%.
Our study showed a relapse rate of 15.6% (n=5 cases) among our study LR group; interestingly, all of them belong to the patients who received five cycles of chemotherapy, with none of our patients who had given four courses experiencing disease relapse.
Given the small number of patients in our study, our findings showed the LR patients treated with four cycles of chemotherapy are not at higher risk of relapse than those treated with five cycles of chemotherapy, which is similar to the data published by the Medical Research Council AML12 trial that concluded that there was no difference in any measure of outcome between children treated with either four or five courses of chemotherapy.
However the prognosis of childhood acute myeloid leukemia has improved, with current survival rates up to 75% similar data was obtained in a national study in Saudi Arabia that involved a multicenter and ran over 193 pediatric patients with acute myeloid leukemia; their survival data showed that the 5-year OS for LR, IR, and HR groups were 72.0 ± 6.9%, 59.8 ± 6.2%, and 45.1 ± 7.4%; respectively (p=0.003); and EFS 50.5 ± 8.0%, 46.3 ± 6.4%, and 23.3 ± 6.4%; respectively.
In this study, the OS and DFS by the time of reporting were found to be 100% in the LR group, who was given four courses, compared to OS of 90% and DFS of 78% in the LR, who was given 5 courses of chemotherapy. These differences in the OS rates between the two arms could be due to the fewer complications and toxic effects from cumulative chemotherapy in the patients given 4 courses of therapy.
The higher survival rate in our study than what is reported from the national and international studies may be due to the small sample size and the short follow-up period from the end of the research. It may need to be recalculated after enough period.
The two mortality cases that occurred in the group who were given 5 courses of therapy happened due to disease recurrence in both with disseminated infection with multi-organ failure in one case and sudden unexplained death in the second. In conclusion, this study showed non-inferiority in survival parameters when giving four courses rather than five courses of chemotherapy in treating LR patients, especially for those who obtained negative MRD after the first cycle induction, with documented lower infection rates and serious toxic effects of chemotherapy.
We can acknowledge that the current study had some limitations as it was retrospective, being done in a single-institute with small sample size, and a short follow-up period, but still, it highlights the need for a further prospective multicenter study to address and optimize the required magnitude of chemotherapy to treat pediatric acute myeloid leukemia with the best clinical outcome and less disease or treatment-related morbidities and mortalities.
Pediatric AML is a rare heterogeneous disease with current survival rates around 70% despite all the advances in intensification of chemotherapy regimens, selective Hematopoietic Stem Cell Transplantation (HSCT), supportive care, and refinement of the risk stratification.
International collaborative clinical trials are significantly essential to provide adequate numbers of patients of this uncommon disorder, and new strategies to improve the prognosis further should focus closely on understanding the molecular landscape along with the biology and genetic background of AML to discover effective target therapies and immunotherapeutic agents, especially when intensifying the currently used conventional chemotherapies may not be possible without major treatmentrelated toxicities raising the crucial need for new therapeutic options.
Great efforts by clinicians are needed in order to address the ongoing therapeutic challenges of reducing toxicity while increasing effectiveness.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: AL-Amer A, Omer H, Saad E, Ahmed O, Daama ALS (2023) Evaluation of the Clinical Outcomes among the Pediatric Low-Risk Acute Myeloid Leukemia Patients Who Received Four versus Five Cycles of Chemotherapy-Single-Center Study. J Leuk. 11:352.
Received: 16-Oct-2023, Manuscript No. JLU-23-27568; Editor assigned: 18-Oct-2023, Pre QC No. JLU-23-27568 (PQ); Reviewed: 07-Nov-2023, QC No. JLU-23-27568; Revised: 14-Nov-2023, Manuscript No. JLU-23-27568 (R); Published: 21-Nov-2023 , DOI: 10.35248/2329-6917.23.11.352
Copyright: © 2023 AL-Amer A, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.