Journal of Research and Development
Open Access
ISSN: 2311-3278
ISSN: 2311-3278
Research Article - (2024)Volume 12, Issue 4
Background: Mobile-based health applications have significantly impacted patients' lives by reducing cardiovascular symptoms. This study evaluates the effectiveness of the Leading Your Future Experience (LYFE) app, a digital health intervention, when combined with Standard of Care (SOC) compared to SOC alone in post-Percutaneous Coronary Intervention (PCI) patients with Coronary Artery Disease (CAD) and/or Acute Coronary Syndrome (ACS).
Methods: This prospective, single-center, randomized controlled trial included 86 patients diagnosed with CAD or ACS who had undergone PCI. Participants were randomly assigned to either the LYFE group (n=42) or the SOC group (n=44). Outcomes were assessed at 1, 3, and 6 months, focusing on Quality of Life (QoL), medication adherence, physical activity, dietary adherence and biochemical parameters.
Results: The LYFE group demonstrated significantly greater improvements compared to the domains of the Dartmouth scale; including physical fitness (-2.0 vs. -0.9), feelings (-1.9 vs. -0.9), daily activities (-1.7 vs. -0.8), pain (-1.9 vs. -0.8), overall health (-1.9 vs. -0.6), and QoL (-1.8 vs. -0.6), (p<0.050). Adherence rates for lifestyle changes were significantly higher in the LYFE group, with 98.0% adhering to exercise and diet, compared to 47.4% and 50.0% in the SOC group, respectively. Medication adherence was also better, with 92.5% of LYFE patients rarely forgetting their medications, compared to 50.0% in the SOC group (p<0.050).
Conclusion: The integration of the LYFE app with SOC significantly enhances patient outcomes, including medication adherence, physical activity, and QoL, compared to SOC alone, and supporting value of digital health interventions in improving cardiovascular care.
Mobile health; Acute coronary syndrome; Digital health interventions; Coronary artery disease; Mobile health; Percutaneous coronary intervention; Quality of life
ACS is a common detrimental presentation of CAD, posing a substantial global burden [1]. This is well supported by the fact that in 2022, there were almost 315 million prevalent cases of CAD worldwide [2]. Studies have shown that Indians are at higher risk of developing CAD and have a higher case-fatality rate compared to the western populations [3]. In patients with ACS, revascularization through PCI remains an exemplar [4]. However, even after revascularization, patients remain at an elevated risk of consecutive cardiovascular events, such as myocardial infarction, stroke, and cardiovascular death, despite innovations in medical care and prevention that have improved survival rates. This underscores a pressing need for secondary prevention to ensure better risk factor management following revascularization in patients with CAD [5].
Secondary prevention through adherence to medical treatment and self-management of risk factors is of significantly important [6]. This can be further reinforced by the fact that European Society of Cardiology (ESC) guidelines for the treatment of ACS, either ST-Segment Elevation Myocardial Infarction (STEMI) or Non-ST-Segment Elevation Myocardial Infarction (NSTEMI), and cardiovascular prevention recommends broad access to a range of evidence-based strategies, during hospitalization and post-discharge for secondary prevention [7-9]. However, at ground level reality, the actual situation often contrasts sharply, largely due to inadequate follow-up after discharge and insufficient communication between patients and their respective cardiologists and other healthcare providers. Therefore, it is important to implement an approach that includes cardiologists and patient-level interventions, encapsulating multifaceted educational, socioeconomic, and technological innovations to boosts long-term adherence to these strategies [10,11]. Patient empowerment and individual-tailored medicine to support lifelong adherence to lifestyle changes and drug therapies can be a surefire solution. Given the widespread use of smartphone devices, the potential for personalized approaches to improve adherence is substantial [12].
In recent years, innovations like mobile- or tablet-based health applications (apps) have significantly impacted patients’ lives. The use of mobile Health (mHealth) apps has attested to their value by helping patients with chronic conditions to manage their symptoms more effectively. These apps offer online education, peer support, lifestyle monitoring and coaching sessions, enabling users to track their health status, foster self-engagement and enhance self-compliance in the disease management, ultimately improving health outcomes [13]. In addition to this, mHealth apps can overcome geographical obstacles in healthcare access by providing real-time responses to patient needs in distant or remote areas [14]. Furthermore, health apps can ease the strain on medical staff by aiding in medication management and intake, as well as monitoring symptoms [15]. In context of cardiovascular health, the use of mobile health apps has demonstrated multifaceted benefits like improved cardiac rehabilitation, elevated adherence to treatment, exercise tolerance, decrease in cardiovascular symptoms, better psychosocial status, and decrease in the overall mortality rate [16]. In line with these benefits, the 2021 International Society for Holter and Noninvasive Electrocardiology (ISHNE)/ Heart Rhythm Society (HRS)/ European Heart Rhythm Association (EHRA)/ Asia-Pacific Heart Rhythm Society (APHRS) expert collaborative statement on mHealth in arrhythmia management: Digital medical tools for heart rhythm professionals: From the ISHNE/ HRS/EHRA/APHRS recommends incorporation of mobile health into routine clinical care to improve cardiovascular outcomes [17]. Considering the context mentioned above, the present study was conducted to evaluate the effectiveness of a clinical evidence-based therapeutic intervention maneuvering the LYFE app software, in combination with SOC compared to only the SOC group among post-PCI patients diagnosed with CAD and/or ACS.
Study design
This prospective, single-center, randomized real-world evidence cohort study was conducted between February 2023 and October 2023, involving patients diagnosed with CAD or ACS who had undergone PCI. The study adhered to the approved protocol by the Institutional Ethics Committee (IEC), Good Clinical Practice (GCP) guidelines, all relevant health authority requirements, and national laws.
Study population
The study included patients aged 18 or above, either sex, who were diagnosed with CAD and/or ACS and have undergone PCI surgery (elective or emergency). Participants were required to agree to the follow-up plan, demonstrate basic reading proficiency in English, Marathi, or Hindi, and provide informed consent before being enrolled in the study.
Study procedure
A total of 86 patients were included in the study; with 42 randomized to the LYFE group and 44 in the SOC group. The study design has been summarized in Figure 1.
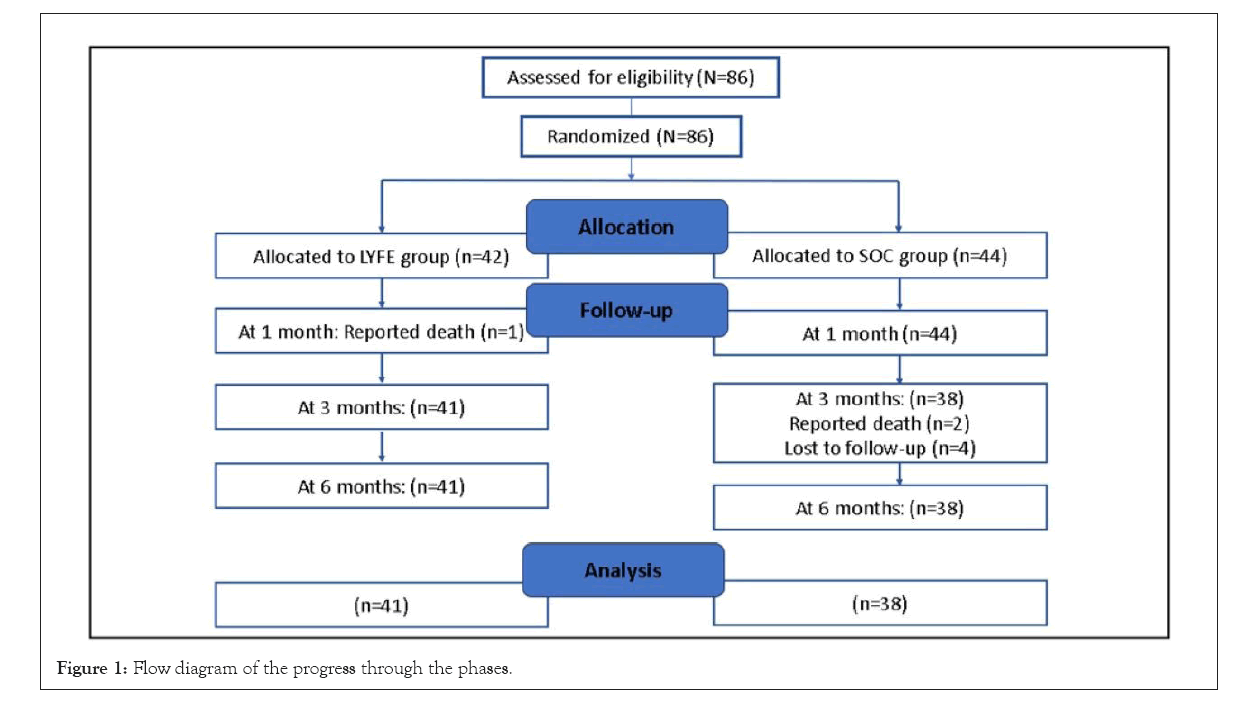
Figure 1: Flow diagram of the progress through the phases.
Intervention
SOC: The SOC group were administered with dual antiplatelet therapy, irrespective of the stent type. Patients with stable CAD were instructed to continue dual antiplatelet therapy post bare-metal or drug-eluting stent implantation. For ACS cases standard treatment protocol involved adding an oral anti-coagulant to a dual antiplatelet regime, with decisions on the duration of dual antiplatelet therapy controlled by bleeding risk and patient-specific standard care.
LYFE application: LYFE app, developed by Lupin Digital Health Pvt Ltd., is a personalized digital heart care program designed by a team of cardiologists. It enables patients to monitor and manage their heart health through a mobile app integrated with distinct connected devices (wireless activity and heart rate tracker, blood pressure monitor, pulse oximeter, glucometer, Smart weighing scale, and ECG handheld). Assimilation of wireless devices enabled patients to monitor and measure their Blood Pressure (BP), heart rate and physical activity. Through this application, patient received reminders regarding their medications, lifestyle changes, and upcoming appointments. This program consists of 7 key aspects: 1) Comprehensive and proactive monitoring that includes a series of auto-scheduled lab tests and teleconsultations with their cardiologists; 2) The conformance lo lifestyle modifications and medications via nudges, which offers actionable, personalized insights and short competitions; 3) Involvement of caregiver through training and a dedicated caregiver app to receive alerts and monitor vitals; 4) Individualized coaching and support from specialized nutritionists and health advisers to assist patients in managing their condition through tailored diet and exercise plans that are contextual to their health status, lifestyle, and preferences; 5) Patient’s and caregiver’s educational module on the particular disease; 6) An emergency response system designed to help patients handle any cardiac emergencies; 7) Access to ambulances that are capable of handling cardiac episodes and pre-determined hospitals based on availability and first-aid education. The overview of LYFE app has been demonstrated in Figure 2.
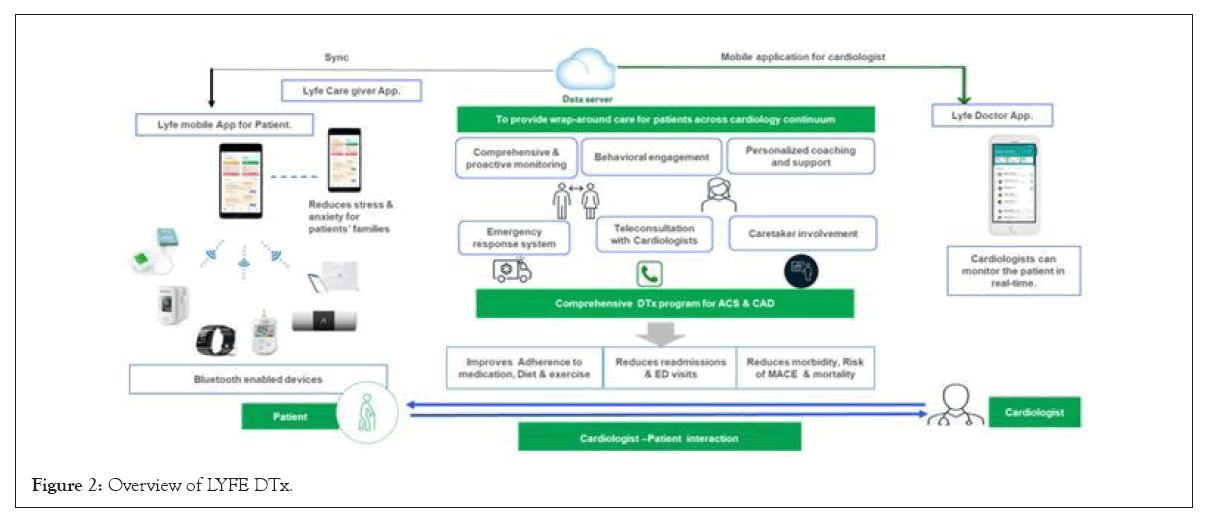
Figure 2: Overview of LYFE DTx.
Follow up
At the end of the first month, the LYFE group reported one death (n=41), and none from the SOC group. At 3 months, four patients were lost to follow-up, and 2 deaths occurred in the SOC group (n=38), with no drop outs in LYFE group. Furthermore, at the end of 6 months, 1 patient dropped from the LYFE group and none from the SOC group, resulting in n=41 for LYFE, and N=38 for SOC. Patients were assessed at 1 month, 3 months, and 6 months by monitoring changes in QoL employing the Dartmouth COOP questionnaire scores, comparing results from the baseline to the conclusion of the study. Moreover, evaluation consisted of tracking changes in weight, Body Mass Index (BMI), vital signs such as BP and Pulse Rate (PR), as well as Laboratory parameters like High-Density Lipoprotein (HDL), Low-Density Lipoprotein (LDL), Triglycerides (TG), Total Cholesterol (TC), Haemoglobin (Hb), Fasting Blood Glucose (FBG), Post-Prandial Blood Glucose (PPBG), Glycated Hemoglobulin (HbA1c) levels and creatinine.
Study endpoints
The primary endpoint of this study was to evaluate the effectiveness of a clinical evidence-based therapeutic intervention maneuvering the LYFE app software, in combination with SOC compared to only the SOC group among post-PCI patients diagnosed with CAD and/or ACS. The evaluation mainly focused on scaling adherence to medication, physical exercises and dietary practices. The secondary objectives were to assess changes in weight, BMI, vital signs such as BP and PR, as well as Laboratory parameters like HDL, LDL, TG, TC, Hb, FBG, PPBG, HbA1c levels and creatinine monitoring hospital admissions and analysing Major Cardiovascular Adverse Events (MACE) such as CV death, non-fatal MI, stent thrombosis, revascularization stroke/Transient Ischemic Attack (TIA), and notable instances of bleeding that occurred throughout the study period.
Statistical analysis
Statistical analyses were conducted using SPSS software (version 21.0; IBM Corp., Armonk, NY, USA). Descriptive statistics were used to perform a statistical analysis of the collected data using the mean and standard deviation for all datasets. To evaluate significant differences in mean changes between the baseline and follow-up periods i.e.1, 3 and 6 months, a paired and independent t-test was used. A p value<0.05 was considered as statically significant within the groups.
Demographic characteristics in patients
A total of 86 patients were included in this study. The mean age of the patients was 51.8 years in LYFE group and 56.5 years in SOC groups. The number of male patients was higher than the female patients in both the groups (LYFE: 76.2% vs. 23.8%, SOC: 72.7% vs. 27.3%). The majority of patients in LYFE (57.1%) as well as SOC group (68.2%) were with a diagnosis of CAD. Around 33.3% of patients in LYFE group and 43.1% of patients in LYFE group has a history of PCI, whereas 9.5% of patients in LYFE group and 11.4% of patients in SOC group had a history of Coronary Artery Bypass Graft (CABG). The majority of patients in LYFE group presented with unstable angina (38.9%) whereas in SOC group the majority of patients presented with unstable angina and NSTEMI (33.3%). Among the comorbidities, most of the patients in both the groups had hypertension; LYFE group (76.2%) and SOC group (68.2%). Upon considering other comorbidities like rheumatoid arthritis, hypothyroidism, and hyperlipidaemia, there was a significant difference between both the groups; LYFE: 28.6%, SOC: 11.4%, p=0.050) as shown in Table 1.
| Demographic characteristics | LYFE (n=42) | SOC (n=44) | p-value* |
|---|---|---|---|
| Age (years), mean (SD) | 51.8(12.0) | 56.5 (11.5) | 0.065 |
| Height (cm), mean (SD) | 164.2 (10.3) | 163.7 (9.3) | 0.813 |
| Weight (kg), mean (SD) | 72.9 (15.8) | 70.2 (12.6) | 0.390 |
| BMI (kg/m2), mean (SD) | 26.9 (4.7) | 26.2 (4.4) | 0.459 |
| Gender, n(%) | |||
| Male | 32 (76.2) | 32 (72.7) | 0.713 |
| Female | 10 (23.8) | 12 (27.3) | |
| Personal habits, n(%) | |||
| Alcohol | 11 (26.2) | 3 (6.8) | 0.015* |
| Tobacco smoking | 7 (16.7) | 5 (11.4) | 0.478 |
| Tobacco chewing | 2 (4.8) | 5 (11.4) | 0.236 |
| Clinical characteristic, n (%) | |||
| Diagnosis, n (%) | |||
| CAD | 24 (57.1) | 30 (68.2) | 0.291 |
| Post-ACS | 18 (42.9) | 14 (31.8) | |
| Previous PCI, n (%) | |||
| Yes | 14 (33.3) | 19 (43.18) | 0.768 |
| No | 28 (66.7) | 25 (56.81) | |
| Previous CABG, n (%) | |||
| Yes | 4 (9.5) | 5 (11.4) | 0.530 |
| No | 38 (90.5) | 39 (88.6) | |
| Indications for surgical intervention (Previous PCI and CABG), n (%) | |||
| Asymptomatic | 0 (0) | 2 (13.3) | 0.106 |
| Unstable angina | 7 (38.9) | 5 (33.3) | |
| N STEMI | 3 (16.7) | 5 (33.3) | |
| STEMI | 3 (16.7) | 3 (20.0) | |
| Others | 5 (27.8) | 0 (0) | |
| Comorbidities, n (%) | |||
| Hypertension | 32 (76.2) | 30 (68.2) | 0.408 |
| Diabetes | 17 (40.5) | 22 (50.0) | 0.375 |
| Both hypertension and diabetes | 13 (13.6) | 16 (36.4) | 0.596 |
| Others (rheumatoid arthritis, CVA/TIA, hypothyroidism and hyperlipidaemia) | 12 (28.6) | 5 (11.4) | 0.045* |
| None | 0 | 0 | - |
Table 1: Comparison of baseline characteristics in the LYFE app. vs. SOC follow-up of patients with CAD and/or ACS who underwent PCI.
| Characteristics | 1st month/30 days | 3rd month/90 days | 6th month/180 days | |||
|---|---|---|---|---|---|---|
| LYFE | SOC | LYFE | SOC | LYFE | SOC | |
| (n=41) | (n=44) | (n=41) | (n=38) | (n=40) | (n=38) | |
| Weight, (kg) | ||||||
| Baseline, mean (SD) | 73.0 (15.9) | 70.2 (12.6) | 73.0 (15.9) | 70.9 (12.9) | 73.1 (16.1) | 70.9 (12.9) |
| Follow-up, mean (SD) | 72.1 (15.6) | 69.4 (12.3) | 73.1 (14.5) | 69.4 (13.3) | 72.7 (14.3) | 68.6 (13.3) |
| Mean change from baseline (95% CI) | 1 (0.6, 1.4) | 0.8 (0.5, 1.2) | 0 (-1.9, 1.9) | 1.5 (0.6, 2.4) | 0.3 (-1.6, 2.3) | 2.4 (1.4, 3.3) |
| Mean change between groups (95% CI) | 2.7 (-3.4, 8.7) | 3.6 (-2.6, 9.9) | 4.1 (-2.1, 10.4) | |||
| p-value* | 0.38 | 0.25 | 0.19 | |||
| Body Mass Index, BMI (kg/m2) | ||||||
| Baseline, mean (SD) | 27.0 (4.8) | 26.2 (4.3) | 27.0 (4.8) | 26.1 (4.5) | 26.9 (4.8) | 26.1 (4.5) |
| Follow-up, mean (SD) | 26.6 (4.7) | 25.9 (4.3) | 27 (4.7) | 25.6 (4.6) | 26.8 (4.3) | 25.2 (4.7) |
| Mean change from baseline (95% CI) | 0.4 (0.2, 0.5) | 0.3 (0.2, 0.4) | 0 (-0.7, 0.6) | 0.6 (0.3, 0.9) | 0.1 (-0.5, 0.7) | 0.9 (0.5, 1.2) |
| Mean change between groups (95% CI) | 0.7 (-1.2, 2.6) | 1.5 (-0.6, 3.5) | 1.6 (-0.4, 3.6) | |||
| p-value* | 0.47 | 0.17 | 0.12 | |||
Table 2: Comparing mean weight and BMI between LYFE vs. SOC groups at 6-month follow-up patients with CAD and/or ACS who underwent PCI.
Mean difference in Dartmouth COOP scale from baseline to 6 months in LYFE and SOC group
The mean differences in the Dartmouth COOP scale were significantly greater in the LYFE group compared to the SOC group in parameters like: Physical fitness (-2.0 vs. -0.9), feelings (-1.9 vs. -0.9), daily activities (-1.7 vs. -0.8), social activities (-1.9 vs. -0.8), pain (-1.9 vs. -0.8), changes in health (-1.6 vs. -0.4), overall health (-1.9 vs. -0.6), and QoL (-1.8 vs. -0.6), with all differences being statistically significant (p<0.05) as show in Figure 3.
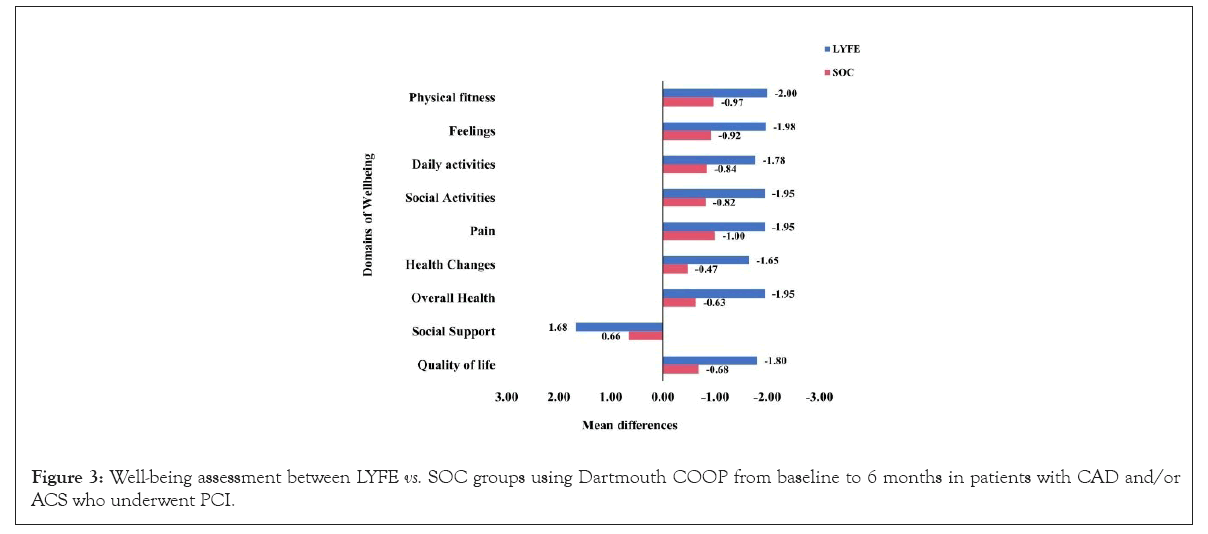
Figure 3: Well-being assessment between LYFE vs. SOC groups using Dartmouth COOP from baseline to 6 months in patients with CAD and/or ACS who underwent PCI.
Since the LYFE care app does not emphasize social support, the mean difference in social support was notably higher in the SOC group compared to the LYFE group (1.6 vs. 0.6), with a p=0.050 as shown in Figure 3.
Comparison of mean weight and BMI between LYFE and SOC
There was no significant change in the mean weight of patients from baseline to 6 months in either group: LYFE (73.0 vs. 72.7) and SOC (70.2 vs. 68.6), p=0.190. Similarly, no significant change in BMI was observed from baseline to 6 months follow-up: LYFE (27.0 kg/m2 vs. 26.8 kg/m2) and SOC (26.2 kg/m2vs. 25.2 kg/m2), with a p=0.120 as shown in Table 2.
Adherence to exercise and diet in LYFE and SOC Groups
At 6 months, almost all the patients adhered to their exercise regimen in LYFE group (98.0%), whereas only 47.4% of patients adhered to their exercise regimen in SOC group. In terms of diet, the number of patients adhering to their prescribed diet was higher in LYFE group (98.0%) than SOC group (50.0%) as shown in Figures 4a-4c.
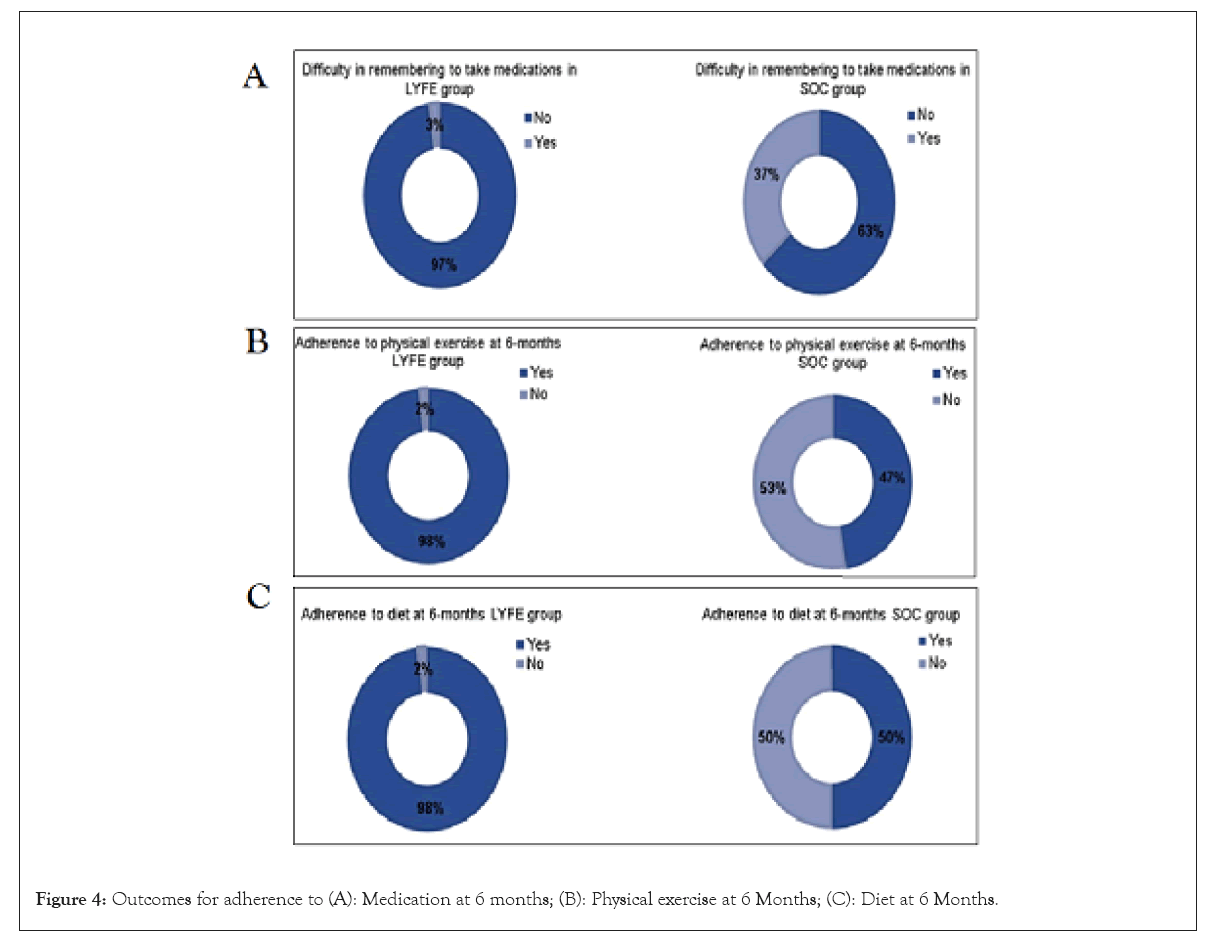
Figure 4: Outcomes for adherence to (A): Medication at 6 months; (B): Physical exercise at 6 Months; (C): Diet at 6 Months.
Comparison of mean Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) and PR between LYFE and SOC
Over the 6-month follow-up period, the LYFE group (125 mmHg vs. 117.2 mmHg) showed a significant reduction in SBP compared to the SOC group (127.8 mmHg vs. 134.1 mmHg). Specifically, LYFE participants experienced a notable decrease in SBP from baseline to 6 months, with a difference of 8.0 mmHg compared to SOC, which was statistically significant (p=0.050). For DBP, the differences were not statistically significant at any follow-up point as shown in Table 3.
| Characteristics | 1st month/30 days | 3rd month/90 days | 6th month/180 days | |||
|---|---|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | LYFE (n=40) | SOC (n=38) | |
| Systolic Blood Pressure, SBP (mmHg) | ||||||
| Baseline, mean (SD) | 125.0 (11.5) | 127.8 | 125.0 (11.5) | 127.6 (18.0) | 125.2 (11.7) | 127.61 (17.93) |
| (18.0) | ||||||
| Follow-up, mean (SD) | 123.4 (17.7) | 125.8 (13.3) | 118.9 (13.6) | 124.5 (12.1) | 117.2 (10.9) | 134.1 (22.2) |
| Mean change from baseline (95% CI) | -1.6 (-3.4, 6.7) | -1.9 (-2.3, 6.1) | -6.1 (0.9, 11.1) | -3.0 (-3.5, 9.6) | -8.0 (3.8, 12.1) | 6.5 (-13.7, 0.6) |
| Mean change between groups (95% CI) | -2.4 (-9.2, 4.2) | -2.6 (-11.2, 0.1) | -5.5 (-24.8, -9.1) | |||
| p-value | 0.47 | 0.44 | 0.05* | |||
| Diastolic Blood Pressure, DBP (mmHg) | ||||||
| Baseline, mean (SD) | 75.9 (9.9) | 78.6 (10.0) | 75.9 (9.9) | 79.3 (10.3) | 75.9 (10.1) | 79.3 (10.3) |
| Follow-up, mean (SD) | 79.2 (13.1) | 77.7 (9) | 78.4 (7.7) | 77.5 (7.8) | 76.7 (7.4) | 82.3 (9.3) |
| Mean change from baseline (95% CI) | 3.2 (-7.6, 1.1) | -0.8 (-2.7, -4.4) | 2.5 (-6.18, 1.16) | -1.7 (-2, 5.5) | 0.8 (-4.3, 2.6) | 3.1 (-6.9, 0.7) |
| Mean change between groups (95% CI) | 1.4 (-3.3, 6.3) | -3.3 (-2.5, 4.3) | 0.9 (-9.4, -1.9) | |||
| p-value | 0.54 | 0.14 | 0.60 | |||
| Pulse Rate, PR (bpm) | ||||||
| Baseline, mean (SD) | 79.9 (8.5) | 77.9 (13.4) | 79.9 (8.5) | 78.32 (14.0) | 79.6 (8.4) | 78.32 (13.95) |
| Follow-up, mean (SD) | 78.7 (11.0) | 79.0 (9.04) | 74.3 (10.3) | 78.6 (11.0) | 77.3 (10.7) | 80.2 (12.6) |
| Mean change from baseline (95% CI) | -1.1 (-2.4, 4.7) | 1.0 (-4.8, 2.7) | -5.5 (2.0, 9.0) | 0.3 (-6.1, 5.4) | -2.2 (-1.8, 6.3) | 1.9 (-7.2, -3.4) |
| Mean change between groups (95% CI) | -4.4 (-9.1, 0.4) | 1.6 (-9.1, 0.4) | -2.9 (-8.1, 2.3) | |||
| p-value | 0.070 | 0.54 | 0.269 | |||
Table 3: Change in mean SBP, DBP, and PR in the LYFE app. vs. standard of care at 6months follow-up of CAD and/or ACS patients who underwent PCI.
At 6 months, blood pressure control was significantly higher in the LYFE group (98.0%) compared to the SOC group (63.2%) as shown in Figure 5. Additionally, when asked about difficulties in remembering to take medications, 92.5% of patients in the LYFE group reported ‘Never or rarely,’ whereas 50.0% of patients in the SOC group reported ‘Once in a while.’ These differences were statistically significant, with a p<0.050.
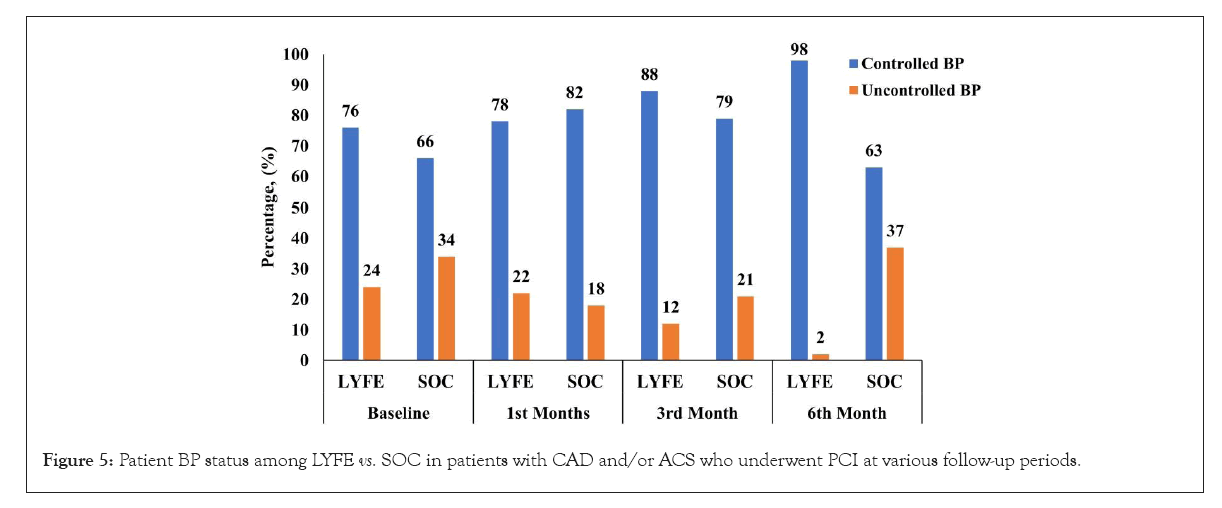
Figure 5: Patient BP status among LYFE vs. SOC in patients with CAD and/or ACS who underwent PCI at various follow-up periods.
Comparison of biochemical assessment between LYFE and SOC
Upon evaluating the biochemical parameters, there was no significant difference in LDL, TC, or creatinine levels between the groups. However, from baseline to 6 months, the LYFE group showed more noticeable reductions compared to the SOC group in several biochemical parameters: HDL levels (LYFE: 39.3 mg/dL vs. 35.2 mg/dL, SOC: 39.1 mg/dL vs. 34.8 mg/dL), TG levels (LYFE: 153.6 mg/dL vs. 143.8 mg/dL, SOC: 156.7 mg/dL vs. 156.7 mg/dL), FBG (LYFE: 137.4 mg/dL vs. 110.5 mg/dL, SOC: 127.7 mg/dL vs. 113.2 mg/dL), and glycated hemoglobin (LYFE: 8.2% vs. 7.1%, SOC: 7.7% vs. 7.1%). Despite these changes, none of the results were statistically significant as shown in Table 4.
| Characteristics | 1 Month | 3 Months | 6 Months | |||
|---|---|---|---|---|---|---|
| LYFE (n=40) | SOC (n=38) | LYFE (n=40) | SOC (n=38) | LYFE (n=40) | SOC (n=38) | |
| Low-Density Lipoprotein, LDL (mg/dL) | ||||||
| Baseline, mean (SD) | 55.5 (39.5) | 63.6 (16.1) | 62.3 (38.6) | 55.6 (21.5) | 65.5 (39.7) | 53.6 (16.4) |
| Follow-up, mean (SD) | 49.7 (25.4) | 60.9 (19.9) | 57.5 (25.5) | 55.6 (21.5) | 67.95 (27.9) | 68.1 (25.2) |
| Mean difference from baseline, (95% CI) | 5.8 (-5.0, 16.6) | 2.7 (-5.2, 10.5) | 4.8 (-8.1, 17.7) | 7.8 (-3.7, 19.4) | -2.41 (-14.3, 9.4) | -14.5 (-30.6, 1.7) |
| p value† | 0.22 | 0.86 | >0.05 | |||
| High-Density Lipoprotein, HDL (mg/dL) | ||||||
| Baseline, mean (SD) | 39.3 (13.1) | 39.1 (13.0) | 39.1 (13.2) | 43.4 (12.6) | 37.3 (11.6) | 34.5 (7.2) |
| Follow-up, mean (SD) | 36.2 (11.9) | 40.4 (13.3) | 37.1 (8.2) | 38.1 (12.2) | 35.2 (8.1) | 34.8 (8.2) |
| Mean difference from baseline (95% CI) |
3.09 (-1.2, 7.4) | -1.33 (-4.8, 2.1) | 2.02 (-2.1, 6.1) | 5.37 (-1.4, 12.2) | 2.12 (-1, 5.3) | -0.31 (-3.3, 2.7) |
| p value† | 0.4 | 0.81 | 0.89 | |||
| Triglycerides, TG (mg/dL) | ||||||
| Baseline, mean (SD) | 153.6 (68.8) | 156.7 (85.9) | 147.2 (57.9) | 162.7 (94.7) | 149.3 (62.3) | 163.2 (71.2) |
| Follow-up, mean (SD) | 121.5 (42.1) | 147 (64.1) | 157.4 (73.1) | 168.4 (72.4) | 143.8 (51.5) | 156.7 (90.2) |
| Mean difference from baseline (95% CI) | 32.1 (1.0, 63.3) | 9.7 (-26.3, 45.8) | -10.2 (-53.4, 33) | -5.5 (-63.2, 52.2) | 5.5 (-15.3, 26.3) | 6.55 (-39.9, 53.0) |
| p value† | 0.22 | 0.73 | 0.59 | |||
| Total cholesterol (mg/dL) | ||||||
| Baseline, mean (SD) | 125.5 (40.3) | 133.7 (25.2) | 132.0 (43.5) | 141.1 (19.2) | 133.5 (44.0) | 121.1 (16.7) |
| Follow-up, mean (SD) | 110.2 (29.9) | 129.7 (31.6) | 127.03 (26.2) | 127.3 (24.5) | 131.9 (31.8) | 133.6 (28.2) |
| Mean difference from baseline (95% CI) | 15.3 (6.1, 24.5) | 4.0 (-6.2, 14.2) | 5.0 (-10.8, 20.7) | 13.8 (1.3, 26.3) | 1.6 (-11.8, 14.9) | -12.6(-32.5, 7.3) |
| p value† | 0.11 | 0.98 | 0.88 | |||
| Haemoglobin , Hb (mg/dL) | ||||||
| Baseline, mean (SD) | 14.1 (2.3) | 14.5 (6.1) | 13.1 (2.4) | 13.0 (1.5) | 13.1 (2.2) | 13.8 (1.5) |
| Follow-up, mean (SD) | 13.7 (2.0) | 12.8 (1.3) | 13.2 (1.6) | 12.7 (1.4) | 12.9 (1.4) | 13.2 (1.6) |
| Mean difference from baseline, (95% CI) | 0.4 (-0.1, 0.8) | 1.6 (-2.2, 5.4) | -0.0 (-1.1, 1.1) | 0.3 (-0.3, 0.8) | 0.2 (-0.7, 1.1) | 0.6 (-0.4, 1.6) |
| p value† | 0.23 | 0.52 | 0.97 | |||
| Fasting plasma glucose, FBS(mg/dL) | ||||||
| Baseline, mean (SD) | 137.4 (71.1) | 127.7 (41.9) | 111.2 (63.2) | 109.3 (25.6) | 121.3 (59.9) | 105.8 (23.9) |
| Follow-up, mean (SD) | 129.2 (43.5) | 139.2 (80.7) | 109.6 (41.61) | 125.1 (51.1) | 110.5 (43.7) | 113.2 (58.5) |
| Mean difference from baseline, (95% CI) | 8.1 (-13.5, 29.8) | -11.5 (-58.0, 34.9) | 1.61 (-11.7, 14.9) | -15.8 (-49.1, 17.5) | 10.8 (-7.2, 28.7) | -5.6 (-35.8, 24.1) |
| p value† | 0.69 | 0.45 | 0.89 | |||
| Postprandial plasma glucose, PPBS (mg/dL) | ||||||
| Baseline, mean (SD) | Na | 193.4 (47.7) | 162.3 (54.16) | Na | 192.5 (85.1) | 190.0 (66.9) |
| Follow-up, mean (SD) | Na | 248.3 (51.0) | 165.6 (98.08) | Na | 142.9 (66.0) | 185.8 (92.6) |
| Mean difference from baseline (95% CI) |
Na | -55 (-65.5, -44.5) | -3.3 (-397.8, 391.3) | Na | 49.6 (-79.4, 178.6) | 4.28 (-1428.5, 1437) |
| p value† | - | - | 0.51 | |||
| Creatinine | ||||||
| Baseline, mean (SD) | 1.0 (0.2) | 1.0 (0.3) | 1.1 (0.4) | 1.0 (0.2) | 1.1 (0.3) | 1.0 (0.3) |
| Follow-up, mean (SD) | 1 (0.21) | 1.1 (0.3) | 1 (0.22) | 1.0 (0.2) | 1.0 (0.2) | 1.1 (0.4) |
| Mean difference from baseline (95% CI) | 0.0 (0.0, 0.1) | -0.0 (-0.2, 0.1) | 0.1 (-0.1, 0.3) | 0.0 (-0.1, 0.1) | 0.1 (0, 0.2) | -0.01 (-0.1, 0.1) |
| Mean difference, (95% CI) | -0.1 (-0.3, 0.1) | 0.04 (-0.2, 0.2) | -0.1 (-0.3, 0.1) | |||
| p value† | 0.46 | 0.72 | 0.63 | |||
| Glycated Haemoglobin, HbA1c (%) | ||||||
| Baseline, mean (SD) | 8.2 (2.51) | 7.7 (1.67) | 7.3 (2.0) | 7.2 (1.8) | 7.6 (2.3) | 7.1 (1.8) |
| Follow-up, mean (SD) | 7.7 (2.1) | 7.5 (1.6) | 6.8 (1.6) | 7.6 (2.38) | 7.1 (1.8) | 7.1 (2.1) |
| Mean difference from baseline (95% CI) | 0.5 (0.0, 1.0) | 0.2 (-0.2, 0.6) | 0.4 (-0.1, 1.0) | -0.3 (-0.9, 0.3) | 0.6 (-0.1, 1.2) | 0.0 (-0.9, 0.9) |
| Mean difference, (95% CI) | 0.2 (-1.3, 1.8) | -0.8 (-2.4, 0.9) | 0.0 (-1.5, 1.4) | |||
| p value† | 0.77 | 0.36 | 0.96 | |||
Table 4: Comparison of biochemical assessment in LYFE vs. standard of care at 6-month follow-up of patients with CAD and/or ACS who underwent PCI.
Comparison of MACE between LYFE and SOC
At the one-month follow-up, one cardiovascular death was reported in the LYFE group, but no deaths were recorded at the six-month follow-up. In the SOC group, two cardiovascular deaths were reported at the three-month follow-up, with no deaths reported at the six-month follow-up as shown in Table 5.
| Characteristics | 1st month/30 days | 3rd month/90 days | 6th month/180 days | |||
|---|---|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | LYFE (n=40) | SOC (n=38) | |
| Clinical outcomes (mortality, myocardial infarction, stroke, target vessel revascularization, heart failure admission and emergency visit), n(%) | 1.0 (2.4%) | 0 (0) | 0 (0) | 2.0 (4.5%) | 0 (0) | 0 (0) |
| p-value* | 0.29 | 0.14 | NA | |||
Table 5: Comparison of clinical outcomes in LYFE vs. standard-of-care patients with CAD and/or ACS who underwent PCI at 6-month follow-up.
Comparison of hospitalization history in LYFE and SOC
At the three-month follow-up, 5.3% of patients in the SOC group were hospitalized, compared to only 2.4% in the LYFE group. By the six-month follow-up, 2.5% of patients in the LYFE group were hospitalized, while no hospitalizations were reported in the SOC group. However, these differences were not statistically significant as shown in Table 6.
| Characteristics | 1st month/30 days | 3rd month/90 days | 6th month/180 days | |||
|---|---|---|---|---|---|---|
| LYFE (n=41) | SOC (n=44) | LYFE (n=41) | SOC (n=38) | LYFE (n=40) | SOC (n=38) | |
| Number of hospitalization, n(%) | 0 (0) | 0 (0) | 1.0 (2.4) | 2.0 (5.3) | 1.0 (2.5) | 0 (0) |
| Mean number of days, n(%) | 0 (0) | 0 (0) | 1 day | 3.5 days | 2 days | 0 (0) |
| p-value | NA | 0.51 | 0.53 | |||
Table 6: Hospitalization history in LYFE vs SOC from baseline to 6-month follow-up in patients with CAD and/or ACS who underwent PCI.
The present study was a unique blend of integration of digital therapeutics and comprehensive monitoring in managing post-PCI CAD and/or ACS patients. This study explores how a personalized digital heart care program can enhance patient outcomes. The LYFE app includes multiple components such as medication reminders, lifestyle modification tracking and emergency response systems, which is a significant advancement over standard care practices. Notable improvements were observed in physical fitness, feelings, daily activities, social activities, pain, changes in health, overall health, and QoL, with all differences being statistically significant (p<0.050). The LYFE group showed superior adherence to exercise and dietary recommendations, as well as the medications, all statistically significant (p<0.050). Hospitalization rates were similar between the LYFE and SOC groups by the six-month follow-up, although the LYFE group showed a lower percentage of hospitalizations at the three-month mark. These findings suggest that integrating the LYFE app with standard care offers substantial benefits in managing cardiovascular health, enhancing QoL, improving medication adherence, and achieving better blood pressure control.
Maintaining suboptimal blood pressure levels are important for secondary prevention of cardiovascular complications. A meta-analysis of 48 randomized trials demonstrated that a 5 mmHg decrease in SBP was associated with approximately a 10% reduction in the risk of major cardiovascular events. These results indicate that a consistent level of pharmacological blood pressure reduction is effective for both primary and secondary prevention of major cardiovascular diseases [18]. A study by Volpi et al., found that the use of mHealth apps led to 92% of patients being fully adherent, 8% being partially adherent, and none being non-adherent (p<0.001) [19]. The study highlighted that mHealth apps can significantly empower patients to manage their health and improve adherence to hypertension treatment, particularly when the app delivers a positive user experience [19]. This was in line with the present study where at 6 months, blood pressure control was significantly higher in the LYFE group (98.0%) compared to the SOC group (63.2%), p<0.050.
A clinical consensus statement from the European Association of Preventive Cardiology states that adequate treatment adherence is important for enhancing outcomes in patients with CVD or those at high risk, as it helps reduce morbidity, mortality, and the financial burden associated with rehospitalizations [20]. A systematic review by Perez-Jover et al., found that mobile apps were both user-friendly and effective for medication management [21]. Patients expressed high satisfaction with these apps, giving them an average rating of 8.1 out of 10. A meta-analysis by Al-Arkee et al., demonstrated statistically significant improvements in medication adherence rates, with an overall effect favoring the app intervention (mean difference of 0.90, p<0.050) [22]. These results were consistent with the present study, where the LYFE app significantly improved medication adherence, as evidenced by 92.5% of LYFE users reporting 'Never or rarely' having difficulty remembering to take their medications, compared to 50.0% in the SOC group (p<0.05)
Nutrition and exercise also significantly influence the development and progression of CVD, likely through increasing inflammatory markers and traditional coronary risk factors [23,24]. LYFE app showed promising results in improving the adherence among patients towards diet and exercise regimen, thus promoting optimal secondary prevention. This can be further reinforced by the fact that the LYFE group showed superior adherence to exercise and dietary recommendations, with 98.0% of patients adhering to their exercise regimen and diet, compared to 47.4% and 50.0% in the SOC group, respectively.
Personality traits such as anxiety, anger, and hostility may affect outcomes in CVD patients, with most studies showing that high levels of anxiety, hostility, and anger are associated with a greater risk of CVD, recurrent cardiovascular events, and worse overall outcomes [23,24]. In this study it was observed that LYFE can help in curbing such emotional distress, as the mean differences in the Dartmouth COOP scale were significantly greater in the LYFE group compared to the SOC group in parameters like: Feelings (-1.9 vs. -0.9), social activities (-1.9 vs. -0.8), pain (-1.9 vs. -0.8), changes in health (-1.6 vs. -0.4), overall health (-1.9 vs. -0.6), and QoL (-1.8 vs. -0.6), with all differences being statistically significant (p<0.05).
The aforementioned literature and clinical insights from this study indicate that digital therapeutics create a valuable pathway for cardiovascular patients, enabling a transition to improved QoL as an alternative to traditional face-to-face cardiac rehabilitation programs [25, 26].
The study demonstrates that integrating the LYFE mobile health application with SOC significantly improves patient outcomes compared to SOC alone in post-PCI patients with CAD and/or ACS. The LYFE app enhanced adherence to medication, physical exercise, and dietary recommendations, which contributed to better management of cardiovascular health. Notably, patients using the LYFE app showed greater improvements in various QoL domains as measured by the Dartmouth COOP Questionnaire. Overall, the LYFE app represents a promising advancement in digital therapeutics, offering a comprehensive approach to patient care that integrates medication management, lifestyle monitoring, and emergency support, which could lead to improved cardiovascular outcomes and enhanced patient QoL.
The study is conducted at a single center, which may limit the generalizability of the findings to broader populations or different healthcare settings. The 6-month follow-up may be insufficient to fully capture the long-term benefits and potential adverse effects of using the LYFE app. With only 86 patients, the sample size is relatively small, which could affect the robustness of the findings and the statistical power of the analyses. There were dropouts in both groups (particularly notable in the SOC group), which could introduce bias and affect the interpretation of the outcomes.
The study demonstrates that the LYFE app significantly improves medication adherence, physical exercise, and diet adherence compared to SOC alone, which are important for patient management post-PCI. LYFE users reported better well-being across multiple domains as measured by the Dartmouth COOP questionnaire, indicating potential benefits in QoL.
Future research should involve multiple centers to validate the findings across different settings and populations, enhancing the external validity of the results. Extended follow-up periods are needed to evaluate the long-term efficacy and safety of digital therapeutics like LYFE in managing cardiovascular health. Further studies could compare LYFE with other digital health interventions or hybrid models combining digital tools with traditional care to assess relative benefits. Future research should evaluate the cost-effectiveness of the LYFE app compared to SOC alone, considering potential economic benefits and healthcare savings.
The authors would like to thank the study investigators, staff, and all participants who took part in the study. Medical writing support Awas provided by Dr. Madhura Donde, Alpha MD Pvt Ltd, Mumbai.
Dr. Anil Potdar: Review and editing (equal);
Dr. Chetan Gharat: Conceptualization (lead); writing–original draft (lead); formal analysis (lead); writing–review and editing (equal);
Dr. Kamlesh Patel: Software (lead); writing–review and editing (equal);
Dr. Nikhil Chougule: Methodology (lead); writing–review and editing (equal).
I confirm that the research study was approved by the Institutional Ethics Committee (ECR/233/Indt/GJ/2015/RR-21).
The data generated and/or analyzed during the trial are available from the corresponding author upon request.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref]
Citation: Potdar A, Gharat C, Patel K, Chougule N (2024). Evaluation of the Effectiveness of an Individualized Smartphone Application-Based Standard of Care Versus Standard Care in CAD and/or ACS Patients Who Underwent PCI: A Real-World Evidence Prospective Study. J Res Dev. 12.277.
Received: 08-Nov-2024, Manuscript No. JRD-24-35055; Editor assigned: 11-Nov-2024, Pre QC No. JRD-24-35055 (PQ); Reviewed: 26-Nov-2024, QC No. JRD-24-35055; Revised: 03-Dec-2024, Manuscript No. JRD-24-35055 (R); Published: 10-Dec-2024 , DOI: 10.35248/2311-3278.24.12.277
Copyright: © 2024 Potdar A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.