International Journal of Advancements in Technology
Open Access
ISSN: 0976-4860
ISSN: 0976-4860
Research Article - (2015) Volume 6, Issue 2
The paper introduces a new aeration technology for stationary tanks with water mass. It is a passive aeration based on the principle of air diffusion, or more precisely oxygen diffusion into water. It uses the principles of Henry's law to increase the effect of diffusion. The basic element of this technology is a superhydrophobic or preferably an ultrahydrophobic surface. The basic characteristic, experimentally verified within this work, is the ability of the hydrophobic surface to bind the gas molecules. This makes the surface simultaneously aerophilic. The work includes the equipment design that utilizes this property. It is a cylinder provided with a double base. The first base closes the cylinder in conventional manner; the second base is formed by a porous membrane, which is fed from the bottom side with the gas (air, oxygen). Due to the aerophilic effect, a thin layer of air is formed on the hydrophobic surface. This layer passes oxygen to the water mass on the principle of diffusion, so that there is a continuous aeration, without the formation of undesirable bubbles. The efficiency of the diffusion increases with increasing hydrostatic pressure in accordance with Henry's law. The fundamental advantage of this principle is the direction of aeration, which in this technology extends from the bottom toward the free surface. This creates suitable conditions for microflora and purity of the water in the tank. The carried out experiments show that the amount of dissolved air depends only on the surface of the porous plate, or a suitably shaped container with a porous surface.This technology can be used both in small volumes (aquariums) as well as in large tanks where the maintenance of the required oxygen concentration in the long term is desired.A common feature is a comparable oxygenation capacity with a submersible aerator operating on the ejector principle.
<Keywords: Hydrophobia; Aeration; Plasma; Experiment
Oxygenation or aeration is a process which ensures the supply of air into a liquid [1,2]. Air bubbles pass through the liquid and partly dissolve [3].
Dissolving air into liquid is a common process of enriching the liquid with oxygen by which more oxygen is provided than can be supplied by the natural rate of aeration, for example in fish farms, waste water treatment, etc., where the amount of oxygen dissolved into the liquid is increased for consumption by living organisms.
The rate of diffusion is temperature and pressure dependent, as described by [4]. This method of air diffusion (bubbles) into the liquid is not economically ideal, as the majority of air passes through the liquid to the surface and only a small portion diffuses into the liquid. The equipment for aeration is in most cases based on the principle of active aeration. Air, or any gas, is injected into a liquid in the forms of bubbles convected via their own buoyancy forces or via liquid flow inertia. Mass transfer takes place during this process [5,6]. The size of the bubbles affects directly the rate of mass transfer and the required energy to inject the bubble. For a better efficiency, the devices are connected with a stirrer and the supplied air is dispersed in the form of very fine bubbles. Mixing of water with bubbles simultaneous stirring of the mixture cause diffusion of the gas into the liquid. The remaining air escapes to the surface. These devices depend on high energy consumption required for the air supply and the stirring performance and large volumes of gas supply. In certain cases, the mixing of water may even have a negative effect. Considering an aquarium, and also possibly similar aquatic habitats, oxygen supply for fish and plants is essential but restricted flow is required for their development. It is necessary to supply aerobic microorganisms with oxygen, for aerobic processes to progress. In the case of stagnant surface water the oxygen enters the solution spontaneously by diffusion from the surface. In such cases it would be possible to utilize the specific properties of surfaces with characteristic super or ultra-hydrophobicity. Materials that repel water simultaneously strongly attract air. When such surface is placed at the bottom of a tank, the gas is spontaneously released into the liquid, leading to saturation of the whole volume through diffusion [7]. Thus a given supply of air at the hydrophobic surface and is completely dissolved. Therefore the effectiveness of such water saturation with air is high relative to the amount of gas supplied. To test this means of aeration, an aeration device was developed and experiments on samples with various values of hydrophobicity were carried out.
Diffusion of gas into a liquid
The kinematics of the gas transfer into a liquid (diffusion) is expressed by the Laplace equation [8], which states that the rate of diffusion in a given time is directly proportional to the air deficiency (or oxygen saturation ratio). Air deficiency is the difference in the concentration at full air saturation and the current concentration.
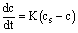 (1)
(1)
Oxygenation capacity
The term “oxygenation capacity” was introduced for the comparison of the performance of the individual aeration devices and is defined by:
OC = K.Cs (2)
Henry's law
Henry's law [8] applies generally to the solubility of gases in liquids as follows: “At a constant temperature, the amount of a given gas that dissolves in a given type and volume of liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.” This law is very important when using air for aeration rather than oxygen, since it specifies the parameter for the permeability of individual gases into liquids. It can be also written as:
Ci = Ki.Pi (3)
The basis of the entire experimental device is a set of an aeration element designed by [9]. It consists of a hydrophobic surface deposited on the starting porous material. The surface layer is in contact with the liquid. Compressed air is fed onto the bottom side of the porous material (Figures 1 and 2).
From past experience we know that the hydrophobic layer binds gas [7]. This creates a thin layer of air on the hydrophobic surface which is stable and in the sense of Henry´s law dissolves into the water. To meet this requirement, it is necessary to maintain the pressure difference between the air and the liquid at the site of the aeration element. The pressure difference is maintained automatically, based on the input differential pressure which characterizes the lifting capacity of the tested layer. The value of the pressure difference depends on the quality of the hydrophobic layer and the matrix used.
The carried gas (air) dissolves in water due to diffusion and therefore continuous feeding is necessary. For this purpose, a 100 liter pressure vessel was mounted to the circuit as well as a control valve connected to the blower (Figure 3).
Description of the measuring techniques and a system diagram
The aeration process is continuously monitored by a concentration sensor placed in the middle of the liquid column. The probe detects the amount of air dissolved in water and the temperature. The values of the aforementioned variables are sampled and transmitted to the evaluation unit every 2 minutes where all the data are backed up and simultaneously displayed.
All experiments were carried out without a free surface, i.e. the upper part of the reactor was closed and separated from the atmosphere by a water seal. The only changes during the experiments were in the differential pressure of the gas (air) supplied under the sample, or in other words the hydrophobic layer was saturated. The differential pressure was adjusted to the maximum limit, so as to avoid the spontaneous formation of bubbles and air runoff upwards the liquid column without dissolution.
Sdp – pressure difference sensor (Figure 3) - differential pressure transmitter DP 705, manufacturers NEWPORT, measuring range 6 kPa (A), accuracy±0.25% of range, current output 4-20 mA, serial number 16524
SkO – measuring of disolved O2 (Figure 3) - dissolved oxygen concentration sensor ENDRESS+HAUSER OXYMAX type COS61D, Decoder unit CM442-10K6/0, the measuring range of 0-20 mg/l, accuracy±1% of range, temperature range -20 a? 60°C
P+R - transducer+relay C/O+solenoid (Figure 3) – converter and regulator of pressure differential analog input 4-20 mA, regulation to constant differential pressure, control of the solenoid valve
The circular samples of foamed corundum ceramics InPor, 60 mm diameter, 20 mm thickness, were first ultrasonically cleaned in distilled water and subsequently dried in open air. The pore size of the InPor bases was measured by Quantachrome Poremaster ver.8.01. The best aeration results were obtained by samples with pore size of 100μm.
An atmospheric plasma nozzle jet generating high frequency nonisothermal plasma was used for sample pretreatment, patented by [11].
A Construction diagram and an Electrical Wiring Diagram of the plasma jet with slit nozzle shown in Figure 5. The plasma jet was connected via an impedance matching unit to radio frequency (RF) generator (13.56 MHz). Argon was utilized as a working gas and delivered at a flow rate of 50 l / min. N2 or O2 were added to the working gas at a flow rate of 0.5 l /min. The length of the working gap of the plasma jet was 120 mm and the width was approximately 1.5 mm. The body of the plasma jet was made of heat resistant and chemically inert mica composite. The power of the RF generator was set to 300 W.
The samples were put on a conveyor belt moving under the plasma nozzle and the following conditions were chosen: sample surface distance from the plasma jet orifice was about 2-3 mm, speed rate of the sample was 1, 3 m/min, the samples were treated twice. For plasma surface pretreatment of samples I2 and I3, argon with an admixture of O2 or N2 was chosen. After the plasma surface pretreatment, only upper layer of the two-component commercial hydrophobizing product Ultra Ever Dry (UED) [12] was sprayed. The surface modification was carried out on both sides.
On each side of the sample, the contact The measurement of the contact angle of water was carried out on the apparatus for evaluation of surface energy See System [10], see Figure 6 and Figure 7. A drop of water with a volume of 2 μl was used for the measurement. The measurement results are summarized in Table 1, the contact angles are averaged.
| Sample | Plasma pretreatment | Product | Curing | Contact Angeleswater | |
|---|---|---|---|---|---|
| side a | side b | ||||
| I1 | - | UED S+V | 50° C | 150,9° | 147,0° |
| I2 | Ar + O2/- | UED V | 50° C | 151,0° | 150,9° |
| I3 | Ar + N2/- | UED V | 50° C | 151,5° | 150,4° |
Table 1: The contact angles for a drop of water with a volume of 2 μl per individual samples of surface-modified foaming corundum ceramics InPor.
The hydrophobic aeration device by [13,14] was installed to the bottom part of the aeration reactor and the whole volume was gradually filled with water. An oxygen sensor was installed at the upper part of the cylinder for the determination of oxygen content in water. The sensor was placed approximately in the center in both the vertical and the horizontal directions.
The measurements were executed after all the control systems were switched on, the appropriate differential pressure in the range of 2 to 70 mbar was set and time was allowed for the system to settle down. The current values of concentration, temperature and time were obtained from the measurements. Based on the theoretical basis provided in the section above, the solubility of the gas at ambient conditions (average temperature and pressure) was calculated. The default value of the pressure was the hydrostatic pressure value.
After the integration of equation (1), reaching the initial conditions, calculation of the integration constant and conversion to the common logarithm we get the equation:
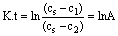 (4)
(4)
By substituting the measured data to equation (4) the progress of the function over time was obtained. By using linear regression on the function, the value K was obtained (Figure 8). From the measurement follows the linear dependence of the lnA on time, which validates equation (1).
The value K is according to equation (2) converted to an OC value and subsequently converted to standard conditions (T = 20°C, p = 101.3kPa) according to [15].
The above shown results demonstrate the status of specific samples, and while it is not explicitly possible to declare, clearly defective types of layers appeared during the test. It can be concluded that from the category of directly measured quantities, the samples labeled I1, I2 and I3 performed best in terms of oxygenation capacity efficiency and simultaneously attained above-average results in durability and functionality of the surface. Table 2 shows the long term tests on one sample that highlights the final oxygen concentration that can be reached.
| Time [hr] | 50 | 100 | 150 | 200 | 250 | 275 |
|---|---|---|---|---|---|---|
| CI1 [mg/l] | 3,40 | 3,72 | 3,96 | 4,15 | 4,32 | 4,49 |
| CI2[mg/l] | 3,48 | 3,94 | 4,28 | 4,60 | 4,86 | 4,90 |
| CI3 [mg/l] | 3,13 | 3,54 | 3,84 | 4,13 | 4,38 | 4,46 |
Table 2: Comparison of the oxygen concentration reached by chosen samples during long term testing.
Among the indirectly measured worse outcomes were inhomogeneity of the surface layers and variable permeability of the individual base layers which resulted in relatively broad range of differential pressures. A general conclusion can be drawn that the samples with fine grain structure are preferred due to their higher resistance and therefore qualitatively higher level of regulation.
Any hydrophobic coating will degrade over time. The degradation is so far described by visual observation and the suppressed ability to saturate liquid with gas. In the first stage, gradual loss of mirrored surfaces (sign of bonded air layer) occurs. The degraded surface is not able to bind the layer of gas (air) and in terms of diffusion becomes idle. In the second stage of the degradation process, gradual seepage of the liquid through the porous matrix occurs. Ultimately, complete flooding of the volume below the sample occurs and thereby aeration ends. The difference between a functional and a degraded hydrophobic surface is shown in Figure 9.
Passive aeration is a technology that uses one of the special properties of ultra-hydrophobic surfaces. This property consists of the hydrophobic surface being also strongly “aerophilic”. Adhesive forces on the ultra-hydrophobic surfaces are capable of "extracting" the gas from the liquid but on the other hand bonding the gas layer on its surface. Now this capability is used in the described technology.
It was shown in this work that it is possible to maintain a relatively thick layer of air on the surface of the hydrophobic membrane. This air dissolves in water by diffusion. If the membrane/porous plate is placed at a sufficient depth where it is exposed to hydrostatic pressure, the dissolution reaches a higher intensity, in accordance with Henry's law. It is therefore obvious that this technology of aeration is more effective than aeration in an open surface tank effected by diffusion from the free surface.
This technology may be used wherever it is necessary to ensure a long-term constant concentration of oxygen, for example in water tanks, aquariums and the like. It is not suitable for use in applications where a short term increase in oxygen concentration is necessary. In such cases, for example bubble aeration may be used. For aeration based on the microbubble theory the inputted air dissolves only partially in water, so that the efficiency of aeration relative to the energy input is very low. The comparison of the commercially used bubble aerators and passive aerator with various types of membranes can be seen in Tables 3 and 4.
| Sample | Notice | Diameter [mm] | Area [m2] | Depth of Submersion [mm] | Volume of water [m3] | Pressure p1[kPa] | Pressure p2[kPa] |
|---|---|---|---|---|---|---|---|
| I1 | measured | 53 | 0,0022 | 920 | 0,018 | 101,0 | 110,316 |
| I2 | measured | 53 | 0,0022 | 920 | 0,018 | 101,0 | 110,056 |
| I3 | measured | 53 | 0,0022 | 920 | 0,018 | 101,0 | 110,496 |
| vz2 | measured | 335 | 0,0881 | 1730 | 0,286 | 101,0 | 127,671 |
| AS055Tr | commercial | - | - | 876 | 14,89 | - | - |
| Equivalent AS055Tr | premise | - | 8,3797 | 920 | 14,89 | 101,0 | 110,056 |
Table 3: The comparison of samples with different diameter ultra-hydrophobic plate I-2 to vz2 and commercial product AS055Tr; P1: atmospheric air pressure [kPa]; P2: pressure at the inlet of the aeration element, primarily given by a hydrostatic pressure of the liquid and the resistance of the ceramic matrix with a hydrophobic surface [kPa].
| Sample | (OC)20 [mg/(m3.h)] | OC[mg/(m3.h)] | C2-C1 [mg/l] | Mass of Dissolved O2 [mg] | Mass of Air [mg] | Gas Energy [J] | Power [mW] |
|---|---|---|---|---|---|---|---|
| I1 | 3,17 | 1436,9 | 2,01 | 36,23 | 1,56E+02 | 0,04 | 0,0003 |
| I2 | 5,75 | 2606,3 | 1,5 | 27,04 | 1,17E+02 | 0,03 | 0,0002 |
| I3 | 4,53 | 2053,3 | 1,89 | 34,07 | 1,47E+02 | 0,03 | 0,0002 |
| vz2 | 225 | 2552,7 | 5 | 1430 | 6,17E+03 | 9,87 | 0,0685 |
| AS055Tr | 21840 | - | 7,75 | 115398 | 8,34E+06 | - | 640000 |
| Equivalent AS055Tr | 21840 | 2606,3 | 7,75 | 115398 | 4,98E+05 | 115,15 | 0,80 |
Table 4: Continuation of the comparison of the commercially used bubble aerators and passive aerator properties.
| cs | solubility of air under given temperature and pressure conditions | 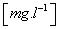 |
| c1,c2 | current air concentration in the water at time t1,t2 | 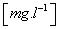 |
| K | volumetric coefficient of the air transfer | 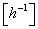 |
| Ci | concentration of water saturation with gas | 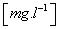 |
| OC | oxygenation capacity, indicates the amount of oxygen per volume unit of aerated water per time unit | 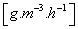 |
| (OC)20 | standardized oxygenation capacity multiplied by the empirical temperature coefficient [10] | 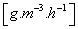 |
| Ki | absorption coefficient, i.e. the solubility of the gas in dependence on temperature | 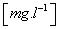 |
| Pi | partial pressure of the gas is directly proportional to the volume percentage in which is gas contained in the serving mixture for aeration | [Pa] |
| i | index that indicates the specific gas in a mixture serving for aeration | [-] |
For passive (no bubble) aeration, the efficiency is maximal because the aeration is accomplished only by diffusion without the formation of bubbles.
The amount of air dissolved depends only on the surface of the porous plate or a suitably shaped container with a porous surface. Tables 3 and 4, the comparison of samples with different diameter of the ultra-hydrophobic plate.
The technology can be used both in small volumes (aquariums) as well as in large tanks where the desired oxygen concentration needs to be maintained in the long term.
Equivalent AS055 represents a hypothetical aeration element with a total area size of approximately 8.5 m2 with a hydrophobic surface that is identical with sample I2. A common feature is the comparable oxygenation capacity with a commercial submersible aerator AS055. Tables 3 and 4 present the basic dimensions and parameters including the calculated values of the amount of sucked air and the energy requirements.
Mass of Dissolved Oxygen - stoichiometric calculation of the mass balance of oxygen dissolved in a given volume of aerated liquid.
Mass of Air - total value of the sucked air mass considering the weight ratio of the individual air components (75,51% N2, 23,16% O2, 1,28% Ar2, 0,05% CO2). In the case of non-wettable surfaces it is a value calculated based on the oxygen concentration measurement in water, for aerator AS055 the total value of sucked air during the test is measured during the testing.
Gas Energy – energy required for the adiabatic compression of gas from the atmospheric pressure p1 to pressure p2.
Power - value of the constant electric power of the equipment that is required for its operation.
Grant Agency of the Czech Republic within project GA101/13-20031S is gratefully acknowledged for the support of this work.