Research Article - (2018) Volume 4, Issue 1
Evaluation of the Effects of Stressful Life on Human Skin Microbiota
*Corresponding Author: Pierre-Yves Morvan, CODIF Technologie Naturelle, La Poultiere BP1, 35610 Roz-sur-Couesnon, France Email:
Abstract
Background: Due to its immune system, the skin constitutes the first barrier against environmental attack such as chemical and physical agents or bacteria. The bacteria, viruses, archaea and fungi present on the superficial layers of the skin correspond to the cutaneous microbiota. Its composition is crucial to the balance of the immune system. It has already been shown that the composition of the microbiota affects the development of diseases such as atopic dermatitis (since an increase in Staphylococcus aureus has been described), but also diabetes and obesity. This microbiota imbalance (or dysbiosis) is mainly related to individual factors (age), diet, environmental (climate) and behavioral factors (hygiene, consumption of antibiotics).
Aim: In our study, we are more interested in the effect of a stressful lifestyle on skin microbiota, and more especially on skin bacteria.
Methods: We studied the skin microbiota from the faces of 70 healthy human subjects (aged 25 to 45 years). Firstly, we worked with 2 groups of 20 volunteers selected according to their stress level, using a validated stress score evaluation, known as the Perceived Stress Scale (PSS). Secondly, we tested the effect of a topical treatment on the skin microflora of a group of 30 volunteers who displayed a high stress index (PSS>27).
Results: We identified a bacterial signature of stressed individuals in comparison to unstressed individuals in term of richness and diversity. We also identified some species that are more prevalent in stressed individuals, especially acidic and anaerobic bacteria, in relation to modified skin parameters (decreased skin pH, increased redness and a higher level of blemishes). We then identified some benefits to the skin microbiota of stressed individuals from a topical treatment, with an improvement in skin parameters (increased pH, reduced redness and fewer blemishes).
Conclusion: This original study on healthy human skin microbiota will serve to direct future research addressing the role of skin microbiota in healthy people, and metagenomic projects addressing the complex physiological interactions between the skin and the microbes that populate this environment.
Keywords: Microbiota; Skin; Stress; Bacteria; Diversity; Imbalance
Introduction
What is the skin microbiota? The symbiosis between micro-organisms and humans developed during evolution at two interfaces: an internal interface which corresponds to our intestines and an external interface which corresponds to our skin (human/environment interface). The skin is the more superficial sheath of our organism. In contact with the external environment, it protects our organism from dehydration and solar radiation. It consists of several layers or strata. We have on the surface of the skin, in intimate connection with the stratum corneum, an innate stratum of microorganisms referred to as the microbiota or sometimes the stratum microbium [1]. The microbiota is made up of all the micro-organisms living in an environment and the microbiome consists of all the genetic material originating from the micro-organisms that live in that environment [2]. The microbiota constitutes an ecosystem of 1,000 billion bacteria, with up to 1 million bacteria per square cm of human skin. We count at least 19 different phylla with 4 major phylla (Actinobacteria, Firmicutes, Proteobacteria and Bacteroidetes), 3 main genera (Corynebacterium, Propionibacterium, Staphylococcus) and more than 500 different species. All of the micro-organisms hosted by the skin constitute the skin microbiota.
What is the function of skin microbiota? It ensures the health and homeostasis of the skin. Microbiota communities on the skin contribute to host immune defence through a variety of mechanisms [3]. Some of those mechanisms inhibit the growth of pathogens by occupying space and producing bactericidal compounds, educating adaptive immunity by tuning local cytokine production and influencing lymphocytes in the epidermis, and by enhancing innate immunity by increasing production of anti-microbial peptides (which decrease inflammatory injury and strengthen the epidermal barrier).
How to study skin microbiota? DNA High Throughput Sequencing has opened a new field of investigation during the past decade. Using this technology, we are able to reveal the global picture of the bacteria living on human skin and the specific skin microbiota fingerprint of every human being. The global picture highlights the variety of bacterial phyla and a specific picture highlights the diversity of bacterial genera present. Each microbiota is unique. Researchers from the Harvard T.H. Chan School of Public Health and colleagues demonstrated that human microbiota has the potential to uniquely identify individuals, much like a fingerprint [4]. In general, the diversity of the cutaneous microbiota is considered to be an advantage because a diversified ecosystem is more resilient than a poorly diversified population [5].
Is the skin microbiota stable? Skin microbiota is determined from birth and varies depending on age, environment, nutrition and body area. Every day, under exposure to UV, pollution or even stress, the equilibrium of the microbiota is threatened and can be disrupted, leading to an imbalance called dysbiosis, which can cause skin disorders or even pathological conditions [5]. Up to now, there has been no data available as to the effects of a stressful lifestyle on the skin microbiota and no information about the relationship between skin microbiota and skin quality.
In a first study, we evaluated the impact of continuous stress on the cutaneous flora of healthy volunteers characterized as being in a state of continuous stress, in comparison with a control group (not characterized by a state of continuous stress). Then, we studied the skin condition and cutaneous flora of stressed women before and after application of a cream containing a combination of marine ingredients (CMI). This CMI is a combination of different components designed to provide a variety of nutrients to ensure the biodiversity and homeostasis of the skin microbiota. It contains sugars and polyols, sources of organic carbon which is the most important constituent of bacteria. Therefore they provide a source of energy for the cutaneous flora. The preparation also contains peptides of sizes between 200 and 3,000 Daltons which correspond to a source of organic nitrogen that provides amino acids for bacterial growth and protein synthesis. It also contains a marine exopolysaccharide (EPS) known for its involvement in intercellular communication and its protective properties against environmental stress. In nature, this biofilm maintains stable environmental conditions by protecting marine bacteria against desiccation. Finally the preparation contains minerals and trace elements involved in many metabolic pathways. This diversity of minerals supports the development of as many different species as possible, with conventional or more specific nutritional needs. In this paper, we will describe the main characteristics of the skin microbiota (species and number) of the stressed group compared to the unstressed group. We will subsequently present the effects of a CMI topical skin treatment applied for 8 days on the skin microbiota and skin parameters of a stressed group.
Materials and Methods
Products
Two formulations were tested per subject in the second study (one product per cheek), one formulation containing the CMI at 1%, and the other consisting of the same formulation without CMI (placebo control). CMI is a combination of 4 marine ingredients containing 1. a solution of an exopolysaccharide obtained by fermentation from a marine planktonic microorganism; 2. an extract of the brown alga Laminaria digitata obtained by lixiviation, filtration and reverse osmosis; 3. an enzymatic extract of the green microalga Chlorella vulgaris; and 4. a marine spring water naturally rich in iron, manganese, zinc and lithium. All the components of CMI are produced by Codif Technologie Naturelle (France). The ratio between each ingredient was previously determined by in vitro testing on bacterial growth (not described in this article). The composition of the 2 formulas is shown in Table 1. Briefly, phase A was heated at 80°C, phase B was heated at 80°C under an emulsifier at 600 rpm, then phase C was added in B under an emulsifier at 1,500 rpm for 15 min. Finally, phase D was added to phases B and C under 2,500 rpm for 15 min. Phase A was emulsified in BCD under 2,500 rpm for 5 min. Phase E was added under 2,500 rpm for 10 min. Phase F was added under 2,500 rpm for 10 min. The cream was cooled with gentle stirring at 35°C and phase G (CMI) was added in the “formula with CMI”, and not in “placebo formula”. Agitation was maintained for 15 min before the obtained creams were conditioned in tubes. The products were applied on a randomized half-face, by the volunteers, twice a day (morning and evening) for 1 week. The subjects had to use a sufficient quantity of cream during each application, as for their day cream.
| Formula with CMI | Placebo Formula | |
|---|---|---|
| A | Cetearyl isononanoate | Cetearyl isononanoate |
| A | Phenoxyethanol | Phenoxyethanol |
| B | Aqua | Aqua |
| B | Propylene glycol | Propylene glycol |
| B | Chlorphenesin | Chlorphenesin |
| C | Acrylates/C10-30 alkyl acrylate crosspolymer | Acrylates/C10-30 alkyl acrylate crosspolymer |
| D | Sodium polyacrylate | Sodium polyacrylate |
| E | Polyacrylate-13, polyisobutene, polysorbate 20, sorbitan isostearate, aqua | Polyacrylate-13, polyisobutene, polysorbate 20, sorbitan isostearate, aqua |
| F | Aqua, sodium hydroxyde | Aqua, sodium hydroxyde |
| G | Aqua, maris aqua, glycerin, laminaria digitata extract, phneoxyethanol, chlorella vulgaris extract, saccharide isomerate, ethylhexylglycerin | ‒–‒ |
Table 1: INCI listings of the 2 formulations investigated.
Panel
In the first study, two groups of volunteers between the ages of 28 and 44 were assessed: a first group of 22 stressed volunteers and a second group of 22 unstressed volunteers. A group of 30 stressed volunteers aged between 20 and 40 years were included in the second study. All the volunteers were healthy females with phototype II to IV and hormonally active. The inclusion criteria specific to the group of "stressed women" were women with a stressful lifestyle (young children, very busy and/or responsible work), believed to have damaged skin and with a Perceived Stress Scale (PSS-14) score>27. The inclusion criteria specific to the group of "unstressed women" were women not experiencing a stressful lifestyle (not working, no or few children or with older children), believed not to have damaged skin and with a PSS-14 score<21. All groups were recruited using a stress questionnaire. The Perceived Stress Scale (PSS) is a scientifically validated scale developed by Sheldon Cohen [6]. It is the most widely used instrument for measuring perception of stress. It is a measure of the degree to which situations in one’s life are appraised as stressful. Fourteen questions were asked of the volunteers who responded using a scale of 1 to 5: 0=never, 1=almost never, 2=sometimes, 3=fairly often and 4=very often.
First study design
Each subject made 2 visits: a pre-inclusion visit (V0), and an inclusion and experimental session (V1). A 48 h wash-out period was observed between the inclusion visit (V0) and the experimental visit (V1). During the first visit (V0), the study coordinator collected demographic and anthropometric data, including age, height and body weight, information about lifestyle, eating habits, level of physical activity and professional activity and asked the subject to fill-in the PSS questionnaire (PSS score=32.6 ± 4.4 for the stressed group and PSS score=14.5 ± 5.4 for the unstressed group, statistically different with p<0.001). During the second visit (V1), volunteers filled in questionnaires to evaluate the quality of their skin using a Visual Analogue Scale (VAS). Skin pH was measured and the skin flora was sampled as described below.
Second study design
This was a randomized, double-blind, placebo-controlled, cross-over, mono centric pilot study. Each subject made 3 visits: a pre-inclusion visit (V0), an inclusion and randomization visit and experimental session (V1), and then an experimental session and end-of-study visit (V2). A 48 h wash-out period was observed between visit V0 and visit V1. During the screening visit (V0), the study coordinator collected demographic and anthropometric data, including age, height and body weight, information about lifestyle, eating habits, level of physical activity and professional activity and asked the subject to fill-in the PSS questionnaire (PSS score=33.8 ± 4.4, n=30). He also gave each subject specific instructions to be followed. During the first experimental session (V1) and the last visit (V2), volunteers filled in questionnaires to evaluate the skin quality and a skin evaluation was performed by the clinician using a Visual Analogue Scale. The pH and redness was measured and cutaneous flora samples were taken from both cheeks, as described below.
Cutaneous sampling and study of the cutaneous flora
Before sampling, the subject was placed in a room for 5 to 10 min under controlled conditions of humidity and temperature (humidity 40% to 60%, temperature 20°C to 22°C). The cutaneous flora were sampled from the cheeks (4 cm2 per sample) using a calibrated method of collection and a non-invasive method of “swabbing”. Bacterial DNA from the biological samples was then extracted using a protocol with double lysis (mechanical by bead-beating and chemical). The resulting DNA solutions were then quantified by fluorimetry. Metagenomic analysis was conducted on a fragment of a sequence amplified by PCR (V3-V4 fragment coding for the 16S ribosomal RNA) using a MiSeq sequencing analysis system from Illumina. Finally, taxonomic classification of resulting sequences was generated using a dedicated bioinformatics pipeline based on the MOTHUR software [7].
Skin pH and redness measurements
Skin pH measurement was performed on cheeks by a qualified technician using a pH-Meter® PH905 probe. Skin redness was evaluated by a qualified technician using a Colorimeter® CL400 probe. All measurements were made under controlled conditions of humidity and temperature (humidity 40% to 60%, temperature 20°C to 22°C).
Volunteer questionnaire
A questionnaire was filled in to assess the condition of the skin, using a Visual Analogue Scale (VAS) from 0 to 100. Four parameters were evaluated by the volunteers: redness of the skin (from “not at all red” to “very red”), uniformity of the complexion (from “not at all uniform” to “very uniform complexion”), health of the skin (from "not at all healthy" to "very healthy"), beauty of the skin (from "my skin is not at all beautiful" to "my skin is very beautiful").
Clinician questionnaire
A questionnaire was completed to assess the condition of the skin, using a Visual Analogue Scale (VAS) from 0 to 100. Two parameters were evaluated by the clinician: redness of the skin (from “not at all red” to “very red”) and cutaneous blemishes (from “no blemishes” to “many blemishes”).
Statistical analysis
For all statistical tests, the 0.05 level of significance was used to justify a claim of a statistically significant effect. Statistical analyses were performed using SAS® software version 9.3 or higher (SAS Institute Inc., Cary, NC, USA). Clinical endpoints (pH, redness and uniformity of complexion), were analyzed using an analysis of variance (ANOVA) model for repeated measurements (SAS® PROC MIXED, statistical model n°1).
Results
Effects of a stressful lifestyle on skin microbiota (Study 1)
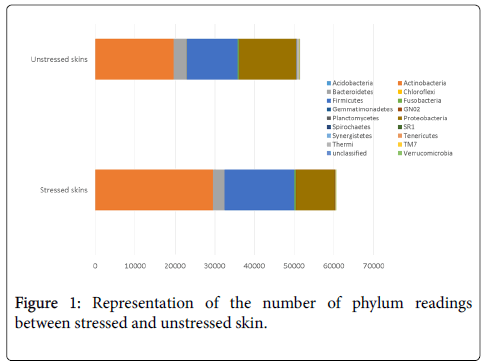
Phyla analysis provides the most global overview we can have of our skin microbiota. And it appears very clearly that continuous psychological stress imbalances skin microbiota. Figure 1 shows that the total number of Actinobacteria and Firmicutes increases in the stressed group compared with unstressed group, and the total number of Bacteroidetes and Proteobacteria decreases in the stressed group versus the unstressed group. The relative abundance of the skin bacteria is also higher in the stressed group than the unstressed group for Actinobacteria (49.41% vs. 42.09%) and Firmicutes (28.99% vs. 26.05%); it is lower for Proteobacteria (15.57% vs. 24.09%) and Bacteroidetes (4.55% vs. 5.40%). The other phyla decrease which means a drop in diversity. Therefore a stressful lifestyle seems to directly impact the homeostasis of the skin microbiota.
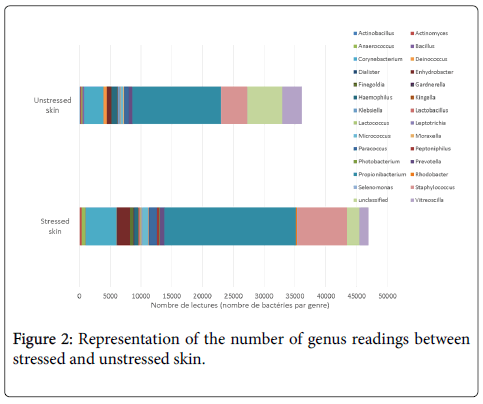
Study of the most differentiating genera shows that at least 30 genera approximately are significantly impacted by stress. Figure 2 shows that the total numbers of Corynebacterium, Propionibacterium and Staphylococcus are increased, and unclassified genera are decreased. The relative abundance is also higher in the stressed group than the unstressed group for Corynebacterium (8.46% vs. 6.05%), for Propionibacterium (36.22% vs. 31.72%) and for Staphylococcus (14.09% vs. 9.19%). The relative abundance for unclassified genera is lower in the stressed group than the unstressed group (3.16% vs. 8.21%). Due to these differences, we can conclude that the microbiota imprint of stressed volunteers is different to unstressed volunteers.
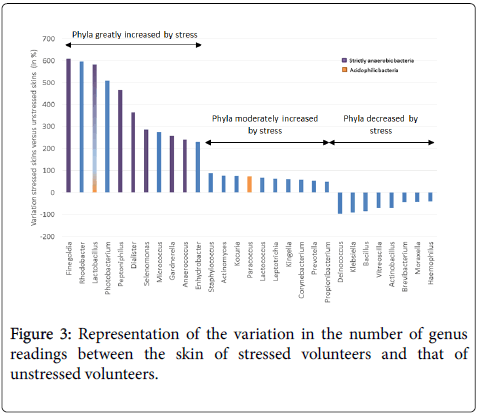
Moreover, based on the most differentiating genera, it appears that stressed skins are characterized by 2 main changes: an increase of 7 bacteria able to survive in strict anaerobic conditions (without O2) and an increase of 2 acidophilic bacteria (Figure 3). For example, Finegoldia, an anaerobic genus potentially pathogenic for the skin, increased by 607% with stress (603 for stressed group vs. 85 for unstressed group). The 3 anaerobic bacteria Peptoniphilus, Dialister (which can lead to infections) and Gardnerella (which becomes pathogenic when associated with anaerobic flora) increased by 470%, 361% and 256%, respectively. They can affect the health of the skin by causing development of symptoms linked to the activation of skin defence systems, thus inducing inflammation. The 2 genera producing lactic acid, Lactobacillus and Lactococcus, increased with stress by 590% and 67%, respectively. This increase indicates acidification of the surface of the skin. By contrast, a decrease in Deinococcus (Thermi) was observed in the stressed group versus the unstressed group (15 vs. 552). This species is known to be resistant to UV and gamma rays, and can therefore decrease this resistance leading to inflammation related symptoms.
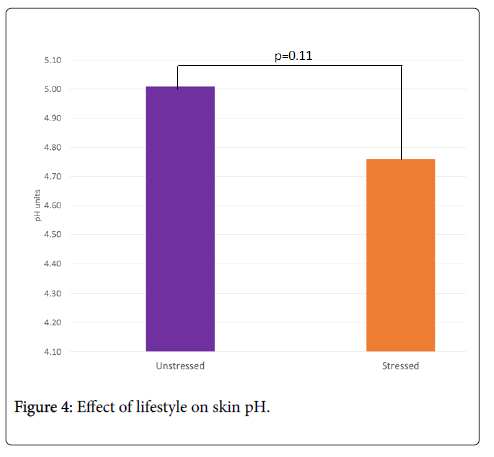
Stress tends to decrease skin pH compared to unstressed skin: 4.76 vs. 5.01, i.e. -5%, p=0.11 (Figure 4). The decrease in cutaneous pH in the stressed group may be related to the presence of acidophilic bacterial flora and a decrease in microbiota diversity. It has been postulated that the greater diversity of microbiota in women versus men may be related to a less acidic pH [8].
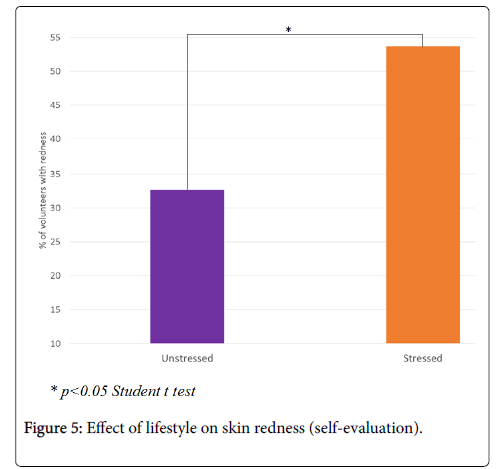
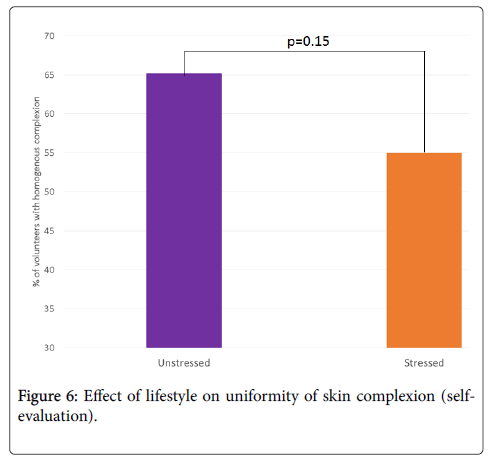
According to the volunteers, the appearance of skin redness is significantly greater in the stressed group compared to the unstressed group: 53.7 vs. 32.7, i.e. +64%, p<0.05 (Figure 5). Moreover, skin complexion in the stressed group tends to be less uniform than in the unstressed group: 55.0 vs. 65.2, i.e. -16%, p=0.15 (Figure 6).
Effect of treatment with CMI on skin microbiota (Study 2)
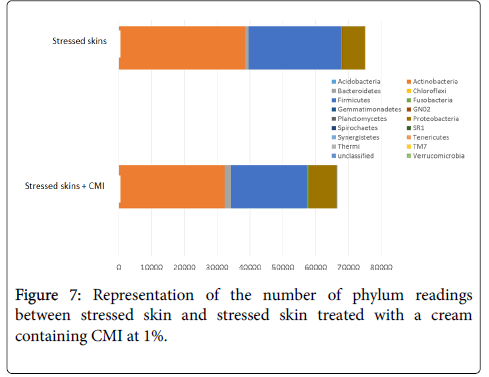
Within just 1 week, treatment with a cream containing CMI decreased Actinobacteria and Firmicutes that had previously been increased by stress and enhanced Proteobacteria and Bacteroidetes that had been diminished by stress (Figure 7).
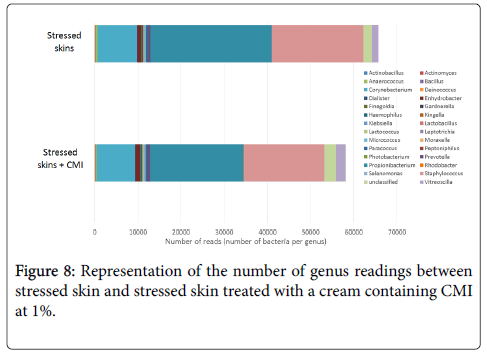
After 1 week of twice-daily application of the cream containing CMI at 1%, the effect of stress on genera also begins to be reversed. Treatment with the cream containing CMI decreased the 3 main genera increased by stress, i.e. Corynebacterium, Propionibacterium and Staphylococcus (Figure 8). In addition, it increased unclassified genera, which reflects an increase in microbial diversity.
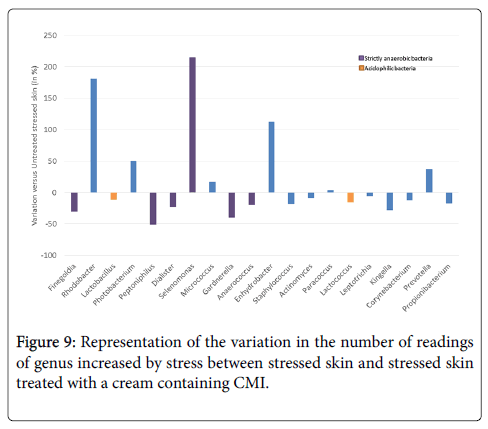
Treatment with the cream containing CMI tends to reverse the effect of stress for most genera: 15 genera were reduced among the 20 genera increased by the stress, i.e. 65% of them were diminished (Figure 9). The few genera for which the effect of stress is not reversed are not pathogenic, such as Rhodobacter, an aquatic and photosynthetic bacterium; Photobacteria, a marine bacteria; Selenomonas and Enhydrobacter which have no known pathogenic potential.
The main genera that we described as being increased by stress are markedly reduced by treatment with the cream containing 1% CMI (Table 2). Some genera were also decreased by the placebo cream (such as Finegoldia and Peptoniphilus), but some genera were not affected by the placebo (such as Dialister) or were even increased by the placebo (such as Gardnerella and Lactococcus).
| Genus | Well known for… | Effect of the cream containing CMI at 1% | Effect of the placebo cream |
|---|---|---|---|
| Genus increased by stress & potentially linked to inflammation | |||
| Finegoldia | Potentially pathogenic for the skin | -30% | -36% |
| Peptoniphilus | Can lead to infections | -51% | -47% |
| Dialister | -23% | 0% | |
| Gardnerella | Becomes pathogenic when associated with anaerobic flora | -40% | +133% |
| Genus increased by stress & potentially linked to acidification of skin pH | |||
| Lactobacillus | Produces lactic acid | -12% | -4% |
| Lactococcus | Metabolizes sugars in lactic acid | -16% | +45% |
| Genus decreased by stress & potentially linked to inflammation | |||
| Deinococcus | Resistance to UV and gamma rays. | +400% | +100% |
Table 2: Effect of treatment with cream containing CMI at 1% or placebo cream on some genera modified by stress.
In these last 2 cases, CMI is actually active on these stress-related genera. In addition, treatment with the cream containing CMI re-enhances stress-reduced genera (such as Deinococcus), and this effect is greater than with the placebo cream.
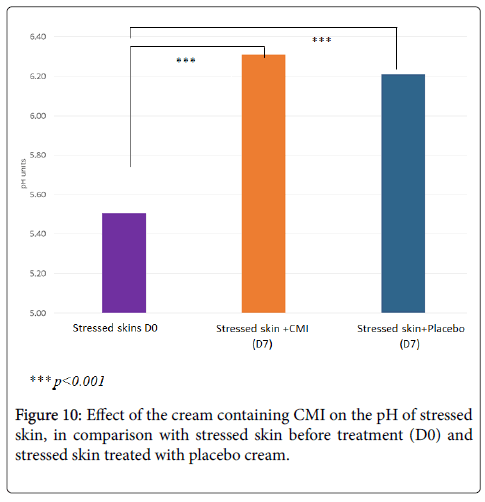
Treatment with the cream containing 1% CMI significantly increases cutaneous pH decreased by stress (+13%, p<0.001) (Figure 10). Its effect is slightly greater than that of the placebo cream (+11%, p<0.001).
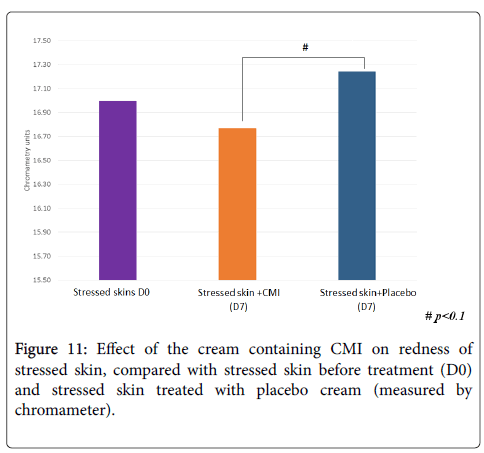
Treatment with the cream containing CMI tends to decrease redness previously accentuated by stress while the placebo tended to increase it (Figure 11). The difference between the product and the placebo is within significant limits (p<0.1). Therefore treatment with CMI reduces redness versus the placebo.
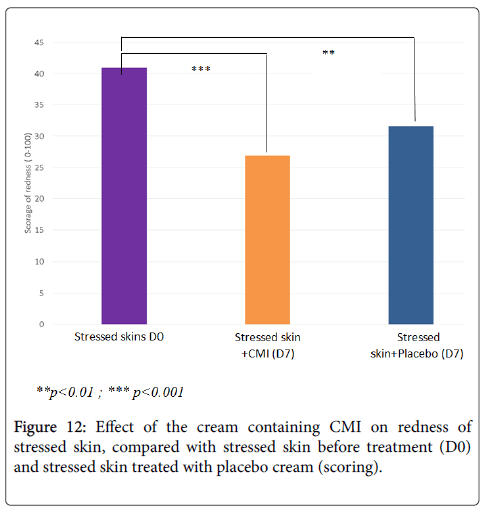
The clinical scoring performed by the beautician confirms that treatment with the cream containing CMI at 1% significantly reduces redness (-34%, p<0.001) accentuated by stress (Figure 12). Its effect is superior to that of the placebo cream (-23%, p<0.01).
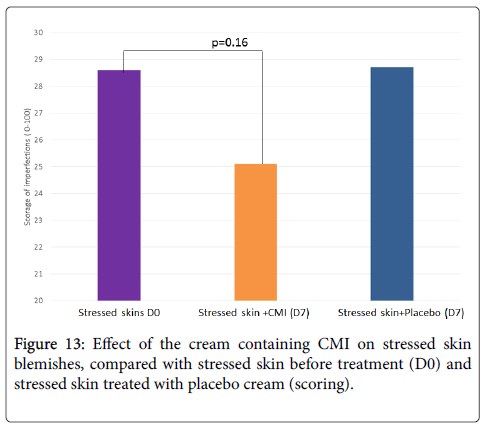
Moreover, treatment with the cream containing 1% CMI tends to reduce skin blemishes (-12%, p=0.16) while the placebo has no effect (Figure 13). Therefore treatment with CMI decreases visible redness and skin blemishes within 1 week.
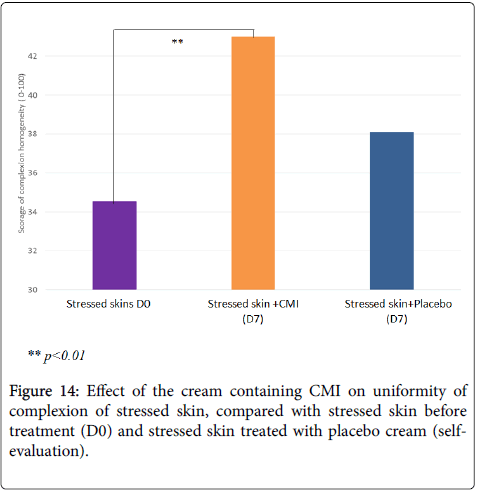
According to the volunteers, treatment with the cream containing CMI at 1% significantly improves the uniformity of the skin complexion (+24%, p<0.01) while the placebo cream has no effect (Figure 14). It also tends to improve the health of the skin (+13%, p<0.1), and the beauty of the skin (+14%, p=0.1), while the placebo has no effect on these 2 parameters (data not shown).
Conclusion
Interpersonal variation was important and confirmed a high degree of complexity in human skin microbiota. A busy lifestyle unbalances the skin’s microbiota, reducing its diversity and increasing acidophilic and anaerobic bacteria. This imbalance of the microbiota is associated with imbalances of the skin such as a lower skin pH, increased redness and more blemishes. Our combination of marine ingredients (CMI) rebalances skin microflora in 7 days. It provides a healthy and balanced diet for skin microbiota, and rebalances skin microbiota disturbed by a busy lifestyle. It reduces anaerobic and acidophilic bacteria potentially involved in skin inflammation, rebalances skin pH and improves the uniform appearance of the complexion. Finally, CMI treats skin disorders by reducing redness and blemishes. The treated volunteers observed a global improvement in the health and the beauty of their skin.
By reversing dysbiosis, CMI creates a new equilibrium previously disturbed by stress.
References
- Grice EA, Kong HH, Renaud G, Young AC, Bouffard GG, et al. (2008) A diversity profile of the human skin microbiota. Genome Res 18: 1043-1050.
- Chen YE, Tsao H (2013) The skin microbiome: current perspectives and future challenges. J Am Acad Dermatol 69: 143–155.
- Sanford JA, Gallo RL (2013) Functions of the skin microbiota in health and disease. Semin Immunol 25: 370-377.
- Franzosa EA, Huang K, Meadow JF, Gevers D, Lemon KP, et al. (2015) Identifying personal microbiomes using metagenomic codes. Proc Natl Acad Sci 112: E2930-E2938.
- Schommer NN, Gallo RL (2013) Structure and function of the human skin microbiome. Trends Microbiol 21: 660–668.
- Cohen S, Kamarck T, Mermelstein R (1983) A global measure of perceived stress. J Health Soc Behav 24: 385-396.
- Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl Environ Microbiol 75: 7537–7541.
- Giacomoni PU, Mammone T, Teri M (2009) Gender-linked differences in human skin. J Dermatol Sci 55: 144-149.
Copyright: © 2017 Morvan PY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.