Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2016) Volume 6, Issue 4
Aims: To assess the antioxidant and antibacterial activities of two locally available natural honey samples, and determine the total phenolic content (TPC) of the honeys. Methods: Antibacterial activity of two local natural honey samples (citrus honey: LNH1 and mango honey: LNH2) was determined against the clinical bacterial isolates of Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enterica serovar Typhi, and Escherichia coli ATCC 25922 strain, by agar-well diffusion method. The antioxidative activity of the honey samples (LNH1 and LNH2) was determined in DPPH system, and the IC50 values were calculated. The bioactive components present in the test honey samples were assessed qualitatively, and the TPC was quantified. Results: The honey samples showed the presence of different bioactive components (flavonoids, steroids, phenol, terpenoids and quinone); the TPCs were estimated in LNH1 and LNH2 were 19.55 µg/ml and 33.3 µg/ml, respectively. In DPPH system, the scavenging activities of LNH1 and LNH2 ranged 43.46 – 75.18 % and 36.09 – 77. 23 %, respectively, with respective IC50 values of 3.83 mg/ml and 6.75 mg/ml. The test honey samples showed antibacterial activity both against gram-positive (S. aureus) and gram-negative (Ps. aeruginosa and S. enteric serovar Typhi) bacterial pathogens, and against the E. coli ATCC 25922 strain. Conclusion: The locally available natural honey may be used as rich source of antioxidant and can be used as effective antibacterial agent in order to combat the bacterial infection to humans.
<Keywords: Natural honeys; Antioxidative activity; DPPH system; IC50 values; Antibacterial activity; Bacterial pathogens
The emergence and dissemination of multiple antibiotic resistant (MAR) pathogenic bacteria are rising global public health concern. Some of the pathogenic bacterial strains including Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli and Salmonella enterica serovar Typhi being MDR, and some even showing resistance to the last-resort antibiotic, due to lack of discovery of new antimicrobials in last few years, cause serious untreatable infection to humans [1-4]. Taking into account the current problem of bacterial multiple antibiotic resistances, exploring the new antimicrobials from natural sources including honey (a natural product formed from the nectars in honeybees’ stomach), to combat the infection caused with the MAR bacteria is of prime significance [5-7]. The honey, which is known as a valued food since ancient, has been in use as a potent therapeutic agent in traditional medicine, and is currently a topic of renewed research interest among the scientists worldwide [7]. One of the most important properties of honey remains its broad-spectrum antibacterial activity [8]. In addition, honey is a well known natural antioxidant, and its antioxidative property has also been studied extensively, by the scientists from different parts of the world [8,9]. As per the report of Chan and Haron [10], the Gelam honey was found effective against Staph. aureus, Staph. epidermidis, Enterococcus faecalis, E. coli, S. enterica serovar Typhimurium, and Klebsiella pneumonia . The earlier studies demonstrated the antibacterial efficacy of five Malaysian honey samples against the nosocomial infection causing MAR isolates of E. coli and Ps. aeruginosa and Staph. aureus , and spore-forming bacterial strain B. cereus , potential to cause food poisoning as well as healthcare associated infection [11-13]. We have already assessed the antibacterial activity of natural (procured from Purulia district, West Bengal state, India) as well as commercial honeys against various bacterial pathogens belonging to both gram- negative and gram- positive [5,6].
One of the most familiar and importunate remedial usage of honey has been the wound dressing, undoubtedly due to its antimicrobial properties [14]. However, distinct variation in the beneficial effect, in terms of antibacterial and antioxidative activities, of honeys has been reported based upon the variation in phytogeographic regions (leading to the chemical composition variation) of honeys collected [15,16]. Moreover, no scientific study has been made in this issue, with natural honey samples from our part of the globe. The current study assesses the antibacterial activity against Staph. aureus, Ps. aeruginosa, E. coli and S. enterica serovar Typhi , and antioxidative capacity of natural honeys available from Malda district (West Bengal state, India); the honeys were assessed for the bioactive components, present in them, too.
Bacterial strains
The test bacterial strains included the clinical isolates of S. enterica serovar Typhi, Ps. aeruginosa , Staph. aureus and standard E. coli ATCC 25922 strain. From NRS Medical College, Kolkata (India), Dr. N. K. Pal, Professor and Head, Department of Microbiology, provided the identified bacterial isolates.
Honey samples
The two unstudied natural honey samples: citrus honey (LNH1) and mango honey (LNH2) (Figure 1), available locally from Malda district (West Bengal state, India), were used. The honey samples were collected from the local apiarists/honey collectors, who obtained honey by draining it conventionally by uncapping the comb frames. The identity of the honeys was confirmed by the honey collector/apiarist based on the geographic area of collection and floral availability at the location of bee hives, within the foraging reach [17]. The pH of the two monofloral honey samples (LNH1 and LNH2) was recorded. The honey samples (LNH1 and LNH2) were diluted with sterilized double distilled water (750 μg/μl), and divided into two equal parts, one of which was autoclaved, at 121°C, and thereafter stored at room temperature, for further work.
Antibacterial activity of honey
Antibacterial activity of the honey samples against the test bacterial isolates was determined by agar-well diffusion method [18], using an inoculum of 108 CFU spread on the surface of the nutrient agar plate, in order to determine the ZDI (zone diameter of inhibition), around the wells (5 mm diameter prepared on the agar plate using sterile plastic borer), each loaded with 50 μl (37.5 mg/well) honey samples; the honey concentration of 56.25 mg/well was also used. incubation at 35°C for 16-18 hrs [8].
Phytochemical analysis
The presence of bioactive components in the honey samples, LNH1 and LNH2, was screened following standard protocol mentioned earlier [19].
Estimation of total phenolic content
The total phenol content (TPC) of the collected honey samples was determined by Folin-Ciocalteu reagent (FCR), using gallic acid (GA) as the standard, as per the protocol described by Alzahrani et al. [20]. Briefly, an aliquot of 200 μl of honey solution (10% in distilled water) was mixed properly with 500 μl of FCR (10% in distilled water), and after 5 min, 1.5 ml of Na2CO3 solution (7.5% in distilled water) was added and mixed. The reaction mixture was incubated at room temperature in a dark place for 30 min, and thereafter absorbance was measured at 725 nm, in UV-Vis spectrophotometer. The GA (10, 20, 40, 60, 80 and 100 μg/ml) was used to construct the standard curve, and the TPC was expressed as mg of GA equivalents (GAE)/gram of honey.
Antioxidative activity
The antioxidant capacity of the honey samples was investigated using an in vitro method: the scavenging assay of 2, 2-diphenyl-1- picrylhydrazyl (DPPH) free radical. The reaction mixture prepared with 0.1 ml of honey sample dissolved in methanol (3-21 mg/ml), methanol (0.3 ml), and 0.3 mM DPPH methanolic reagent ((0.4 ml)), was incubated in darkness, for 30 min, at room temperature. The absorbance value of the reaction mixture was read using UV-Vis spectrophotometer, at 517 nm. The control was prepared as above without honey, and the baseline correction was done using methanol. The radical scavenging activity of honey (for each of the honey concentrations), which was expressed as the inhibition percentage, was calculated with the formula [8]:
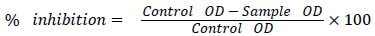 , and the IC50 value of individual samples was determined as per the protocol mentioned earlier [8].
, and the IC50 value of individual samples was determined as per the protocol mentioned earlier [8].
The pH values of the light coloured honey sample (LNH1) and dark coloured honey sample (LNH2) were 5.2 and 5.0, respectively. The qualitative analysis of phyto-components revealed the presence of flavonoids, steroids, phenol, terpenoids and quinone, and absence of glycosides, in both the honey samples tested.
The antibacterial activity of the honey samples are represented in Figures 2 and 3. The honey sample LNH1 showed growth inhibitory action against gram-positive bacteria: S. aureus (ZDIs: 28-32 mm, for non-autoclaved honey and 27-28 mm, for autoclaved honey), as well as gram-negative bacteria: E. coli ATCC 25922 (ZDIs: 30-35 mm, for non-autoclaved honey and 28-33 mm, for autoclaved honey), Ps. aeruginosa (ZDIs: 25-30 mm, for non-autoclaved honey and 24-28 mm, for autoclaved honey) and S. enterica serovar Typhi (ZDIs: 26-28 mm, for both non-autoclaved and autoclaved honeys). The gramnegative bacteria, E. coli ATCC 25922 and S. enterica serovar Typhi, were sensitive to the honey sample LNH2, having ZDIs 31-34 mm, for both non-autoclaved and autoclaved honeys, and 18-22 mm and 17-20 mm, respectively, for non-autoclaved and autoclaved honeys; for Ps. aeruginosa , ZDIs ranged 19-22 mm, for non-autoclaved honey and 18-19 mm, for autoclaved honey (at 48 h incubation). Due to the action of honey sample LNH2, the ZDIs obtained against Staph. aureus , at 48 h incubation, ranged 15-21 mm.
Figure 2: Antibacterial activity of LNH1 (citrus honey: autoclaved and non-autoclaved) and LNH2 (mango honey: autoclaved and non-autoclaved) against the gram-negative clinical bacterial isolates. Antibacterial activity of the honeys against E. coli ATCC 25922 strain is also shown. ZDI: zone diameter of inhibition.
The GA standard curve prepared in the determination of TPC values of honey samples is shown in Figure 4. The TPCs of the honey samples (LNH1 and LNH2) were calculated as 19.55 μg/ml and 33.3 μg/ml, respectively.
The scavenging activity (% inhibition) of the test honey samples (LNH1 and LNH2), as determined in DPPH system, is represented in Figure 5. The lowest inhibition caused by LNH1 and LNH2 honey samples were 43.46% and 36.09%, respectively, at concentrations 3 mg/ml, while the highest scavenging activities (75.18% and 77.23%, respectively) were found due to 15 mg/ml and 18 mg/ml concentrations of honey samples, respectively. The highest concentration of the honey samples used was 18 mg/ml, at which the scavenging activities were decreased to 69.61% for LNH1 and increased to 77.23% for LNH2. The IC50 values calculated were 3.83 mg/ml and 6.75 mg/ml for LNH1 and LNH2 honeys, respectively.
As per the authors’ knowledge, this is the first study that investigates the antibacterial and antioxidative activities of locally available natural honeys from this part of the globe. In this study, two gram-negative (S. enterica serovar Typhi and Ps. aeruginosa ) and one gram-positive (Staph. aureus ) clinical bacterial isolates, along with the E. coli ATCC 25922 strain, were considered as the representatives that are common cause of infection, both nosocomially and in the community, to humans [21-24]. Such bacterial strains are reported to be associated with food-borne illness and are resistant to multiple antibiotics [22-25]. The honey samples, having low pH values (pH: 5.0-5.2), did not contain any pathogenic microbial contamination, based upon the results of plate culture of the samples in different agar media.
The broad antibacterial spectrum of honey has been demonstrated in several studies reported earlier. Firdose et al. [26] reported three different honeys (neem, forest and acacia) with effective antimicrobial properties against antibiotic resistant bacteria (MRSA and MDR Ps. aeruginosa ). The thyme honey has been reported to be the most efficacious growth inhibitory agent against B. subtilis (at 6%), Streptococcus pyogenes (at 7.33%), E. coli (at 7.66%) and Ps. aeruginosa (at 9.50%) [27], while the Libyan eucalyptus honey had ZDIs 21 – 31 mm and 20 – 31 mm, respectively for E. coli and Staph. aureus isolates [28]. The ZDIs for the gram-negative (E. coli and Ps. aeruginosa ) and gram-positive (Staph. aureus ) test bacteria ranged 10-18 mm, from the action of Scaptotrigona bipunctata honey, and 8-13 mm, following treatment with Scaptotrigona postica honey [15]. Three different honey samples from Apis dorsata, Apis indica and Apis florae had inhibitory activities against Staph. aureus , Ps. aeruginosa and Klebsiella pneumoniae showing ZDIs 13-20, 13-14, 18-22 mm, respectively [29]. The apiarist honey as well as the honey packer honey showed growth inhibition activity against E. coli, K. pneumoniae and Ps. aeruginosa , and the average ZDIs of 17 mm for honey samples provided by the apiarists and 13 mm for honey samples procured from the honey packers have been noted [30]. Five natural honey samples from three countries (UAE, Yemen and Pakistan) had ZDIs 11-23 mm for Staph. aureus ATCC 29213 and 10-17 mm for Ps. aeruginosa ATCC 27453 standard strains, while for the clinical isolates, the ZDIs were recorded as 15-24 mm, 14-22 mm, 8-15 mm and 8-18 mm, respectively, for MRSA, Staph. aureus , E. coliand Ps. aeruginosa [31]. The ZDIs of honey samples, from Algeria, tested ranged 44-46 mm for Streptococcus pyogenes (S. pyogenes ) and Staph. aureus , with MICs 12-95% and 25-73%, respectively [32]. As has been reported by AINamma [33], that the honey sample had higher growth inhibitory activity against gram-negative bacteria (S. enterica serovar Typhi, Ps. aeruginosa and E. coli ) compared to the other test microorganisms. In our previous investigation, the two commercial honey samples (Dabur and Patanjali), showed greater activities against the gram-positive isolate of Staph. aureus (ZDIs: 24 mm and 30 mm, respectively) than against the gram-negative bacteria (E. coli , P. vulgaris , Ps. aeruginosa ) with respective ZDIs 11-13 mm and 15-21 mm [8]. However, in the current study, the honey sample LNH1 showed antibacterial activity in the order: E. coli > S. aureus > S. enterica serovar Typhi > Ps. aeruginosa , while the honey sample 2 was effective against S. enterica serovar Typhi and E. coli , at 24 h, and against Ps. aeruginosa and Staph. aureus at 48 h incubation. Thus, mixed results were obtained when the issues of growth inhibitory activities of honeys, against grampositive and gram-negative bacteria, have been considered; such variation in the honeys antibacterial activities has been linked to multiple issues: source and chemistry of honey, and geographic and seasonal (spatial and temporal) variation in honey collection [19,21,34].
The locally available natural as well as commercially available processed honeys (around the globe) are reported to contain antioxidant components (such as TPC, flavonoids, etc.), and to exert antioxidative activities with varied capacities [35,36]. The honey samples procured from seven counties had TPCs 17-66 mg GAE/g, and the antioxidative capacity was recorded higher for the sample with higher quantities of phenolic compounds [37]. From Croatia, Saric et al. [38] reported the IC50 values of chestnut honey as 14.24-24.56, acacia honey as 52.06-176.57 mg/ml, and the TPCs as 180-292.3 mgGAE/kg of chestnut honey and 6.67-142.2 mgGAE/kg of acacia honey. The lowest TPC was registered for Romanian honeydew honey, as 107.9 mgGAE/100g, and the highest for Polish honeydew honey, as 215.6 mgGAE/100g, showing excellent antioxidative activity with IC50 values 3.1-5.05 % for Romanian honeydew honey and 2.39 - 5.11 % for Polish honeydew honey [39]. The thyme honey has been found to display a highest phenolic (1138.53 mg GAE/kg) with potent antioxidative activity in DPPH system (IC50 value: 5.52 mg/ml) [27]. The antioxidant contents and DPPH values were estimated for Chinese honey samples from various floral sources (TPC: 9.57 - 136.17 mg GAE/100 g) and (antioxidant activity: 22.40-92.5%H); the values were inversely proportional to each other (TPC and DPPH scavenging activity), depending on their colour intensity [40]. The IC30 values of the raw and processed honeys ranged 126 - 625.79 μg/ml, with highest activity being noted in Trigona honey from Trivandrum, Kerala, India, having 97.21% inhibition at 500 μg/ml DPPH system [41,42] recorded higher TPC of chestnut honey (8.01 mg GAE/100 g) compared to the multifloral honey (4.48 mg GAE/100 g), with DPPH free radical scavenging activities of 19.04-71.92 as the IC50 values (μg/ml).The TPCs were found to be highest in raw neem honey (373.2 mg GAE/g) followed by raw forest honey (297.5 mg GAE/g) and raw acacia honey (205.6 mg GAE/g), as per the report of Firdose et al. [26]. As we have reported earlier, the commercial honeys had antioxidative activities, in DPPH system, with IC50 values 66.73-132.24 mg/ml, which were higher than the IC50 values (3.83 mg/ml and 6.75 mg/ml) of the natural honey samples (TPC: 19.55 μg/ml and 33.3 μg/ml) used in the current study, in the same system, and hence the local honeys are more effective as the antioxidant agents. The presence of steroids, terpenoids and quinones has been detected in two commercial honeys (India), as has been reported earlier [8], while in the current study the bioactive phytochemicals detected in the natural honeys included flavonoids, steroids, phenol, terpenoids and quinone, which (along with other components) possibly contributed to their antibacterial and antioxidative activities.
The local natural honeys, in this study, the citrus honey and mango honey, thus can be utilized as an excellent dietary source of antioxidants as well as the antibacterial agents, and hence duly be active for both therapeutic and prophylactic application. Such natural honeys might lead to the preparation of broad-spectrum antibacterial agents to combat the emergence of bacterial antibiotic resistance. However, further studies are essential in the issues of chemical analysis of the bioactive components of honey determining the antibacterial and antioxidative activities.