
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2020)Volume 10, Issue 2
Background: Luteolin is a flavonoid with some useful pharmacological properties yet its safety profile has not been
fully delineated .Objectives of the study were to investigate the effects of luteolin on blood and liver functions, and
make necessary recommendations regarding therapeutic use of luteolin.
Methods: A total of 33 adult albino rats (average weight) were used in this study. Oral LD50 of luteolin was carried
out with 13 rats using modified Lorke’s method. The other 20 rats were divided into 4 groups comprising of 5 rats
per group (n=5 per group) for biochemical, haematological and histopathological studies which were carried out for
28 days. Group I was used as control and received orally 10 ml/kg of distilled water. Groups II,III and IV also orally
received 50 mg/kg,100 mg/kg and 200 mg/kg of luteolin respectively. Blood samples were collected from each rat in
the different groups before treatment, on 14th and 28th days respectively and after repeated daily dosing. The liver of
the rats were extracted and examined for histological changes.
Results: Results show that all doses of luteolin had significant (p<0.05) elevation of some blood indices (RBCs, PCV,
Hb) while WBCs was not elevated. There was significant (p<0.05) increase in liver enzymes such as ALT, AST and
ALP.
Conclusion: The study indicates that high doses ( ≥ 200 mg/kg) and prolonged use of luteolin may increase hepatic
enzyme activity while low doses ( ≤ 50 mg/kg) and are hepatoprotective. No significant liver damage was observed
based on histopathological results.
Luteolin; Flavonoid; Liver; Blood
ALT: Alanine Aminotransferase; AST: Aspartate amino- Transferase; ALP: Alkaline Phosphatase
Although luteolin have several pharmacological properties with a promising therapeutic potential, its safety profile has not been fully delineated. Luteolin is a flavone, a type of flavonoid found in combination with glycosides. It is present in several plant products, including broccoli, pepper, and celery [1] as well as olive oil (Oleaeuropaea L.), peppermint (Menthapiperita L.), (Thymusvulgaris L.), rosemary (Rosmarinusofficinalis L.), oregano (Origanumvulgare L.) and lettuce (Lactucasativa L.) . It is also found in T. chebula which is called the “King of medicines” due to its efficacy in healing, with a wide spectrum of biological, antiulcerogenic, neuroprotective , antioxidant, and antibacterial activities [2]. Luteolin and its derivatives have been isolated from a wide variety of natural sources; these isolates such as luteolin glycosides and luteolin rutinoside possess several pharmacological activities [3,4]. Apart from the natural isolates, synthetic and semi-synthetic luteolin have been obtained through different methods. Some methods require use of catalysts and metal complexation. Luteolin, like other flavonoids have many medicinal properties. Studies with mice suggest that luteolin treatment serves as a key role in improving the psychological stress-induced cognitive impairments [5,6]. This indicates that luteolin may enhance brain function, memory and cognition. Moreover, neuroprotective effects of luteolin has been documented in the management of epilepsy, Alzheimer’s disease, autism spectrum disorders, Parkinson disease as well as traumatic brain injury. Such pharmacological effects of luteolin have been linked to its ability to ameliorate neuroinflammation. Some clinical trials show the effectiveness of luteolin on diabetes mellitus type II, and some kinds of cancers [7].
However, notwithstanding the antioxidant property of luteolin, safety profile of luteolin remains unclear. Recent reports suggest that luteolin and its analogues may be deleterious to mammalian cells due to their effects on the endocrine system. This is likely attributed to the estrogenic activity of luteolin and its ability to antagonize progesterone receptor activation [8]. Presently, there are negligible clinical trials evaluating the therapeutic effects of luteolin in a large cohort. Furthermore, there is growing need for natural products that will take the place of synthetic drugs in the management of some chronic disease states. Therefore, this study investigated the effect of luteolin on the hepatic and hematopoietic systems in albino rats.
Animals
Male albino rats weighing between 180 g and 200 g were used in the study. These animals were bred and housed in the Animal House of the Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Anambra State, Nigeria.
The animals were separated and acclimatized in a cage for ten days, maintained at room temperature with adequate ventilation and naturally illuminated environment. They were fed with commercial rat chow (Vital Feeds Nigeria Limited) ad libitum, and liberally supplied with distilled water. The animals were handled according to the Guide for the Care and Use of Laboratory Animals as established by the National Research Council (NRC) [9].
Materials
The reagents used were: Aspartate aminotransferase (AST) Teco Diagnostic kit, Alanine aminotransferase (ALT), Alkaline phosphatase (ALP), deionized water and distilled water. Major equipment instruments used include: Spectrophotometer (Jenway, UK), Microhematocrit (Jenalab Medical, England), The plant extract used was pure sample of luteolin (Chengdu Biopurify Phytochemicals Ltd, BP0896, Lot: PRF7031021,>95% purity).
Determination of acute toxicity (LD50) by oral route
The modified Lorke ’ s method [10] was used in the determination of oral acute toxicity of luteolin with a total of 13 rats. Two phases were involved in the process. In phase I, the rats were divided into three groups (n=3 per group) and their weight determined. Luteolin was orally administered to each of the animals using cannula in the dosages of 10, 100 and 1000 mg/kg per group respectively. The animals were constantly monitored for the next one hour, intermittently for the next 3 hours and over a total period of 24 hours for behavioural changes and mortality.
In Phase II, four groups (n=1) of rats received 2000, 3000, 4000 and 5000 mg/kg of luteolin respectively. Behavioural changes and mortality were recorded.
Administration of luteolin
A total of 20 adult albino rats was used in this study. They were weighed and grouped into 4 of 5 rats per group (n=5). Group I served as the control and was orally given 10 mg/kg distilled water. Group II was given 50m g/kg luteolin, group III received 100 mg/kg while group IV received 200 mg/kg orally respectively. All luteolin administered was a single daily dose and lasted for 28 days. Blood sample was collected from each animal through ocular-puncture and was analysed for liver and haematological parameters. Blood sample was collected from each animal before treatment (pre-treatment), on day 14 and day 28.
Parameters (Blood and Liver markers)
Liver parameters (ALT, ALP, AST), were colorimetrically determined using the procedure described by Tecodiagnostics [11,12] with absorbances at 340 nm, 600 nm and 340 nm. Packed cell volume (PCV) was determined by microhematocrit method similar to Bull et al., 2017.Haemoglobin (Hb) was done using Sahlis method [13], while white blood cell (WBC) and red blood cell (RBC) were carried out by haemacytometric method as described by Reddy et al. [14]. Histological examination of the liver was done as described by Bancroft et al. [15].
Data analysis
Data obtained were expressed as Mean ± SEM (Standard Error Mean). Statistical analyses was performed by one-way analysis of variance (ANOVA) and p values<0.05 were considered statistically significant.
Figures 1-4 shows the photomicrographs of histological sections of rat’s liver. Figure 1 shows normal liver of the control rat (H&EX100) whereas Figures 2 and 3 show visibly normal livers at dose 50 mg/kg of luteolin and 100 mg/kg (H&EX100) respectively).
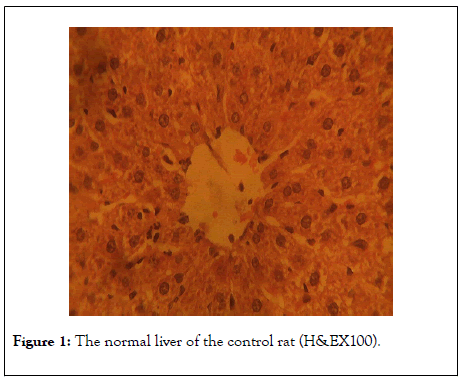
Figure 1: The normal liver of the control rat (H&EX100).
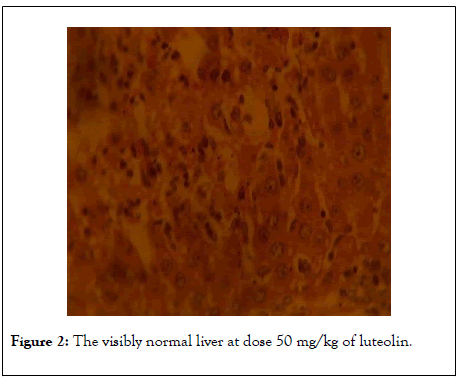
Figure 2: The visibly normal liver at dose 50 mg/kg of luteolin.
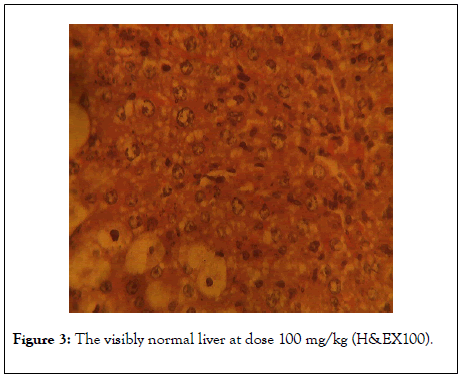
Figure 3: The visibly normal liver at dose 100 mg/kg (H&EX100).

Figure 4: Slightly altered liver tissue at 200 mg/kg of luteolin (H&EX100).The arrows show the regions of alterations.
Figure 4 shows slightly altered liver tissue at 200 mg/kg of luteolin (H&EX100).The arrows show the regions of alterations.
Table 1 shows the result of acute toxicity (LD50) of orally administered luteolin obtained by Lorke [10]. Phase (I) had three groups of animals (n=3) while phase (II) had four groups (n=1) of animals. No death was recorded in the study.
| Group | Dose(mg/Kg) | NOD | |
|---|---|---|---|
| Phase I | 1 | 10 | 0/3 |
| 2 | 100 | 0/3 | |
| 3 | 1000 | 0/3 | |
| Phase II | 1 | 2000 | 0/1 |
| 2 | 3000 | 0/1 | |
| 3 | 4000 | 0/1 | |
| 4 | 5000 | 0/1 |
Note: SOT=Sign of Toxicity, NOD=No of Death. LD50> 5000 mg/kg
Table 1: Acute Toxicity (LD50) of orally administered luteolin.
Table 2 displayed the mean liver parameters (ALT, AST and ALP), indicating administered doses of luteolin per mg/kg measured on 14th and 28th days respectively. In comparison with pre-treatment values, there was significant (p<0.05) increase in ALT on 14th day for doses of 50, 100 and 200 mg/kg respectively.
| Parameters (Liver) | Luteolin (mg/kg) | Duration of Administration Luteolin | ||
|---|---|---|---|---|
| Pre-treatment | 14th Day | 28thDay | ||
| Control ALT | 0 | 23.90 ± 0.10 | 23.60 ± 5.90 | 26.53 ± 3.56 |
| 50 | 32.60 ± 8.80 | 55.07 ± 16.76* | 54.20 ± 0.00* | |
| 100 | 32.60 ± 8.80 | 53.03 ± 10.19* | 38.93 ± 4.95ns | |
| 200 | 33.75 ± 7.65 | 70.73 ± 17.67* | 49.40 ± 11.77ns | |
| Control AST | 0 | 24.95 ± 1.15 | 29.50 ± 5.90 | 39.57 ± 3.27 |
| 50 | 33.75 ± 7.65 | 23.60 ± 5.90ns | 40.30 ± 0.00ns | |
| 100 | 67.95 ± 8.85 | 35.37 ± 10.19* | 51.43 ± 4.91ns | |
| 200 | 76.80 ± 17.70 | 58.93 ± 21.24* | 54.50 ± 7.04* | |
| Control ALP | 0 | 17.20 ± 0.90 | 30.20 ± 1.20 | 46.40 ± 15.96 |
| 50 | 17.20 ± 0.90 | 26.60 ± 1.20* | 29.10 ± 0.00* | |
| 100 | 16.47 ± 0.17 | 27.80 ± 1.20* | 25.57 ± 2.04* | |
| 200 | 17.20 ± 0.90 | 29.00 ± 2.08* | 30.70 ± 6.78 | |
Note: ALT= Alanine Aminotransferase, AST= Aspartate amino-Transferase, ALP=Alkaline Phosphatase, Number of animals per group(n)=5, *=significant (p<0.05), ns=not significant (p>0.05).
Table 2: Mean ± Parameters of ALT (IU/L), AST (IU/L) and ALP (IU/L).
AST indicates significant (p<0.05) decrease on 14th day on both 100 mg/kg and 200 mg/kg as well as on 28th day of 200 mg/kg. There was significant (p<0.05) increase in all the doses in both the 14th and 28th day when compared with the pre-treatment.
From the mean blood parameters measured in table 3, there was significant (p<0.05) increase in packed cell volume (PCV) at 100 and 200 mg/kg doses on the 14th day when compared with pre-treatment.
| Parameters (Blood) | Luteolin (mg/kg) | Duration of administration luteolin | ||
|---|---|---|---|---|
| Pre-treatment | 14th Day | 28th Day | ||
| Control | 0 | 33.8 ± 0.97 | 43.0 ± 0.70 | 36.8 ± 1.74 |
| PCV | 50 | 36.2 ± 0.37 | 36.8 ± 0.58ns | 34.4 ± 0.93ns |
| 100 | 36.8 ± 0.49 | 43.8 ± 1.39* | 33.6 ± 0.9ns | |
| 200 | 31.8 ± 0.37 | 45.4 ± 2.01* | 36.0 ± 0.84ns | |
| Control | 0 | 11.28 ± 0.33 | 14.34 ± 0.23 | 12.26 ± 0.58 |
| Hb | 50 | 12.06 ± 0.11 | 12.28 ± 0.20ns | 11.48 ± 0.28ns |
| 100 | 12.26 ± 0.16 | 14.60 ± 0.47* | 11.20 ± 0.31ns | |
| 200 | 10.60 ± 0.13 | 15.16 ± 0.67* | 12.02 ± 0.29ns | |
| Control | 0 | 5.62 ± 0.16 | 6.12 ± 0.12 | 6.14 ± 0.29 |
| RBC | 50 | 6.04 ± 0.07 | 7.16 ± 0.12* | 5.72 ± 0.15ns |
| 100 | 6.14 ± 0.09 | 7.30 ± 0.23* | 5.60 ± 0.15ns | |
| 200 | 5.30 ± 0.05 | 7.54 ± 0.34* | 5.98 ± 0.13ns | |
| Control | 0 | 4.94 ± 0.35 | 4.85 ± 0.33 | 5.12 ± 0.21 |
| WBC | 50 | 5.14 ± 1.13 | 4.39 ± 0.17ns | 5.90 ± 0.19ns |
| 100 | 4.66 ± 0.31 | 4.95 ± 0.06ns | 4.83 ± 0.08ns | |
| 200 | 5.76 ± 0.56 | 5.29 ± 0.47ns | 5.60 ± 0.55ns | |
Note: PCV=Packed Cell Volume, Hb= Haemoglobin, RBC=Red Blood Cell, WBC=White Blood Cell, ns=not significant (p>0.05), *= significant (p<0.05), number of animals per group (n)=5
Table 3: Mean ± Parameters of PCV (%), Hb (g/dl), RBC(x106 /μL) and WBC (x 103 /μL).
All doses of luteolin administered on 28th day showed no significant increase in PCV of blood. Also, there was significant increase (p<0.05) in hemoglobin (Hb) on administered doses of 100 mg/kg and 200 mg/kg of luteolin while all administered doses of luteolin on 28th day were not significant (p>0.05).
The mean red blood cell (RBC) showed significant (p<0.05) increase on all doses administered on 14th day only. The mean white blood cells (WBC) measured showed no significant increase in all the doses of luteolin administered.
In the result, the acute toxicity value (LD50) of the pure luteolin sample was above 5000 mg/kg. This implies that the flavonoid is not acutely toxic. This was also evident as no death was recorded at the different concentrations of luteolin administered. Oral (LD50) of luteolin obtained might be considered practically non-toxic based on Hodge and Sterner scale of toxicity [16]. However, it might not be harmless or absolutely non-toxic on chronic consumption especially at high doses. This is because U.S Environmental Protection Agency, as compiled by Paris et al. [17] classified any chemical whose LD50>5000 mg/kg in category (IV) which is interpreted as optionally harmful if swallowed.
The result of haematological indices shows significant (p<0.05) increases in mean red blood cells, packed cells volume and haemoglobin concentration when compared to the pretreatment at doses 100 mg/kg and 200 mg/kg on the 14th day. The 28th day result was not significant suggesting that luteolin might have favourable effect on the blood indices. This is supported by earlier studies on oral consumption of bioflavonoids which have shown to be effective in preventing oxidative stresses which damage red blood cells as also observed by Asgary [18]. Moreover, there was no significant (p>0.05) increase in White Blood Cells at doses of 50 mg/kg, 100 mg/kg and 200 mg/kg. This implies absence of infection or immune stimulation which is in agreement with the antimicrobial effects of luteolin as studied by Tormakangas et al. [19].
There were significant (p<0.05) increases in the liver function parameters tested, ALT, AST and ALP in the animals treated on both the 14th and 28th days of treatment when compared with the pre-treatment and the negative control. These increases in liver enzymes with increase in doses of luteolin and duration of treatment suggest that high doses and prolonged use of luteolin increase liver enzyme activity. This result is in agreement with study by Lopez-Lazaro [20]; they opined that chronic accumulation of the flavonoid in the liver could be cytotoxic to the hepatocytes and possibly lead to DNA damage. The histopathological results virtually show no significant damage to the liver. However, prolong use and increased doses may show damage.
Result of the study indicates that high doses and prolonged use of luteolin may increase hepatic enzyme activity while low doses are hepatoprotective. However, the study opined that chronic use at high doses could result to liver damage.
The study is mainly animal study.Ethical approval was obtained from the research and ethics committee of Faculty of Pharmaceutical Sciences,NnamdiAzikiwe University, Awka, Nigeria.
CKO: Conceived of the study. CPI and CAA: Designed and supervised the study. ACO: Procured the kits and carried out the dissection of tissues. CEO: Carried out the acute and subchronic toxicity study, collected, and its analyzed interpreted the data statistically and wrote the manuscript. JNO: carried out major corrections on the manuscript. All authors read and approved the final manuscript.
We acknowledge the Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Nigeria for use of facilities.
Citation: Orji CE, Okpoko CK, Agbata CA, Okoyeh JN, Okeke AC, Ihekwereme CP (2020) Evaluation of the Effect of Luteolin on the Hepatic and Hematopoietic Systems in Albino Rats. J Clin Toxicol. 10:434. DOI: 10.35248/ 2161-0495.20.10.434
Received: 28-Feb-2020 Accepted: 13-Mar-2020 Published: 20-Mar-2020 , DOI: 10.35248/2161-0495.20.10.434
Copyright: © 2020 Orji CE, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.