
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2023)Volume 6, Issue 3
Background: The association between Hypertension (HTN) and type 2 Diabetes Mellitus (DM) frequently leads to left ventricular Diastolic Dysfunction (DD).
Objective: The main objective of our study was to diagnose Left Ventricular Diastolic Dysfunction (LVDD) in hypertensive and diabetic patients with normal transthoracic echocardiography.
Methods: We aim to test whether DD can readily be unveiled as early as in the subclinical stage in diabetic hypertensive asymptomatic patients, even before echocardiography can do so. We compared the values of NT-pro BNP (as a marker of increased filling pressures) before and after the treadmill stress test, in hypertensive patients with and without Diabetes Mellitus (DM) and normal subjects. All had normal systolic and diastolic functions at rest and after the stress test, according to the recommendations of the ESC.
Results: The results from our study showed a significant increase in NT-pro BNP after the stress test, but only in hypertensive patients with diabetes.
Conclusion: Compared with echocardiography, measuring the changes in NT-pro BNP after the stress test in hypertensive and diabetic patients with class A heart failure, could be a tool for diagnosing DD much earlier in the evolution of the disease. This is an important finding because these patients are difficult to distinguish from those with normal left ventricle function, based only on resting NT-pro BNP or echocardiography. In this way, they can benefit much earlier from specific therapies to mitigate future cardiovascular events.
Subclinical diastolic dysfunction; Exercise-induced diastolic dysfunction; Natriuretic peptides; NT-pro BNP; Diabetes mellitus; Hypertension
HPT: Hypertension; DM: Diabetes Mellitus; DD: Diastolic Dysfunction; ESC: European Society of Cardiology; NP: Natriuretic Peptide; NT pro-BNP: N terminal pro-Brain Natriuretic Peptide; BNP: Brain Natriuretic Peptide; ECG: Electrocardiogram; BMI: Body Mass Index; eGFR: estimated Glomerular Filtration Rate; LVDD: Left Ventricular Diastolic Dysfunction; HBP: High Blood Pressure
Cardiovascular diseases represent the main complications that account for the high morbidity and mortality seen in patients with type 2 diabetes, with up to 9 million deaths from 2000 to 2009. For a long period of time in the history of the disease, they remain asymptomatic and consequently diagnosed late [1].
Elevated levels of BNP and NT-pro BNP are markers of increased parietal stress. They are used in diagnosing and tailoring the management of congestive heart failure [2]. The ESC guidelines included these biomarkers in the risk stratification for evolution and complications in heart failure patients [3].
The association between exercise and the release of NP (BNP and NT pro-BNP) has already been proven in patients with and without cardiovascular diseases [4,5]. Moreover, elevated NT-pro BNP levels were identified postexercise in hypertensive patients with left ventricular hypertrophy [6].
We performed an observational study with Prof. Dr. Th. Burghele's Hospital, Bucharest, Romania. The study was approved by the ethics committee of the institution.
We included 45 subjects, divided into three groups: Group 1 with 15 healthy subjects; Group 2 with 15 hypertensive patients and Group 3 with 15 hypertensive and diabetic patients.
The patients were more than 5-years old and had controlled hypertension and type 2 diabetes. They also had a normal ejection fraction and diastolic function (according to the recommendations of ESC) [3]. Those with known ischemic cardiovascular diseases, abnormal rest or stress ECG tests, left ventricle hypertrophy (increased left ventricle mass), atrial fibrillation, dilated cardiac chambers, moderate-severe valvulopathies, ejection fraction <50%, or moderate-severe chronic diseases (renal, lung or liver) were excluded. All patients included in the study signed informed consent. We measured the levels of NT-pro BNP three times: at rest, immediately after the stress test, and 24 h after the stress test. We also collected anthropometric data and serum creatinine to estimate GFR.
Statistical analysis
We performed the statistical analysis and graphics using Analyse-it software version 6.10 (Microsoft Excel add-on, Analyse-it Software, Leeds, United Kingdom). Due to the small cohort, continuous variables had a non-Gaussian distribution and are presented as medians and interquartile ranges. Categorical variables are presented as numbers and relative frequencies. For continuous variable comparisons between groups, we used nonparametric tests. For categorical variables, we used the chi-square test. We considered statistical significance at a p-value less than 0.05. We calculated BNP variability after the test as the difference between BNP at rest and BNP immediately after the stress test as a marker for the release of stored BNP. BNP variability 24 h after the test was the difference between BNP at rest and BNP 24 h after the stress test as a marker for the de novo production of BNP. We also computed BNP variability after the test and 24 h after the test.
Socio demographic status of the respondents
Cohort characteristics are presented in Table 1. We noted that patients with HBP and DM were older with a higher BMI and a lower eGFR, all of which were statistically significant. To clarify this presumed bias in our cohort, we tested the impact of age, BMI, and eGFR on BNP at rest (Table 2).
| Parameter | All (n=45) | Healthy (n=15) | HBP (n=15) | HBP+DM (n=15) | P value |
|---|---|---|---|---|---|
| Age | 51 (49; 54) | 49 (47.2; 50) | 52 (50; 54) | 54 (51; 57) | <0.01 |
| Male | 24 (53.3%) | 7 (46.66%) | 9 (60%) | 8 (53.33%) | 0.76 |
| BMI | 30 (28;31) | 28 (27.2; 29.8) | 29 (28; 31) | 31 (30; 32) | <0.01 |
| eGFR | 72 (68; 81) | 84 (78.5; 86.7) | 70 (69.2; 73.7) | 68 (62.5; 72) | <0.01 |
| BNP-1 | 115 (69; 157) | 115 (77; 137) | 61 (51; 119) | 174 (84; 186) | 0.016 |
| BNP-2 | 125 (81; 201) | 115 (63; 140) | 88 (61; 133) | 268 (195; 281) | <0.01 |
| BNP-3 | 120 (74; 176) | 118 (55; 147) | 88 (68; 109) | 197 (175; 284) | <0.01 |
| Variability BNP-2 | 16 (-2; 55) | 2 (-8.8; 14.3) | 7 (-10.7; 20.2) | 94 (51; 126.8) | <0.01 |
| Variability BNP-3 | 6 (-11; 46) | -5 (-23.8; 14.8) | -3 (-17.7; 14.3) | 92 (20.5; 127.7) | <0.01 |
| Variability after test and after 24h | 0 (-16; 37) | 12 (-28.7;39.5) | -2 (-8;23.8) | 0 (-67;83.5) | 0.9772 |
Table 1: Overall cohort characteristics and their distribution among the studied groups.
| Age | BMI | eGFR | ||
|---|---|---|---|---|
| BNP-1 (at rest) | R squared | 0.03 | 0.07 | 0.08 |
| P-value | 0.22 | 0.067 | 0.038 | |
Table 2: Correlation between BNP-1 and potential confounders (cohort).
The data from Table 2 show that although age, BMI and eGFR had different statistical significance, their influence on BNP-1 were different: age and BMI values were insignificant (R2<0,1), whereas eGFR correlated statistically but with a very low significance (p=0.046). Overall age had only a 3% influence, BMI had only a 7% influence and eGFR had only an 8% influence on BNP-1. However, when we analyzed the influence of the three parameters on the levels of BNP-1 in each group separately, we noticed that eGFR did have an influence of 29%, with good statistical significance (Table 3 and Figure 1).
| Group-1 | Group-2 | Group-3 | |
|---|---|---|---|
| BNP-1 | BNP-1 | BNP-1 | |
| eGFR | R2=0.005 | R2=0.107 | R2=0.291 |
| p=0.79 | p=0.23 | p=0.038 | |
| BMI | R2=0.028 | R2=0.001 | R2=0.054 |
| p=0.54 | p=0.90 | p=0.4 | |
| Age | R2=0.03 | R2=0.01 | R2=0.0137 |
| p=0.53 | p=0.98 | p=0.67 |
Table 3: Correlation between BNP-1 and age, BMI and eGFR in each group.
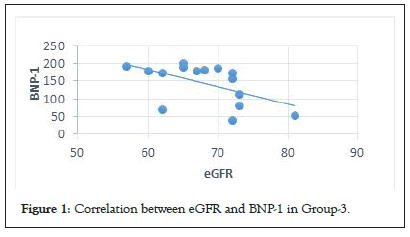
Figure 1: Correlation between eGFR and BNP-1 in Group-3.
We noticed that NT-pro BNP changes were significant after the stress test and persisted even after 24 hours only in patients with HBP and DM (Group-3). The amplitude of the variability in this group was 10 times higher than that in the other 2 groups (Figures 2a-2d).
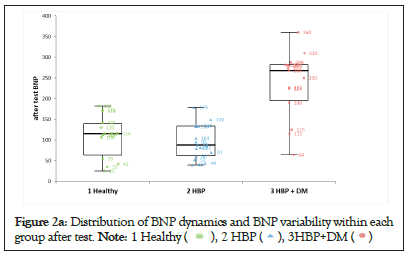
Figure 2a: Distribution of BNP dynamics and BNP variability within each
group after test. 
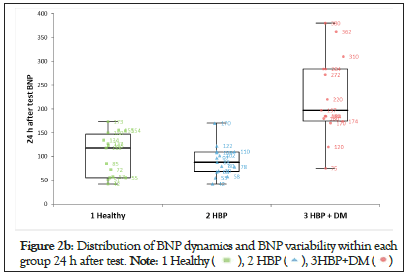
Figure 2b: Distribution of BNP dynamics and BNP variability within each
group 24 h after test. 
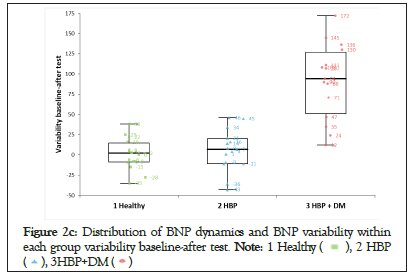
Figure 2c: Distribution of BNP dynamics and BNP variability within
each group variability baseline-after test. 
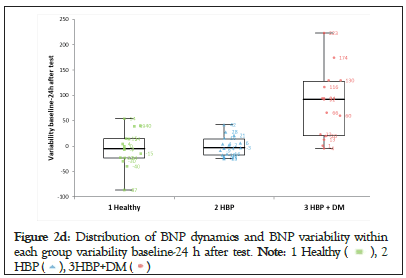
Figure 2d: Distribution of BNP dynamics and BNP variability within
each group variability baseline-24 h after test. 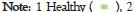
We applied the one-sample t-test for the mean of differences (Table 4). The statistics showed a significant change for the entire cohort, for both BNP-2 and BNP-3 compared with BNP-1. Once again, this result was intensely biased by the changes in Group-3. When the test was applied to each group separately, only the changes in NTpro BNP in Group-3 showed statistical significance for both BNP-2 and BNP-3. In the other two groups analyzed separately, there was no statistical significance.
| Differences | Cohort | Group 1 | Group 2 | Group 3 |
|---|---|---|---|---|
| BNP2-BNP1 | p<0.0001 | p=0.42 | p=0.20 | p<0.0001 |
| BNP3-BNP1 | p=0.002 | p=0.84 | p=0.40 | p=0.00013 |
Table 4: Statistical significance for the mean of differences.
Impairment of left ventricular diastolic performance in patients with normal systolic function is the first step in developing heart failure [3-5].
Alteration of left ventricular diastolic function and heart failure with preserved ejection fraction have been described in diabetic patients in the general population and hospitalized diabetic patients alike [6]. The same abnormalities exist in many other diseases, such as hypertension, left ventricle hypertrophy, stenotic valvular diseases and coronary disease. In all of these diseases, patients often present with exertional dyspnea. [7-11].
Many previous studies that addressed heart changes in diabetic and hypertensive patients used only the chocardiographic criteria recommended by the ESC for diagnosing DD, both at rest and exercise. Echocardiography proved helpful in identifying DD in many patients, but its sensitivity is less than 80% (the correct measurements depend on many factors). Even fewer studies used the gold standard method namely, catheterization. More importantly, all of them included patients already known for having DD, patients with dyspnea, or known cardiac diseases (stage B heart failure) [12-16].
Natriuretic Peptides (NP) are another means proposed by the ESC for evaluating ventricular filling pressures and DD [21,22]. They have been used in several studies with different types of patients [17,18]. Some of them concluded that the levels of NP at rest are suboptimal tests for identifying preclinical left ventricular dysfunction [19].
In contrast, when comparing the measurements of NT-pro BNP before and after exercise, Paun N and co. recently showed that they are a better means of diagnosing DD than echocardiography [5,6]. They demonstrated a higher sensitivity of NT-pro BNP changes after effort as a marker for increased diastolic filling pressures in asymptomatic hypertensive patients, especially those with left ventricle hypertrophy.
In the present study, we intended to see if, in the absence of left ventricular hypertrophy, hypertensive patients with type 2 DM can develop myocardial parietal stress after exercise, despite the normal systolic and diastolic echocardiographical indexes at rest and after the stress test (heart failure stage A).
The morphological changes induced by diabetes and hypertension within the myocardium in the earliest stages of the disease show increased variability. Hence, variable responses of the myocytes can be seen after stress [22,23]. The type and severity of these abnormalities needed to cause an increase in myocardial wall stress high enough to initiate the secretion of NP, is not well known. However, even before an increased left ventricular mass can be detected, microscopic abnormalities have been described to account for increased left ventricular stiffness.
There are patients in whom echocardiography is not able to identify mild changes in the left ventricular filling pressures. Therefore, we sought to diagnose DD as early as possible before echocardiography can identify it (before any structural or Doppler changes appear), by using the levels of NT-pro BNP as a marker of filling pressures.
We enrolled a cohort of 45 participants, of which 15 were healthy subjects and 30 were asymptomatic hypertensive patients (15 of whom had type 2 noninsulin-dependent DM) (Table 1). All of them had both normal ejection fraction (EF>50%) and normal measurements for diastolic function at rest and after exercise, demonstrating normal filling pressures (E/e' <10, LAVi<30 ml/m2, TR with velocity <2, 8 m/s). Both HTN and DM were older than five years and controlled by medication.
The participants were divided into three groups: Group 1 (healthy subjects), Group 2 (hypertensive patients) and Group 3 (hypertensive and diabetic patients); other cardiovascular, renal, lung or liver diseases were excluded. The values of NT-pro BNP were measured before the treadmill test (BNP-1 variables), within 15 minutes after the stress test (BNP-2 variables) and 24-30 hours after the test (BNP-3 variables). The BNP-2 values reflect the cytoplasmic deposits of NT-pro BNP ready to be released whenever myocardial wall stress increases; on the other hand, BNP-3 values reflect the de novo synthesis of NT-pro BNP, which requires a longer period of time to develop [24,25].
The values of BNP-1 were analyzed for the entire cohort, showing no statistically significant differences (p=0.016) (Table 1). However, in Group 3, the values were higher than those seen in the other two groups (mean values 174 pg/dl vs. 115 pg/dl vs. 61 pg/dl). To rule out a bias related to possible factors, different from those involved in causing myocardial wall stress, as a cause for these higher values even at rest, we looked into the relationship between NT-pro BNP values and other factors well-known for influencing the blood levels of NP, such as age, Body Mass Index (BMI), and estimated Glomerular Filtration Rate (eGFR). The statistics showed no relationship when all participants were taken into account (R2<0.01, Table 2). However, when we separately analyzed each group of patients, we noticed a significant negative correlation between BNP-1 and eGFR, only in Group 3 (Table 3 and Figure 1). Taken together, this observation with the fact that the echocardiographic indexes at rest were similar in all groups (within normal limits as we mentioned above), allowed us to consider that the higher values of BNP-1 in Group 3 were not induced by a subclinical DD present at rest, but rather in relation to eGFR.
The values of NT-pro BNP after the stress test for the entire cohort showed that BNP-2 and BNP-3 were significantly increasedcompared with BNP-1 (p<0.01) (Table 1). This could suggest that regardless of the clinical characteristics of the patients, there was an increase in myocardial wall stress responsible for this result. A subanalysis for each group found that only patients in Group-3 had a highly statistically significant increase in NT-pro BNP, which was accountable for the results seen for the entire cohort. In the other two groups analyzed separately, there was no statistical significance for the changes in NT-pro BNP. The distribution of NT-pro BNP after the stress test (Table 1 and Figures 2a-2d) showed that both the values of BNP-2 and BNP-3 in Group-2 (hypertensive patients), were very similar to those in Group- 1 (normal subjects). Consequently, the changes in NT-pro BNP after the stress test in patients from Group-2 were not statistically significant. In Group-3, the values of BNP-2 and BNP-3 showed a different distribution from both Group-1 and Group-2 with a clear statistical significance and a 10 times higher variability than the other 2 groups.
Notably, there were large differences in the levels at rest of NT-pro BNP in all groups. The same differences were seen even after the stress test. Some patients had the highest values immediately after the test, while others had the highest values only 24 hours later. There were few patients in whom the levels of NT-pro BNP did not change significantly or even decreased.
The dynamics of NP synthesis and secretion are highly variable in accordance with the expression of the genes coding for them and their regulators and with the type of stimuli that trigger their activation [26].
Considerable individual variations in NT-pro BNP levels in both normal subjects and apparently stable HF patients have been found. Numerous studies, including those on transgenic mice, have demonstrated that mutational modifications lead to different responses of myocytes to specific stimuli [27].
Of all stimuli, myocardial wall stress is by far the most powerful for inducing the transcription and secretion of NT-pro BNP. The major determinant of intramyocardial pressure was proven to be stretching the tissues within the interstitial spaces, which are closely related to the extracellular matrix composition [28-30].
Therefore, to mitigate the influence of this large variability on our statistics, we looked not only into the absolute values of NT-pro BNP, but also into the type and magnitude of changes induced by the treadmill test. For instance, in patients with low levels of NTpro BNP at rest, we can assume that there is no DD, even when associated with low absolute values after the stress test, lower than those seen in other patients. However, the increased levels caused by the effort could be significant enough to prove that parietal stress developed. In this way, we can wrongly rule out the DD based only on the absolute values of NT-pro BNP.
Hence, we analyzed the statistical significance of the differences between the levels at rest of NT pro-BNP (BNP-1) and those after the stress test (BNP-2 or BNP-3). They showed for the entire cohort a significant change for both BNP-2 and BNP-3. Once again, this result was intensely biased by the changes in Group-3. When the test was applied to each group separately, only the changes in Group-3 showed statistical significance for both BNP-2 (BNP-2 vs. BNP-1) and BNP-3 (BNP-3 vs. BNP-1). In the other two groups, there was no statistical significance.
Our study proves that asymptomatic type 2 noninsulin-dependent diabetic patients who associate hypertension with normal left ventricular function do have a subclinical DD which that can be unmasked only by exercise. In some of these patients, echocardiography is not diagnostic and we propose NT-pro BNP for identifying the increased filling pressures, instead.
The remaining levels of NT-pro BNP had no discriminative power for differentiating a subclinical DD from a normal diastolic function in all our patients. Only by measuring exercise-induced levels of NT-pro BNP, were we able to identify such an early stage of DD. Moreover, the magnitude of increase after the test can be useful in differentiating normal subjects from diseased subjects, especially in those with low levels of NT-pro BNP at rest.
Even small but significant increases, less than those used in decompensated heart failure or acute dyspnea, could become diagnostically significant.
In conclusion, we consider that these patients should be reclassified as having stage B heart failure and benefit from adequate treatment. We also consider that the levels in the blood of NT-pro BNP, before and after the stress test, should be incorporated into the usual practice for early diagnosis of heart involvement in asymptomatic type 2 DM patients with HTN.
In this section, you can acknowledge any support given that is not covered by the author contribution or funding sections. This may include administrative and technical support or donations in kind (e.g. materials used for experiments).
For research articles with several authors, a short paragraph specifying their individual contributions must be provided. The following statements should be used Conceptualization, N.P., U.G..A. C; methodology, N. P, C.N.; software S.S.B.; validation, C. N, V.J., A.M.I.; formal analysis, N.P, A.C, S.S.B.; investigation, C.N.; resources, N.P.U.G.; data curation, C.N, S-S.B. ; writing-N.P., U.G. writing-review and editing, V. J, S.S.B.; visualization, A.M. I.; supervision, V.J.A. MI; project administration, N.P; funding acquisition, N.P.
Data available on request due to ethical restrictions. The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical restrictions.
Citation: Paun N, Jinga V, Iliesiu AM, Uscoiu G, Capitanescu A, Nicolae C, et al. (2023) Exercise-induced Secretion of NT-Pro BNP in Asymptomatic Hypertensive Patients with Type 2 Diabetes Mellitus: An Early Sign of Subclinical Ventricular Dysfunction. J Clin Chem Lab Med. 6:266.
Received: 19-May-2023, Manuscript No. JCCLM-23-24275; Editor assigned: 22-May-2023, Pre QC No. JCCLM-23-24275 (PQ); Reviewed: 05-Jun-2023, QC No. JCCLM-23-24275; Revised: 12-Jun-2023, Manuscript No. JCCLM-23-24275 (R); Published: 19-Jun-2023 , DOI: 10.35248/JCCLM.23.6.266
Copyright: © 2023 Paun N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.