
Journal of Clinical and Cellular Immunology
Open Access
ISSN: 2155-9899

ISSN: 2155-9899
Research Article - (2023)Volume 14, Issue 4
It is constantly sought to understand the different mechanisms of action of the immune system to establish potential efficient therapeutic targets for different clinical situations. Thus, the present study aims to expand the knowledge about the protective effect of physical exercise on the immune system of patients with chronic renal failure, associating it with the incidence of infection by COVID-19. The present study was based on a narrative systematic review of the literature and understands, elucidate, and discuss the subject. The main result found was that regular, moderate-intensity exercise modulates immunological parameters in CKD patients, evidenced by decreased inflammatory factors such as Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-18 (IL-18) and Tumor Necrosis Factor-α (TNFα), and increased Interleukin-10 (IL-10) and Interleukin-4 (IL-4). Decreases in C-reactive protein and intercellular Adhesion Molecule-1 (ICAM-1) were also seen, associated with increased activity of leukocytes, Natural Killer (NK) cells, and CD8+ T cells. Based on this, moderate and continuous exercise is a protective therapy for kidney patients, causing a decrease in the risk of morbidity and mortality caused by COVID-19 infection.
Physical exercise; Chronic kidney disease; COVID-19; Immune system
Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), was first diagnosed in December 2019 and subsequently spread internationally, resulting in a pandemic. In June 2022, this disease caused the death of more than 6 million people worldwide [1].
Chronic Kidney Disease (CKD) generates inflammation leading to mortality from cardiovascular problems. However, no evidence showed the relation of CKD and increase of mortality from COVID-19 [2]. Immunosuppression associated with this disease may lessen the hyper inflammatory state described in COVID-19. On the other hand, immune dysfunction and a high incidence of comorbidities (such as underlying CKD diseases such as metabolic syndromes, cardiovascular diseases and arterial hypertension, and diabetes) can lead to a worse clinical course [3,4].
Some studies conducted after the onset of the COVID-19 pandemic did not find evidence that individuals with CKD had a greater or lesser chance of contracting the virus. The prevalence of CKD in individuals with COVID-19 ranged from 0.4 to 49.0%. The risk of hospitalization appears to be higher in CKD patients with COVID-19 infection compared to those without the disease, and patients with advanced stages of CKD were at increased risk of hospitalization. Numerous systematic reviews bring data confirming that mortality from COVID-19 in individuals with CKD is higher when compared to individuals without CKD [5].
Wittmer, et al. have described the importance of physical exercise concerning the prognosis and clinical outcome of COVID-19 infection. Individuals with CKD have unfavorable clinical outcomes and persist with some generalized impairments in post-COVID-19 physical function. These factors highlight the importance and benefits of physical exercise for patients with CKD and the general population, both in the physical condition before illness and during the COVID-19 infection, considering that periods of isolation or hospitalization favor the loss of physical fitness and muscle mass [6].
Therefore, several studies have suggested starting an exercise routine or early mobilization in these individuals during hospitalization. However, it is important to note the exercise intensity, it is worth mentioning that continuous training of moderate intensity improves biomarkers of immune function and can optimize the functional integrity of the immune system to prevent or mitigate the severity of infection, especially among vulnerable populations with immuno compromised conditions. On the other hand, prolonged high-intensity exercise leads to immunosuppression, consequently aggravating the patient's condition. Thus, the individualized prescription of exercise is fundamental [6].
Thus, the present study aims to expand the knowledge about the protective effect of physical exercise on the immune system of patients with chronic renal failure, associating it with the incidence of infection by COVID-19.
Pathophysiology of chronic kidney disease
According to Kidney Disease Outcomes Quality Initiative (KDOQI) and Kidney Disease, Chronic Kidney Disease (CKD) is defined as the presence of kidney damage or reduced kidney function for three months or more [7]. The degree of injury can be understood according to the clinical condition, from hematuria to renal failure requiring dialysis. The variation in clinical presentation is justified by how the kidney responds to injuries [8]. This lesion can be diagnosed through imaging tests, renal biopsy, abnormal urinary sediment markers, or elevation in the albumin secretion rate, and mainly through the Glomerular Filtration Rate (GFR). Patients diagnosed with CKD must be constantly monitored to measure their renal function. This assessment should be performed using GFR based on serum creatinine, thus allowing recognition of the degree of renal injury.
The reference values for the confirmatory diagnosis of CKD should comprise rates lower than 60 ml/min/1.73 m2 for longer than three months [9]. If these rates are maintained for less than three months, the diagnosis is not established, as it may be suggestive of other diseases, such as Acute Renal Failure. CKD is divided into five stages according to the estimated GFR, as follows: G1= ≥ 90 mL/min/1.73 m2, considered within the reference parameters; G2=60 to 89 mL/min/1.73 m2, slightly decreased; G3a=45 to 59 mL/min/1.73 m2, slightly to moderately reduced; G3b=30 to 44 mL/min/1.73 m2, moderately to severely decreased; G4=15 to 29 mL/min/1.73 m2, severely decreased; G5=<15 mL/min/1.73 m2, renal failure, and G5D=dialysis [7].
In stages 1 and 2, respectively, GFR is within reference standards and slightly reduced, defined by kidney damage due to albuminuria. Steps G1-G4 is considered conservative therapeutic measures, such as changes in lifestyle, use of pharmacological therapies, and continuous monitoring of biochemical markers. In the last classification stage, G5 represents the need for options that perform renal filtration [10]. Patients classified as G3a remain in volume balance. However, they become less efficient in the metabolic response to rapid sodium intake, making them more susceptible to fluid overload [8].
End-Stage Renal Disease (ESKD) is identified from GFR patterns below 15 mL/min per 1.73 m2, i.e., considered group 5 in disease staging. Thus, due to ESKD, it is essential to precede the discussion of substitutive renal therapies from numbers lower than 30 mL/min per 1.73 m2 of GFR to examples of renal transplants and dialysis. Based on the reduction mentioned above in the glomerular filtration rate, it is necessary to refer the patient to professionals with advanced clinical skills in CKD [7].
Assuming the clinical care for renal replacement therapies, the patient must be guided regarding the burdens and bonuses of the conduct. Some of these selections comprise hemodialysis, peritoneal dialysis, or kidney transplantation. Kidney transplants are indicated in the case of ESKD because the successful procedure promotes an increased quality of life and reduces mortality compared to maintenance dialysis [8]. Peritoneal dialysis consists of placing a catheter that allows the exchange of the dialysate through the placement of a dialyzing substance. In turn, hemodialysis is intended for the elimination of potentially harmful substances directly from the blood, thus being performed through tunnel hemodialysis catheters, arteriovenous grafts, and arteriovenous fistulas [8].
With the advancement of research and technology, treatments for CKD have also evolved, offering pharmacological and nonpharmacological approaches. Based on literature, numerous guidelines indicate that using Renin-Angiotensin-Aldosterone System (RAAS) blockers should be the first-line option for treating systemic arterial hypertension in CKD patients. By controlling blood pressure, disease progression is delayed, increasing the chances of successful treatment and maintaining stable blood pressure [11].
In 2019 the first Randomized Controlled Trial (RCT) focused on treating CKD in patients with diabetes was published. It was followed by the CREDENCE study (Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy), which successfully analyzed the use of canagliflozin, a Sodium-Glucose Cotransporter 2 (SGLT2) inhibitor [11].
The use of pharmacological agents for CKD treatment improves health patients, especially when there are complicating factors such as hypertension and diabetes, and help slowly down the progression of the disease. In 2020, the DAPA-CKD study (Dapagliflozin in Patients with Chronic Kidney Disease) demonstrated, for the first time, the use of dapagliflozin, which showed a relative risk reduction of approximately 39% [12].
Some non-pharmacological measures include a balanced diet with sodium restriction, controlled protein intake, weight control, and physical activity. Physical activity plays an important role in non-pharmacological treatment and improves cardiovascular function affected by CKD. Physical activity helps control blood pressure without required medications and becomes an excellent option for those in regular exercise despite having the disease. Additionally, weight control combined with a balanced diet, reduces the strain on the kidneys associated with excess weight [12].
Chronic kidney disease and COVID-19 involvement
People with CKD present a susceptibility to being infected by the 2019 coronavirus, and when infected, they demonstrate more susceptibility to developing the most severe form of the disease. They are also at greater risk of death. This high mortality is related to indicators such as high levels, the ratio of neutrophils to lymphocytes and high levels of systemic immune inflammation [13].
Cardiovascular events triggered by hypoalbuminemia represent one of the main mortality factors in patients with chronic renal failure. This low albumin concentration in the body may result in unbalanced nutrition combined to an inflammatory process [14].
Hypoalbuminemia triggers an elevated risk for patients with severe SARS-CoV-2 infection, regardless of age and comorbidities. Albumin is a protein capable of retaining water in the systemic circulatory system and maintaining sufficient blood pressure to irrigate major organs such as kidneys and lungs [15].
The loss of kidney function caused by SARS-CoV-2 infection is related to worsening the clinical picture and high mortality rates in the hospital environment. This fact represents a warning for patients with chronic kidney disease, as the complications caused by COVID-19 result in a high overproduction of inflammatory cytokines (IL-6, TNF-alpha) that causes multiple dysfunction syndrome organs and hypercoagulability [16]. Hyper coagulation in critically ill patients with COVID-19 infection, and mechanics, admitted to the ICU is one of the leading causes of risk and death [15].
Several other pathophysiological mechanisms are presented to exemplify the damage caused to the renal system during the post-acute phase of COVID-19 infection. These damages in the renal system include injury to renal tubular cells, damage to structures and cells that line the inside of blood vessels, micro or macro vascular injury, glomerular podocyte injury, immune-inflammatory mediators, and activation of the complement system.
Furthermore, the development of acute kidney injury during the early phase of covid-19 facilitated by chronic kidney disease that result in high mortality rates (Figures 1 and 2) [17].
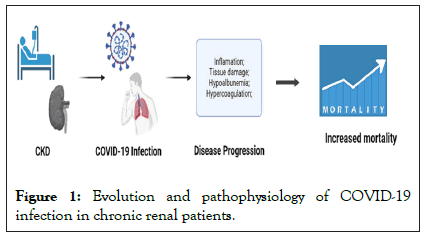
Figure 1: Evolution and pathophysiology of COVID-19 infection in chronic renal patients.
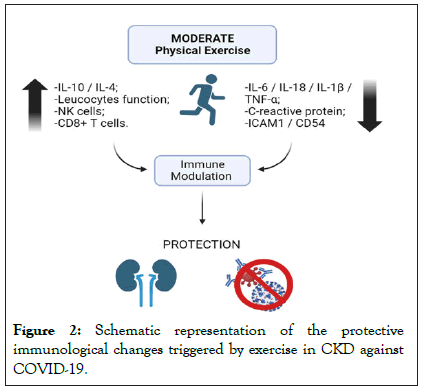
Figure 2: Schematic representation of the protective immunological changes triggered by exercise in CKD against COVID-19.
Through direct or indirect pathways, the disease caused by the SARS-CoV-2 virus impaired renal anatomy and physiology, so the severity of these factors can vary over time. These effects are present during hospitalization or after hospital discharge, such as pathophysiological occurrences may favor frequent episodes of generalized infection, development of recurrent acute kidney injury and increased risks and worsening of chronic kidney disease. Furthermore, chronic kidney disease facilitates the entry and installation of COVID-19 in the body and increases the risk of acute kidney injury caused by the viral infection [17].
Effects of physical exercise on chronic kidney disease
According to the World Health Organization, nearly four to five million deaths per year could be avoided with the practice of physical exercises. Furthermore, the preventive role played by physical activity in cardiovascular disease, type 2 diabetes, hypertension, mental illness, and cancer further emphasizes its importance for improving joint health and people's quality of life. Given this, the WHO recommends that healthy adults between 18 and 64 years of age and adults with chronic diseases practice more than 300 minutes of moderate-intensity physical activity or more than 150 minutes of high-intensity physical activity per week to obtain beneficial health results [18].
The effects of physical exercise on healthy individuals have been known and monitored for a long time. Therefore, the beneficial results from physical activities are documented in numerous scientific studies and are encouraged by several health professionals precisely because of the beneficial effects provided by them, such as the reduction of inflammatory processes, increased life expectancy, prevention of diseases and injuries, control of chronic diseases, muscle strengthening, in addition to countless other factors [18].
With the vastness of scientific advances in recent decades, the look at the immune system has not been left aside. It is imperative to consider the fundamental role played by the immune complex of each individual and which external attitudes favor its effectiveness. Of the numerous interventions, it is known that the association between physical activities directly influences the vast composition of our body's defense cells. It is known that the effect of physical exercise and muscle contraction provides an increased release of anti-inflammatory [19] and proinflammatory cytokines, depending on the intensity and duration of the activity, the reduction in neutrophil chemotaxis, which is normalized 48 hours after exercise, increased circulation of leukocytes released by the medulla, inflammatory suppression by decreasing the expression of Toll-like receptors on macrophages, recruitment of NK cells, and increased concentration of lymphocytes in the vascular bed. It is also understood that the influence of physical activity implies an increase in circulating leukocytes in the bloodstream even 24 hours after starting activities [20].
When physical exercises are applied to CKD patients by sequence, the effects are similar. Studies on the subject show that there is a significant improvement in the general clinical picture of patients, evidenced by the following findings: antiinflammatory effect by reducing the release of inflammatory cytokines [21] and the number of basophils, significant increase in total cholesterol, HDL, and LDL (a factor that contributes to the elimination of endotoxins), decrease in cardiac risk (mainly due to the elimination of excess potassium) [22]. However, a study observed an increase in infectious complications secondary to immune system disorders due to the lowering of the patient's inflammatory potential.
In addition, studies indicate Silva, et al., [23] a significant increase in the improvement of the quality of life of CKD patients, evidenced by parameters pre and post-exposure to exercise such as decreased pain, increased functional capacity, increased motor performance and resistance, reduced fatigue and normalization of vital signs (heart rate, respiratory rate, and pain).
Therefore, as mentioned above, the practice of physical exercises, both acute and chronic, has actual results for the body as a whole. Studies in patients on dialysis have shown a high number of pro-inflammatory cytokines. Therefore, these individuals with kidney diseases are more susceptible to suffering complications in their clinical conditions from infections due to a deficient immune system. In this way, physical activities explicitly aimed at this type of public can serve as a preventive method for the injuries caused by chronic kidney disease [24].
For that reason, the practice of recurrent physical activity and variable intensity can bring beneficial results for individuals with known pathologies, such as CKD. The immune response and anti-inflammatory effects of physical exercises are known and substantiated through clinical evidence. Therefore, physical activity for patients with chronic kidney disease can be an excellent indication that promotes health and improves the individual's quality of life.
Influence of physical exercise on the immune response in people with chronic kidney disease and COVID-19
The immune system is characterized as innate and adaptive. The innate immunity is characterized by cells that do not have a specific antigen and need an immunological memory, among which neutrophils, eosinophils, mast cells, basophils, monocytes, macrophages, and natural killer cells stand out Natural Killer (NK) and various types of Dendritic Cells (DCs). In comparison, adaptive immunity has a very specific relationship with antigens and develops immunological memory being mediated by lymphocytes [25].
Some evidence demonstrates that the infection triggered by SARS-CoV-2 modulates the activation of innate and adaptive responses, resulting in resistant inflammatory reactions in the disease. Thus, through the lack of control of the inflammatory response, it is possible to identify local and systemic tissue damage in patients with the pathology [26].
In this manner, the infection originating from SARS-CoV-2, leads to serious complications when targeting the adaptive immune system, causing eosinopenia and lymphopenia in severe cases, and the interruption of antibody production and a high reduction of responses in cells T, such as T CD4+ and CD8+, B cells and Natural Killer (NK) cells [27].
Furthermore, the mechanisms contributing to lymphopenia include the effects of the cytokine environment, with the severity of the disease arising from the relationship between the levels of pro-inflammatory cytokines and the individual's cellular immune profile. In cases where inflammation is not brought under control by the adaptive immune system within 7 to 10 days of infection, distortion of the adaptive response occurs resulting in cytokine storm syndrome.
This hyperactivation of cytokines is caused by an imbalance in the regulation of the immune system and has a greater observation in critically ill patients with COVID-19, with higher levels of pro-inflammatory cytokines being identified in the serum. The lymphopenia results from the apoptosis of lymphocytes, resulting in critical immune dysfunctions. Moreover, the elevation of neutrophil levels indicates the progression of the severity of the disease, which leads to cause neutrophilia and the increase in the neutrophil/lymphocyte ratio, relating to the poor clinical prognosis.
Faced with immune changes, the recruitment of other proinflammatory cells such as granulocytes and macrophages results in a cascade of cytokine secretion and leukocyte recruitment, making the systemic environment inflamed from the macrophage activation syndrome [28]. The activation and maintenance of the immune system against the COVID-19 infection drives the systemic environment with high levels of inflammation. Furthermore, the inflammatory process plays a major role in the damage generated by the COVID-19.
In line with chronic kidney disease, the individual has a significant immune dysregulation compared to the general population. It is estimated that patients with CKD have high levels of pro-inflammatory cytokines, and these present chronically persistent inflammation during the course of the disease, which is closely related to the pathology's mortality process [29,30].
The patient with CKD becomes a high-risk group in the COVID-19 pandemic, due to hospitalization by hemodialysis intervention increasing the risk of contracting the virus in the hospital. COVID-19 in positive cases since the individual already has elevated inflammatory levels and immune dysfunction [31,32].
Measurement of circulating levels of immune-inflammatory mediators, as well as evaluation of polymorphisms of the genes that encode these immune-inflammatory mediators, have shown that CKD patients exhibit a pro-inflammatory phenotype that is accentuated as the kidney injury progresses. CKD patients have elevated levels of pro-inflammatory cytokines, of which Interleukin-1 (IL-1), Interleukin-6 (IL-6), and Tumor Necrosis Factor-α (TNFα) stand out, which are involved in the response and antigen presentation, mainly by T helper lymphocytes [33].
Likewise, chronic kidney patients presenting systemic inflammation and immune compromise, the effects of COVID-19 infection become more severe, due to the phenomenon called cytokine storm. These two situations concomitantly alter the pathophysiological aspects significantly and consequently justify the high mortality rates from COVID-19 in renal patients [34].
Given strengthening the immune system some measures and alternatives should be considered. Among the nonpharmacological measures, one can highlight the adoption of a balanced diet associated with regular physical exercise [35].
Physical exercise is considered one of the pillars in the prevention and promotion of health and is also a great ally in the rehabilitation and treatment of various pathological conditions. There is numerous evidence of the positive effect of physical exercise on chronic health conditions, such as Diabetes, Hypertension, Obesity, Cancer, Chronic Obstructive Pulmonary Disease (COPD), and CKD, but although lesser, current evidences also suggest that physical exercise can be beneficial in reducing infections by communicable diseases, including pathologies of viral origin [36].
It is noteworthy that both acute and chronic exercise can affect the immune system [37]. When talking about the immune response resulting from physical exercise, as well as the therapeutic benefits of its practice, the type of exercise, its intensity, its duration, and its frequency must be taken into consideration.
Moderate-intensity physical exercise, both resistance and aerobic, is beneficial for maintaining and improving the immune system. It is evidenced that this intensity of exercise in a program with a frequency of three days a week positively affects the immune system, justifiable by the increased leukocyte function, increased chemotaxis, degranulation, cytotoxic activity, phagocytosis, and oxidative activity of neutrophils and macrophages, and increased cytolytic activity of NK cells and NK cell-activating lymphocytes [38,39]. In addition, there are reduced concentrations of proinflammatory cytokines such as IL-6, TNF-α, and IL-1β, the increase of cytotoxic activity of NK and TCD8+ cells, neutrophil function and proliferation of B [40].
Several physiological mechanisms are involved in the antiinflammatory effects of exercise, among them: reduction of visceral fat and increased production of cortisol, adrenaline, prolactin, and growth hormone to influence the flow of leukocytes; decreased expression of TLR in immune cells; and increased levels of anti-inflammatory myokines influenced by skeletal muscle contraction [41]. Moreover, moderate-intensity exercise provides decreased pro-inflammatory mediators, mainly IL-18, IL-6, C-reactive protein, TNF-α, IL-1β and exacerbated increases in IL-10 and IL-4. High levels of IL-10 can neutralize the effects of pro-inflammatory cytokines and prevent the infiltration of these cells into the tissues, thus decreasing the levels of adhesion molecules such as ICAM-1(CD54), providing a beneficial immune modulation [42].
When referring to moderate intensity in the practice of physical exercise, practices of 45 minutes to 1 hour on average, and weekly basis of 3 days, are adopted. In this context, aerobic exercises need to be performed between 60% and 70% of the volume of maximum oxygen consumption or between 65% and 75% of the maximum heart rate [43]. Resistance exercises need to be performed in an average zone of 60% of the 1-rep test with the maximum load for each muscle group [44].
Thus, taking into consideration the benefits of physical exercise for the modulation between cytokines, cells and other anti and pro inflammatory components, suggest that moderate practice in individuals with CKD improves the strengthening of the immune system and decreases the chances of infection and severe evolution of COVID-19. Finally, moderate and continuous physical exercise is a protective therapy for these patients, causing a decrease in morbidity and mortality risks [45-47].
Some evidence showed the susceptibility of CKD patients to develop severe forms of COVID-19 infection. In this way, the prevention and treatment strategies for CKD patients must be designed to avoid permanent sequelae and higher mortality in this population. Adjusting the pharmacological treatment is essential, at the same time that non-pharmacological strategies are extremely important. Moderate-intensity physical exercise modulates the immune system and helps in the recovery of these individuals. Thus, moderate-intensity physical exercise is a nonpharmacological tool that might be helpful in the prevention and treatment of CKD patients affected by COVID-19 infection.
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Puhle JG, da Silva VC, Soares AA, Kroth JV, Gallina MM, Cavasin MG, et al. (2023) Exercise-Mediated Immune Protection in Chronic Kidney Disease Decreases Infection and Severe Evolution of COVID-19. J Clin Cell Immunol. 14:692.
Received: 30-Jun-2023, Manuscript No. JCCI-23-25336; Editor assigned: 03-Jul-2023, Pre QC No. JCCI-23-25336 (PQ); Reviewed: 17-Jul-2023, QC No. JCCI-23-25336; Revised: 24-Jul-2023, Manuscript No. JCCI-23-25336 (R); Published: 31-Jul-2023 , DOI: 10.35248/2155-9899.23.14.692
Copyright: © 2023 Puhle JG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.