Review Article - (2016) Volume 2, Issue 2
Exopolysaccharides Production by Lactic Acid Bacteria
*Corresponding Author: Pinar Sanlibaba, Associate Professor, Department of Food Engineering, Ankara University Engineering Faculty, Ankara, Turkey, Tel: + 90-312-203-3300 Email:
Abstract
Exopolysaccharides (EPSs) are high molecular weight and biodegradable polymers. They are biosynthesized by a wide range of bacteria. Lactic acid bacteria (LAB) are also able to produce EPSs. EPSs can be classified into two groups. These are homopolysaccharides and heteropolysaccharides. Homopolysaccharides are polymers which are composed of one type of monosaccharide. Heteropolysaccharides are polymers of repeating units. They are composed of two or more types of monosaccharides. Producer microorganisms don’t use the bacterial EPSs as energy sources. EPSs have been used in the production of several fermented foods such as thickeners, stabilizers, emulsifiers and gelling or water-binding agents. In addition, EPSs have some positive effects on health. These are to have antitumor effects, immune-stimulatory activity and to lower blood cholesterol. Incubation temperature and time, growth medium, acidity of growth medium and type of strain have an impact on EPSs production.
In this review, EPSs production by LAB, including chemical composition, structure, biosynthesis, genetics and application of EPSs produced by LAB is discussed
Keywords: Exopolysaccharides; Lactic acid bacteria; Chemical structure; Application; Biosynthesis
Introduction
Exopolysaccharides (EPSs) are formed monosaccharide residues of sugar and sugar derivatives. They are produced by plants, algae, fungi and bacteria [1]. Deep-sea hydrothermal vents, Antarctic ecosystem, saline lakes and geothermal springs are extreme environmental and several microorganisms isolated from these areas. Potential sources of valuable biopolymers including EPSs have recently isolated from there [2]. Among the various EPSs producing bacteria, lactic acid bacteria (LAB) have gained special attention. LAB is generally recognized as safe (GRAS) microorganisms and also, their capabilities to produce EPSs have wide diversity of structures without health risk [3]. EPSs produced by LAB have recently got an increasing amount of attention because of their health benefits to the consumers. These advantageous effects are antitumor, anti-ulcer, antioxidant activities, cholesterollowering activity, and immune-stimulating activities [4]. Although some EPSs form a biofilm that causes hygiene problems, other EPSs derived from LAB play crucial role in improving the rheology, texture, mouth feel of fermented food formulations in food industry [1,5]. Bioflocculants, bio-absorbents, heavy metal removal agents, drug delivery agents, and others are the new application of EPSs [6].
Microbial EPSs generally exist in two forms depending on their locations: 1) cell-bound EPSs which closely adhere to the bacterial surface, as capsular (cEPSs), 2) released EPSs that release into the surrounding medium, as free EPSs (fEPSs) [7,8]. EPSs produced from LAB are often distinguished as ropy or non-ropy EPS [9]. Composition of the medium (carbon and nitrogen sources, vitamins, minerals and so on), LAB strains and growth conditions (temperature, agitation, incubation time, pH, oxygen tension and so on) are important factors for the total yield of EPSs produced from LAB. Optimization of the growth environment is critical point, if maximal EPSs productions by LAB strains are achieved [6,10]. Complex media usually resulted in higher EPSs yields. But defined media have been used to elucidate EPSs metabolism, product biosynthesis, and limiting factors such as the carbon/nitrogen ratio and so on [9]. The EPSs production increases during the exponential phase and no further production is observed in the stationary growth phase [11]. One of the main disadvantages of the EPSs-producing LAB is the small amount of polymer synthesized, which varies from 25-500 mg/L. The highest production levels reported so far were obtained for the mesophilic strains Lactobacillus rhamnasus 9595M (1200 mg/L) and Lactobacillus sakei 0-1 (1375 mg/L) [12]. Streptococcus, Lactococcus, Pediococcus, Lactobacillus, Leuconostoc and Weissella species among LAB strains are frequently produced EPSs [12,13]. Also, some non-starters LAB like Bifidobacteria are able to produce EPSs [14]. Elective methods for improving the commercial scale production and field application of microbial biopolymers are; 1) optimizing the fermentation conditions, 2) biotechnological tools involving genetic and metabolic engineering, 3) the exploration of cheap fermentation substrates for their production [15].
This article reviews focuses on EPSs production by LAB, including chemical composition, structure, biosynthesis, genetics and application of EPSs produced by LAB.
Chemical Composition and Structure of Exopolysaccharides
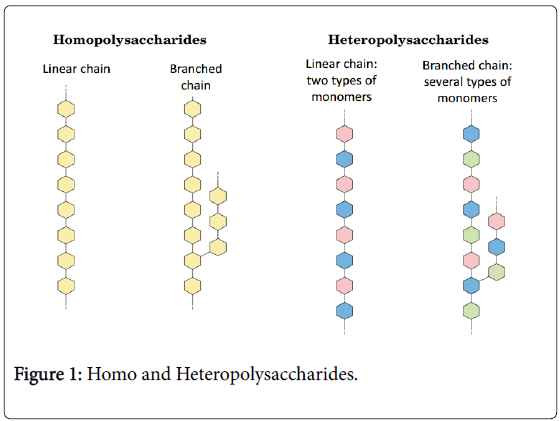
EPSs are long-chain molecules. These molecules are polysaccharides consisting of branched, repeating units of sugars or sugar derivatives. Glucose, galactose and rhamnose in different ratios are mainly appeared in these sugar units [15]. In consideration of the characteristics of EPSs, they are largely varied in molecular structure and mass, molecular size, charge, and as a consequence, in their rheological properties. EPSs from LAB are highly various polymers and can be classified following different criteria. One of the most used classification criteria is based on their monomer composition. Basically, depending on the composition of the repeating units and biosynthesis pathway, EPSs can be classified by two parts. These parts are homopolysaccharides (HoPS) or heteropolysaccharides (HePS) (Figure 1) [9]. Molecular mass of HePS ranges from 104 to 106 Da, which is generally lower than the average molecular mass of HoPS (up to approximately 107 Da) [16]. As it is seen, the HePS production levels from LAB are generally lower than those for HoPS [17,18]. Also, producer microorganisms don’t use the bacterial EPSs as energy sources [19].
Homo-exopolysaccharides
HoPS are the most studies on EPSs from LAB in the last decade. Some of characteristics of HoPS have been described such as the isolation of HoPS-producing strains, the molecular and structural characterization of these EPS, studies on their biosynthetic enzymes, and the HoPS application in food. Weissella genera are mainly produced HoPS [18].
The HoPS consist of one type of monosaccharide (Figure 1). To give an example, α-D-glucans, β-D-glucans, fructans, alternan, reuteran, pullulan, levan, inulin, curdlan, mutan, and others like polygalactan. As a matter of fact, most of them share the attribute of being synthesized by extracellular glycansucrases. These enzymes use sucrose as the glycosyl (fructose or glucose) donor [20]. The features of their primary structure (the pattern of main chain bonds, molecular weight and so on) and branch structure are constituted the main differences between the HoPS. Two important groups of HoPS are produced by LAB (Table 1). One of them is α-glucans. They are namely dextrans and mutans. Leuconostoc mesenteroides subsp. mesenteroides and Leuconostoc mesenteroides subsp. dextranicum produced dextrans and Streptococcus mutans and Streptococcus sobrinus produced mutans. Glucans can be sub-classified into (i) α-glucans [dextran: α-DGlc( 1,4); mutan: α-D-Glc(1,3); alternan: (α-D-Glc(1,6)/α-D-Glc(1,3); and reuteran: α-D-Glc(1,4)/α-D-Glc(1,6) with α-D-Glc(1,4)/α- DGlc(1,6) branching points], and (ii) β-glucans [β-D-Glc(1,3) with side chain linked (1,2)]. Second group is fructans, namely levan. S. salivarius produced levan [2]. Fructans can be classified into (i) levantype: β-D-Fru(2,6), and (ii) inulin-type: β-D-Fru(2,1), being both β- fructans. In addition, polygalactans are also HoPS. They contain a pentameric repeating unit of galactose. These polymers are more uncommon. They were only described for the strain Lactococcus lactis subsp. lactis H414 and two strains of Lactobacillus delbrueckii subsp. bulgaricus (CRL 406 and 142) [18].
| EPS | Strain | |
|---|---|---|
| α-D-glucans | Dextran | Leuconostocmesenteroides subsp. mesenteroides |
| Leuconostocmesenteroidessubsp. dextranicum | ||
| Mutan | Streptococcus mutans | |
| Streptococcus sobrinus | ||
| Alternan | Leuconostocmesenteroides | |
| Reuteran | Lactobacillus reuteri | |
| β-glucans | Pediococcus spp. | |
| Streptococcus spp. | ||
| Fructans | Levans | Streptococcus salivarus |
| Inulin-type | Streptococcus mutans | |
| Leuconostoccitreum | ||
| Lactobacillus reuteri | ||
| Polygalactan | Lactococcus lactis subsp. lactis H414 | |
| Lactobacillus delbrueckii subsp. bulgaricus (CRL 406 and 142) |
Table 1: Homopolysaccharides produced by lactic acid bacteria [17,18].
A specific substrate, such as sucrose is required the production of HoPS. Assembly of the monosaccharide units occur outside the bacterial cell [17]. The yields of HoPS produced by LAB are low as compared with other bacterial EPSs. EPSs formation by Weissella sp. and Lactobacillus sanfranciscenis strains reached levels up to 16 and 5 g EPSs/kg dough, respectively [21].
Hetero-exopolysaccharides
The majority of EPSs produced by LAB are HePS, containing three to eight repeating units composed of two or more monosaccharides (Figure 1) [22]. The HePS are produced by a great variety of mesophilic and thermophilic LAB. Lactococcus lactis subsp. lactis, Lactobacillus rhamnosus and Lactococcus lactis subsp. cremoris, Lactobacillus sakei, Lactobacillus casei are the major mesophilic LAB. Additional, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus acidophilus, Lactobacillus helveticus and Streptococcus thermophilus are considered to be the major representatives of thermophilic LAB [23].
The HePS are composed of either linear or branched repeating units. These units are often contained a combination different types of monosaccharide such as D-glucose, D-galactose and L-rhamnose. In a few cases, N-acetylyglucosamine, N-acetylgalactosamine or glucuronic acid are played a part of HePS. Sometimes non-carbohydrate substituents such as phosphate, acetyl and glycerol are present in HePS. Gellan, xanthan and kefiran are some examples of HePS [17].
The composition of the monosaccharide subunits and the structure of the repeating units are showed very little structural similarities. Also, these are not to be species-specific. Under optimal culture condition, 0.15-0.6 g/L HePS yield occurs. The molecular mass of these HePS polymers ranges between 1.0 × 104 and 6.0 × 106 Da [10]. In general, the LAB have produced a wide variety of HePS, however they have shown wide range of variations. A few bacterial isolates such as Streptococcus thermophillus, Lactococcus lactis subsp. cremoris, Lactobacillus delbrueckii subsp. bulgaricus, Lactobacillus casei and Lactobacillus plantarum found to produce 50-350, 80-600, 60-150, 50-60 and about 140 mg/liter of HePS. The maximum amount of HePS recovery has been noticed for Lactobacillus rhamnosus RW-9595 and Lactobacillus kefiranofaciens WT-2B by 2275 and 2500 mg/liter, respectively [23]. Structurally, HePS may be ropy or mucoid [10].
Biosynthesis of Exopolysaccharides by Lactic Acid Bacteria
Biosynthesis of bacterial EPSs is a complex process involving large number of enzymes and regulatory proteins [10]. Basically, EPSs biosynthesis can be categorized into three main steps: Firstly, carbon substrate is assimilated. Secondly, the polysaccharides are synthesized intracellular location and finally, they exudation out of the cell [24]. Biosynthesis of EPSs in LAB has four main steps starting with sugar transport into cytoplasm, synthesis of sugar-1P, polymerization of repeating unit precursors and lastly EPS transport outside the cell [25].
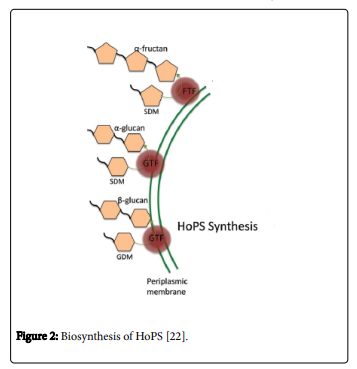
The HoPS synthesis is a relatively simple process (Figure 2).
Some characteristics of HoPS synthesis are here: no active transportation stages in the synthetic pathway, no energy expenditure and requirement of biosynthesis of the extracellular enzymes. These extracellular enzymes are named glycosyltransferases and fructosyltransferase (FTF or fructansucrase). Glycosyltransferases are termed GTF or glycansucrases. This enzyme utilizes glucose. Fructosyltransferase are called FTF or fructansucrase. Also fructose is utilized by this enzyme. This sugars uses as the glycosyl donor during HoPS synthesis [26].
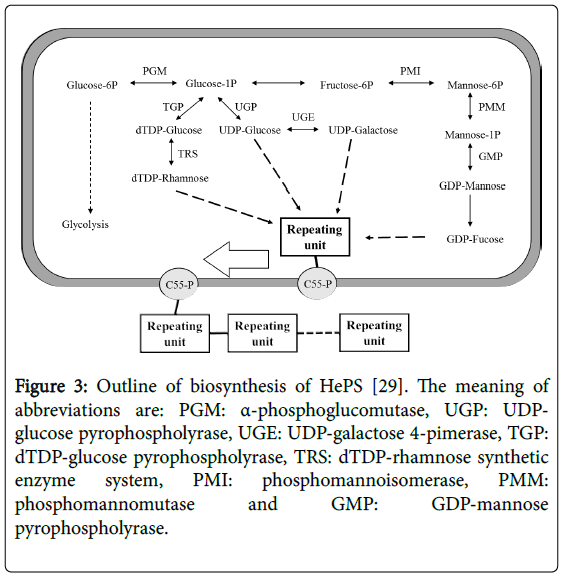
Sugar transportation, sugar nucleotide synthesis, repeating unit synthesis, and polymerization of the repeating units formed in the cytoplasm are the four main steps of the synthesis of HePS. The HePS synthesis mechanism is more complicated. In this process, the precursor nucleotide units such as UDP-GalNac, GDP-fructose, dTDPrhamnose, UDP-galactose and UDP-glucose are synthesized intracellularly from glucose-1-phosphate and fructose-6-phosphate [22,26]. Additionally, several enzymes and/or proteins are involved in the biosynthesis and secretion of HePS. The sugar nucleotides play an essential role in HePS biosynthesis. Importances of these sugar nucleotides, derived from sugar-1-phosphates are; 1) their role in sugar activation (Sugar activation is necessary for monosaccharide polymerization, 2) sugar interconversions (epimerization, decarboxylation, dehydrogenation and so on). Together with the sugar activation and modification enzymes are played a vital role in the formation of the building blocks and the final EPS composition [27]. HePS biosynthesis is energy consuming process. There are some steps which energy used in this process; 1) One ATP is required for the conversion of sugar to sugar phosphate, 2) another is needed for synthesis of each nucleotide and 3) one more is required for phosphorylation of isoprenoid C55 lipid carrier (Figure 3) [28,29].
Figure 3: Outline of biosynthesis of HePS [29]. The meaning of abbreviations are: PGM: α-phosphoglucomutase, UGP: UDPglucose pyrophospholyrase, UGE: UDP-galactose 4-pimerase, TGP: dTDP-glucose pyrophospholyrase, TRS: dTDP-rhamnose synthetic enzyme system, PMI: phosphomannoisomerase, PMM: phosphomannomutase and GMP: GDP-mannose pyrophospholyrase.
Genetics of Exopolysaccharides Production
Genetic determinants of EPSs can be placed either on a plasmid or on a chromosomal DNA [30]. The eps genes encode the proteins required for EPSs synthesis. Production of EPSs in the mesophilic LAB strains is located on plasmid origin. An example is Lactococcus strains. The production is a less stable [9,10]. The main reasons for an observed instability of EPSs can be summarized: 1) the plasmid location of the eps cluster and 2) the presence of mobile insertion sequence (IS - e.g. ISS1, IS981) elements [22]. The eps genes in thermophilic strains of Streptococcus and Lactobacilli are situated on chromosomally so the production is more reliable [9,10]. Strain specific eps gene cluster determines according to EPSs biosynthesis and the assembly of their repeating units. With respect to the EPSs specific gene cluster coding for EPS production and secretion, Streptococcus thermophilus Sfi 6 [31] and Lactococcus lactis NIZO B40 [32] were first identified and characterized strains. Generally, the EPSs synthesis genes are located on plasmids rather than the chromosome in the most LAB strains [24].
In general, all LAB strains follow a highly conserved basic pathway for intracellular EPS synthesis [22]. The organization of the EPSs gene clusters are classified into four regions. Regulatory genes are in the first region. The second codes for proteins proposed to be involved in determining polymer chain length. Following this region, genes similar to glycosyl transferases (GTs) are involved. GTs is specifically important enzyme for biosynthesis of the EPS repeating unit. The GTs connects the EPS repeating unit by serial transfer of nucleotide sugar residues onto a lipid carrier or onto a growing chain. Finally, encompasses genes involved in transport and polymerization [28,30,33]. Many gene structures for EPSs synthesis from various LAB have been isolated and identified, e.g., Streptococcus thermophilus, Streptococcus macedonicus, Lactococcus lactis subsp. cremoris, Lactobacillus delbrueckii subsp. bulgaricus but the understanding on exact mechanisms at genomic level, e.g., assembly of repeating units or regulatory mechanisms of EPSs yield is still limited [9]. These LAB strains contain a number of glycosyl transferases in the EPSs gene cluster, which provide many chemical structures of the repeating unit, and hence their EPSs have wide diversity of physicochemical properties [33]. Also, several primer pairs have already been described, such as primers targeting regulatory genes, genes involved in chain length determination and genes coding for glycosyl transferases [34].
Application of Exopolysaccharides of Lactic Acid Bacteria in the Food Industry
Application of EPSs is in various industries such as medicine, cosmetics, pharmaceuticals and cosmetics. Also, microbial EPSs can be used in food industry. The areas of usage are; 1) control viscosity and modify flow, 2) Improve texture, mouth feel and freeze-thaw stability, 3)Thickeners softeners, 4) Suspending agents, 5) Low calories food products, 6) Dietary fibers, 7) Films and coating agents, 8) Salad dressings, 9) Frozen food icing, 10) Moisturizing agents [23].
LAB have been used to a large variety of products like milk, meat and vegetable because of their improving preservation, sensorial characteristics and nutritional value. Also, EPSs from LAB have technological significance in the production of several fermented products. These technological advantages are to improve the rheology, texture, mouth feel of fermented food formulation [17]. EPSs can also be used as a source of oligosaccharides and sugar monomers [2]. A variety of functional oligosaccharides can be produced by LAB. These oligosaccharides have huge industrial application as prebiotics, nutraceuticals, sweeteners, humectants, drug against colon cancer, immune stimulators and so on [10]. Phage attack, nutrient shortage, osmotic stress, antagonists, toxic compounds, and desiccation are defined some important stress factors. Also, the major physiological function of EPSs is believed to be biological defenses against these various stresses [3,11]. Applications of functional EPSs and oligosaccharides from LAB are summarized in Table 2. In conclusion, some EPSs produced LAB have showed ability being used as a viscosifer, thickener, emulsifier or stabilizer in the food industry [23].
| EPS | Uses |
|---|---|
| Dextran | As adjuvant, emulsifier, carrier and stabilizer in food and pharmaceutical industries, plasma substitute, matrix of chromatography column, anticoagulant, paper industry, metal plating processing, for enhanced oil recovery, biomaterials. |
| Alternan | Prebiotics, sweetener in confectionaries, low viscosity bulking agent and extender in foods. |
| Reuteran | Used in Bakery. |
| Levan | Prebiotics, antitumor property, hypocholesterolaemic agent, ecofriendly adhesive, Biothickener in food industry. |
| Inulin | Prebiotics, nourishes gut mucosal cells and inhibits pathogens, for targeted drug delivery against colon cancer, substitute of fat in food products. |
| Kefiran | Improves viscoelastic properties of acid milk gels, antimicrobial and wound healing properties, ability to lower blood pressure and cholesterol in serum, capacity to retard tumor growth, enhance immunity of gut. |
| Oligosaccharides | Prebiotics, nutraceutical, alternative of antibiotics, food additives, humectants, prevention of colon cancer, treatment of chronic constipation, reduce lipid level in blood, in skin cosmetics. |
| Glucan | Adjunct culture in cheese. |
| β and α glucans | Starter culture. |
| Novel hetero-EPS | Emulsifying and flocculating activities, antioxidant, anti-biofilm. |
Table 2: Applications of functional exopolysaccharides and oligosaccharides from lactic acid bacteria [10].
EPSs from LAB are especially essential in the fermented dairy products such as yoghurt, drinking yoghurt, cheese, fermented cream and milk based desserts [35]. If the use of “ropy” starters containing EPS forming strains Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus utilizes in yoghurt, there are some advantages improving texture, avoiding syneresis and increasing the viscosity of the yoghurt. In addition, EPSs-forming LAB have been used to improve the rheological characteristics of dairy products [17]. The EPSs producing LAB starters can be applied to produce hydrocolloids. They are food ingredients and used in the manufacture of fermented milk products and cheese [26].
Conclusion
EPSs are microbial polysaccharides. They are released outside of the bacterial cell wall. Among the bacteria, lactic acid bacteria produce a wide variety of EPSs. EPSs produced from LAB strains have received special attention as valuable compounds, because of their potential economic applications and positive effects on health benefits in humans and livestock. Economic applications are included natural, safe-food additives or natural functional food ingredients increasing the possibility to replace or reduce the use of external hydrocolloids.
The research interest in LAB production of EPSs is continuously growing. It is focused on characterization and expression analysis of the EPS gene cluster, on using low cost substrates and improving downstream processing.
References
- Dilna SV, Surya H, Aswathy RG, Varsha KK, Sakthikumar DN, et al. (2015) Characterization of an exopolysaccharide with potential health-benefit properties from a probiotic Lactobacillus plantarum RJF4. LWT-Food SciTechnol 64: 1179-1186.
- Freitas F, Alves VD, Reis MA (2011) Advances in bacterial exopolysaccharides: from production to biotechnological applications. Trends Biotechnol 29: 388-398.
- Surayot U, Wang J, Seesuriyachan P, Kuntiya A, Tabarsa M, et al. (2014) Exopolysaccharides from lactic acid bacteria: structural analysis, molecular weight effect on immunomodulation. Int J BiolMacromol 68: 233-240.
- Kim Y, Oh S, Yun HS, Oh S, Kim SH (2010) Cell-bound exopolysaccharide from probiotic bacteria induces autophagic cell death of tumour cells. LettApplMicrobiol 51: 123-130.
- Lee SJ, Kim JH, Jung YW, Park SY, Shin WC, et al. (2011) Composition oforganic acids and physiological functionality of commercial makgeolli. Korean J Food SciTechnol 43: 206-212.
- Zajsek K, Gorsek A, Kolar M (2013) Cultivating conditions effects on kefiran production by the mixed culture of lactic acid bacteria imbedded within kefir grains. Food Chem 139: 970-977.
- Wang K, Li W, Rui X, Chen X, Jiang M, et al. (2014) Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J BiolMacromol 63: 133-139.
- Fontana C, Li S, Yang Z, Widmalm G (2015) Structural studies of the exopolysaccharide from Lactobacillus plantarum C88 using NMR spectroscopy and the program CASPER. Carbohydr Res 402: 87-94.
- Mende S, Rohm H, Jaros D (2016) Unfluence of exopolysaccharides on the structure, texture, stability and sensory properties of yoghurt and related products. Int Dairy J 52: 57-71.
- Patel S, Majumder A, Goyal A (2012) Potentials of exopolysaccharides from lactic Acid bacteria. Indian J Microbiol 52: 3-12.
- Ismail B, Nampoothiri KM (2010) Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch Microbiol 192: 1049-1057.
- Mozzi F, Savoy de Giori G, Font de Valdez G (2003) UDP-galactose 4-epimerase: a key enzyme in exopolysaccharide formation by Lactobacillus casei CRL 87 in controlled pH batch cultures. J ApplMicrobiol 94: 175-183.
- Patel A, Prajapati JB (2013) Food and Health Applications of Exopolysaccharides produced by Lactic acid Bacteria. Adv Dairy Res 1: 107.
- Malang SK, Maina NH, Schwab C, Tenkanen M, Lacroix C (2015) Characterization of exopolysaccharide and ropy capsular polysaccharide formation by Weissella. Food Microbiol 46: 418-427.
- Welman AD, Maddox IS (2003) Fermantation performance of an exopolysaccharide-producing strain of Lactobacillus delbruckii subsp. bulgaricus. J IndMicrobiolBiotechnol 30: 661-668.
- Kristo E, Miao Z, Corredig M (2011) The role of exopolysaccharide produced by Lactococcuslactis subsp. cremoris in structure formation and recovery of acid milk gels. Int Dairy J 21: 656-662.
- Ruas-Madiedo P, Hugenholtz J, Zoon P (2002) An owerview of the functionality of exopolysaccharides produced by lactic acid bacteria. Int Dairy J 12: 163-171.
- Torino MI, Font de Valdez G, Mozzi F (2015) Biopolymers from lactic acid bacteria. Novel applications in foods and beverages. Front Microbiol 6: 834.
- Tok E, Aslim B (2010) Cholesterol removal by some lactic acid bacteria that can be used as probiotic. MicrobiolImmunol 54: 257-264.
- Monsan P, Bozonnet S, Albenne C, Joucla G, Willemot RM, et al. (2001) Homopolysaccharides from lactic acid bacteria. Int Dairy J 12: 675-685.
- Galle S, Arendt EK (2014) Exopolysaccharides from sourdough lactic acid bacteria. Crit Rev Food SciNutr 54: 891-901.
- Ryan PM, Ross RP, Fitzgerald GF, Caplice NM, Stanson C (2015) Sugar-coated: exopolysaccharides producing lactic acid bacteria for food and human health applications. Food Funct 6: 679-693.
- Bajpai VK, Rather IA, Majumder R, Shukla S, Aeron A, et al. (2016) Exopolysaccharide and lactic acid bacteria: Perception, functionality and prospects. Bangladesh J Pharmacol 11: 1-23.
- Donot F, Fontana A, Baccou JC, Schorr-Galindo S (2012) Microbial exopolysaccharides: Main example of synthesis, excretion, genetics and extraction. Carbohydrate Polymer 87: 951-962.
- Becker A (2015) Challenges and perspectives in combinatorial assembly of novel exopolysaccharide biosynthesis pathways. Front Microbiol 6: 687.
- Juvonen R, Honkapää K, Maina NH, Shi Q, Viljanen K, et al. (2015) The impact of fermentation with exopolysaccharide producing lactic acid bacteria on rheological, chemical and sensory properties of pureed carrots (Daucuscarota L.). Int J Food Microbiol 207: 109-118.
- De Vuyst L, Degeest B (1999) Heteropolysaccharides from lactic acid bacteria. FEMS Microbiol Rev 23: 153-177.
- Madhuri KV, Prabhakar KV (2014) Microbial Exopolysaccharides: Biosynthesis and Potential Applications. Orient J Chem 30: 1401-1410.
- Harutoshi T (2013) Exopolysaccharides of Lactic Acid Bacteria for Food and Colon Health Applications. INTECH Open Access Publisher, Croatia.
- Provencher C, La Pointe G, Sirois S, Van Calsteren MR, Roy D (2003) Consensus-Degenerate Hybrid Oligonucleotide Primers for Amplification of Priming Glycosyltransferase Genes of the Exopolysaccharide Locus in Strains of the Lactobacillus casei Group. Appl Environ Microbiol 69: 3299-3307.
- Stingele F, Neeser JR, Mollet B (1996) Identification and characterization of the eps (Exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol 178: 1680-1690.
- Van Kranenburg R, Marugg JD, van Swam II, Willem NJ, de Vos WM (1997) Molecular characterization of the plasmid encoded eps gene cluster essential for exopolysaccharidebiosyntheisis in Lactococcuslactis. MolMicrobiol 24: 387-397.
- Dan T, Fukuda K, Sugai-Bannai M, Takakuwa N, Motoshima H, et al. (2009) Caharacterization and expression analysis of the exopolysaccharidesgenecluster in Lactobacillus fermentum TDS030603. BiosciBiotechnolBiochem 73: 2656-2664.
- Van der Meulen R, Grosu-Tudor S, Mozzi F, Vaningelgem F, Zamfir M, et al. (2007) Screening of lactic acid bacteria isolates from dairy and cereal products for exopolysaccharide production and genes involved. Int J Food Microbiol 118: 250-258.
- Duboc P, Mollet B (2001) Applications of exopolysaccharides in the dairy industry. Int Dairy J 11: 759-768.
Copyright: © 2016 Sanalibaba P, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.