Journal of Thermodynamics & Catalysis
Open Access
ISSN: 2157-7544
ISSN: 2157-7544
Research Article - (2015) Volume 6, Issue 1
Apparent molar volume ( φV ) and viscosity B-coefficients were estimated for potassium chloride, potassium bromide and potassium iodide in aqueous mixture of L-Proline from measured solution density (ρ) and viscosity (η) at 298.15, 308.15 and 318.15 K at various electrolyte concentrations. The experimental density data were evaluated by Masson equation and the derived data were interpreted in terms of ion-solvent and ion-ion interactions. The viscosity data has been analyzed using Jones-Dole equation and the derived parameters, B and A, have also been interpreted in terms of ion-solvent and ion-ion interactions respectively. The structure-making or breaking capacity of the electrolyte under investigation has been discussed in terms of sign( ∂φV0 / ∂T )P.
Keywords: Potassium chloride; Potassium bromide; Potassium iodide; L-Proline; Ion-solvent interaction
Studies on densities (ρ) and viscosities (η) of electrolyte solutions are of great importance in characterizing the properties and structural aspects of solutions. The addition of an electrolyte to an aqueous organic solution alters the pattern of ion solvation and causes phenomenal changes in the behaviour of the dissolved electrolyte. Hence studies on the limiting apparent molar volume and viscosity B-coefficients of electrolyte provide us valuable information regarding ion-ion, ion-solvent and solvent-solvent interactions [1-3]. It has been found by a number of workers [4-6] that the addition of an electrolyte could either make or break the structure of a liquid. As the viscosity of a liquid depends on the intermolecular forces, the structural aspects of the liquid can be inferred from the viscosity of solutions at various electrolyte concentrations and temperature.
In this paper we have attempted to report the limiting apparent molar volume ( 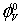 ), experimental slopes (
), experimental slopes ( 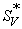 ) and viscosity B-coefficients for potassium chloride, potassium bromide and potassium iodide in aqueous mixture of L-Proline at 298.15, 308.15 and 318.15 K. Since potassium ion being a common cation for all of the electrolytes under investigation, the present work enables us to have a qualitative comparison of the role of anion in aqueous L-Proline in terms of various derived parameters obtained from viscosity (η) and density (ρ) measurements.
) and viscosity B-coefficients for potassium chloride, potassium bromide and potassium iodide in aqueous mixture of L-Proline at 298.15, 308.15 and 318.15 K. Since potassium ion being a common cation for all of the electrolytes under investigation, the present work enables us to have a qualitative comparison of the role of anion in aqueous L-Proline in terms of various derived parameters obtained from viscosity (η) and density (ρ) measurements.
Materials
L-Proline (SD. Fine Chemicals) was purified by standard methods [7]. The purity of the solvent was checked by measuring the viscosity (η) and density (ρ) at 298.15 K which was in good agreement with the literature values. Doubly distilled, degassed and deionised water with a specific conductance of 1 × 10-6/Ω/cm was used. Potassium chloride, potassium bromide and potassium iodide (Sigma-Aldrich, Germany) were purified by re-crystallizing twice from conductivity water and then dried in a vacuum desiccator over P2O5 for 24 h before use. The experimental values of viscosity (η) and density (ρ) of aqueous mixtures of 0.01 M, 0.03 M and 0.05 M L-Proline at different temperatures are listed in Table 1.
| Temperature (K) | ρ×10-3( kg/m) | η(mPa.s) | |
|---|---|---|---|
| 0.01 M L-proline | |||
| 298.15 K | 0.99753 | 0.909 | |
| 308.15 K | 0.99605 | 0.771 | |
| 318.15 K | 0.99466 | 0.639 | |
| 0.03 M L-proline | |||
| 298.15 K | 0.99902 | 0.916 | |
| 308.15 K | 0.99763 | 0.778 | |
| 318.15 K | 0.99604 | 0.645 | |
| 0.05 M L-proline | |||
| 298.15 K | 1.00045 | 0.923 | |
| 308.15 K | 0.99941 | 0.786 | |
| 318.15 K | 0.99772 | 0.652 | |
Table 1: Density (ρ, kg/m) and viscosity (η, mPa.s) of aqueous mixtures of 0.01 M L-proline, 0.03 M L-proline, 0.05 M L-proline at different temperatures.
Apparatus and procedure
Densities (ρ) were measured with an Anton Paar density-meter (DMA 4500M) with a precision of 0.0005 g/cm3. The calibration was done by double-distilled water and dry air and uncertainty in density was ± 0.00005 g/cm.
The measurements were done in a thermostat bath controlled to ± 0.01 K. Viscosity (η) was measured by means of Brookfield DV-III Ultra Programmable Rheometer with spindle size-42 having an accuracy of 1.0% and fitted to a Brookfield Digital Bath TC-500 at 298 K using density and viscosity values from the literature [8-10]. The uncertainty in viscosity measurements is within ± 0.003 mPa s. The mixtures were prepared by mixing known volume of pure liquids in airtight-stopper bottles and each solution thus prepared was distributed into three recipients to perform all the measurements in triplicate, with the aim of determining possible dispersion of the results obtained. Adequate precautions were taken to minimize evaporation loses during the actual measurements.
The electrolyte solutions studied here were prepared by mass and the conversion of molality into molarity was accomplished [3] using experimental density values. The experimental values of concentrations (c), densities (ρ), viscosities (η), and derived parameters at various temperatures are reported in Table 2.
| c(mol/dm3) | ρ×10-3( kg/m) | η (mPa.s) |
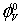 ×106 ×106( m3/mol ) |
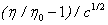 (kg1/2 mol-1/2) (kg1/2 mol-1/2) |
|---|---|---|---|---|
| Potassium chloride in aqueous mixture 0.01 M L-proline | ||||
| 298.15K | ||||
| 0.0151 | 0.99804 | 0.918 | 40.7724 | 0.081 |
| 0.0302 | 0.99862 | 0.923 | 38.4219 | 0.089 |
| 0.0452 | 0.99924 | 0.927 | 36.6533 | 0.093 |
| 0.059 | 0.99985 | 0.931 | 35.1369 | 0.1 |
| 0.0752 | 1.00059 | 0.935 | 33.7388 | 0.104 |
| 0.0903 | 1.00132 | 0.938 | 32.4331 | 0.106 |
| Potassium chloride in aqueous mixture 0.01 M L-proline | ||||
| 308.15K | ||||
| 0.0151 | 0.99651 | 0.779 | 44.1206 | 0.084 |
| 0.0302 | 0.99709 | 0.784 | 40.094 | 0.097 |
| 0.0452 | 0.99773 | 0.789 | 37.3202 | 0.11 |
| 0.059 | 0.99835 | 0.793 | 35.4719 | 0.117 |
| 0.0752 | 0.99914 | 0.798 | 33.3263 | 0.128 |
| 0.0903 | 0.99988 | 0.802 | 31.9725 | 0.134 |
| Potassium chloride in aqueous mixture 0.01 M L-proline | ||||
| 318.15K | ||||
| 0.0151 | 0.99509 | 0.647 | 46.1479 | 0.102 |
| 0.0302 | 0.99565 | 0.653 | 41.7753 | 0.126 |
| 0.0452 | 0.99628 | 0.659 | 38.6617 | 0.147 |
| 0.059 | 0.99693 | 0.664 | 35.9803 | 0.161 |
| 0.0752 | 0.99774 | 0.67 | 33.4493 | 0.177 |
| 0.0903 | 0.99858 | 0.675 | 30.951 | 0.187 |
| Potassium bromide in aqueous mixture 0.01 M L-proline | ||||
| 298.15K | ||||
| 0.0153 | 0.99853 | 0.922 | 53.5574 | 0.116 |
| 0.0303 | 0.99959 | 0.931 | 50.8657 | 0.139 |
| 0.0454 | 1.00071 | 0.94 | 48.7479 | 0.16 |
| 0.0607 | 1.00189 | 0.948 | 46.9048 | 0.174 |
| 0.0759 | 1.00311 | 0.957 | 45.1597 | 0.192 |
| 0.0912 | 1.00438 | 0.966 | 43.5138 | 0.208 |
| Potassium bromide in aqueous mixture 0.01 M L-proline | ||||
| 308.15K | ||||
| 0.0153 | 0.99692 | 0.783 | 62.103 | 0.126 |
| 0.0303 | 0.99796 | 0.792 | 55.8276 | 0.156 |
| 0.0454 | 0.99911 | 0.801 | 51.3776 | 0.183 |
| 0.0607 | 1.00036 | 0.81 | 47.6963 | 0.205 |
| 0.0759 | 1.00168 | 0.819 | 44.4546 | 0.226 |
| 0.0912 | 1.00305 | 0.828 | 41.8137 | 0.245 |
| Potassium bromide in aqueous mixture 0.01 M L-proline | ||||
| 318.15K | ||||
| 0.0153 | 0.99542 | 0.651 | 69.3779 | 0.152 |
| 0.0303 | 0.9964 | 0.661 | 61.4873 | 0.198 |
| 0.0454 | 0.99754 | 0.671 | 55.3595 | 0.235 |
| 0.0607 | 0.9988 | 0.681 | 50.4901 | 0.267 |
| 0.0759 | 1.00015 | 0.69 | 46.2727 | 0.29 |
| 0.0912 | 1.00162 | 0.701 | 42.2061 | 0.321 |
| Potassium iodide in aqueous mixture 0.01 M L-proline | ||||
| 298.15K | ||||
| 0.0151 | 0.99887 | 0.926 | 77.1285 | 0.152 |
| 0.0302 | 1.00029 | 0.941 | 74.3642 | 0.203 |
| 0.0451 | 1.00175 | 0.955 | 72.0751 | 0.238 |
| 0.0604 | 1.00329 | 0.968 | 70.1716 | 0.264 |
| 0.0755 | 1.00486 | 0.982 | 68.3442 | 0.292 |
| 0.0906 | 1.00647 | 0.995 | 66.6517 | 0.314 |
| Potassium iodide in aqueous mixture 0.01 M L-proline | ||||
| 308.15K | ||||
| 0.0151 | 0.99714 | 0.788 | 93.7994 | 0.179 |
| 0.0302 | 0.99851 | 0.803 | 84.3485 | 0.239 |
| 0.0451 | 1.00001 | 0.818 | 77.8489 | 0.287 |
| 0.0604 | 1.00168 | 0.833 | 72.2998 | 0.327 |
| 0.0755 | 1.00341 | 0.847 | 67.9012 | 0.359 |
| 0.0906 | 1.00525 | 0.861 | 63.7203 | 0.388 |
| Potassium iodide in aqueous mixture 0.01 M L-proline | ||||
| 318.15K | ||||
| 0.0151 | 0.99559 | 0.656 | 104.5438 | 0.217 |
| 0.0302 | 0.99691 | 0.672 | 91.3819 | 0.297 |
| 0.0451 | 0.99839 | 0.688 | 82.9873 | 0.361 |
| 0.0604 | 1.0001 | 0.703 | 75.4455 | 0.408 |
| 0.0755 | 1.00189 | 0.718 | 69.5956 | 0.45 |
| 0.0906 | 1.00387 | 0.733 | 63.5555 | 0.489 |
| Potassium chloride in aqueous mixture 0.03 M L-proline | ||||
| 298.15K | ||||
| 0.015 | 0.99921 | 0.923 | 61.945 | 0.062 |
| 0.0301 | 0.99949 | 0.927 | 58.9421 | 0.069 |
| 0.0451 | 0.99984 | 0.931 | 56.3604 | 0.077 |
| 0.0603 | 1.00027 | 0.935 | 53.7704 | 0.085 |
| 0.0754 | 1.00075 | 0.938 | 51.5348 | 0.087 |
| 0.0907 | 1.00137 | 0.942 | 48.5495 | 0.094 |
| Potassium chloride in aqueous mixture 0.03 M L-proline | ||||
| 308.15K | ||||
| 0.0151 | 0.99772 | 0.785 | 68.7139 | 0.073 |
| 0.0301 | 0.99795 | 0.79 | 64.0361 | 0.089 |
| 0.0451 | 0.99829 | 0.795 | 59.9847 | 0.103 |
| 0.0603 | 0.99871 | 0.799 | 56.6853 | 0.11 |
| 0.0754 | 0.99924 | 0.804 | 53.2104 | 0.122 |
| 0.0907 | 0.99979 | 0.808 | 50.6903 | 0.128 |
| Potassium chloride in aqueous mixture 0.03 M L-proline | ||||
| 318.15K | ||||
| 0.0151 | 0.99619 | 0.652 | 64.8067 | 0.088 |
| 0.0302 | 0.99659 | 0.658 | 56.4412 | 0.116 |
| 0.0451 | 0.99712 | 0.664 | 50.6549 | 0.139 |
| 0.0604 | 0.99784 | 0.669 | 44.7281 | 0.151 |
| 0.0756 | 0.99866 | 0.675 | 39.7752 | 0.169 |
| 0.0907 | 0.99964 | 0.679 | 34.7144 | 0.175 |
| Potassium bromide in aqueous mixture 0.03 M L-proline | ||||
| 298.15K | ||||
| 0.015 | 0.99923 | 0.928 | 105.103 | 0.107 |
| 0.0301 | 0.99983 | 0.938 | 92.0902 | 0.138 |
| 0.0452 | 1.00074 | 0.947 | 80.857 | 0.159 |
| 0.0603 | 1.00193 | 0.956 | 70.5692 | 0.178 |
| 0.0754 | 1.00332 | 0.965 | 61.7272 | 0.195 |
| 0.0905 | 1.00486 | 0.975 | 54.1642 | 0.214 |
| Potassium bromide in aqueous mixture 0.03 M L-proline | ||||
| 308.15K | ||||
| 0.0151 | 0.99774 | 0.79 | 111.9319 | 0.126 |
| 0.0302 | 0.99842 | 0.802 | 92.8868 | 0.178 |
| 0.0453 | 0.99948 | 0.812 | 78.0739 | 0.205 |
| 0.0604 | 1.00089 | 0.822 | 64.8203 | 0.23 |
| 0.0755 | 1.00258 | 0.832 | 53.1259 | 0.253 |
| 0.0906 | 1.00448 | 0.842 | 42.9908 | 0.273 |
| Potassium bromide in aqueous mixture 0.03 M L-proline | ||||
| 318.15K | ||||
| 0.0151 | 0.99612 | 0.657 | 114.1186 | 0.152 |
| 0.0302 | 0.99691 | 0.669 | 90.3578 | 0.214 |
| 0.0453 | 0.99818 | 0.68 | 71.7285 | 0.255 |
| 0.0604 | 0.99985 | 0.691 | 55.7207 | 0.29 |
| 0.0755 | 1.00185 | 0.702 | 41.6985 | 0.322 |
| 0.0906 | 1.00412 | 0.713 | 29.3384 | 0.35 |
| Potassium iodide in aqueous mixture 0.03 M L-proline | ||||
| 298.15K | ||||
| 0.0149 | 0.99928 | 0.934 | 148.643 | 0.161 |
| 0.0302 | 1.00032 | 0.95 | 122.8749 | 0.214 |
| 0.0456 | 1.0018 | 0.965 | 104.7895 | 0.251 |
| 0.0604 | 1.00356 | 0.98 | 90.4247 | 0.284 |
| 0.0755 | 1.00571 | 0.996 | 76.8781 | 0.318 |
| 0.0906 | 1.00802 | 1.012 | 66.0675 | 0.348 |
| Potassium iodide in aqueous mixture 0.03 M L-proline | ||||
| 308.15K | ||||
| 0.0149 | 0.99781 | 0.796 | 154.2313 | 0.19 |
| 0.0302 | 0.99878 | 0.813 | 127.989 | 0.259 |
| 0.0456 | 1.00027 | 0.829 | 107.9414 | 0.307 |
| 0.0605 | 1.00225 | 0.845 | 89.2142 | 0.35 |
| 0.0756 | 1.00455 | 0.861 | 73.9113 | 0.388 |
| 0.0907 | 1.00701 | 0.879 | 61.9273 | 0.431 |
| Potassium iodide in aqueous mixture 0.03 M L-proline | ||||
| 318.15K | ||||
| 0.0149 | 0.99612 | 0.663 | 161.2379 | 0.229 |
| 0.0302 | 0.99723 | 0.68 | 126.7934 | 0.312 |
| 0.0456 | 0.99899 | 0.697 | 101.1548 | 0.378 |
| 0.0605 | 1.00115 | 0.714 | 81.1575 | 0.435 |
| 0.0756 | 1.00374 | 0.731 | 63.5879 | 0.485 |
| 0.0907 | 1.00674 | 0.749 | 47.3012 | 0.535 |
| Potassium chloride in aqueous mixture 0.05 M L-proline | ||||
| 298.15 K | ||||
| 0.0147 | 1.00058 | 0.931 | 66.192 | 0.071 |
| 0.0298 | 1.00085 | 0.937 | 61.6009 | 0.088 |
| 0.0449 | 1.00122 | 0.942 | 57.8579 | 0.097 |
| 0.0602 | 1.00167 | 0.947 | 54.712 | 0.106 |
| 0.0754 | 1.00215 | 0.952 | 52.421 | 0.114 |
| 0.0904 | 1.00271 | 0.957 | 49.9364 | 0.123 |
| Potassium chloride in aqueous mixture 0.05 M L-proline | ||||
| 308.15 K | ||||
| 0.0147 | 0.99955 | 0.794 | 65.5701 | 0.084 |
| 0.0298 | 0.99998 | 0.8 | 55.9323 | 0.103 |
| 0.0449 | 1.00064 | 0.806 | 47.6291 | 0.12 |
| 0.0602 | 1.00141 | 0.812 | 41.7613 | 0.135 |
| 0.0754 | 1.00234 | 0.817 | 36.1032 | 0.144 |
| 0.0903 | 1.00348 | 0.823 | 29.8654 | 0.157 |
| Potassium chloride in aqueous mixture 0.05 M L-proline | ||||
| 318.15 K | ||||
| 0.0147 | 0.99787 | 0.66 | 64.981 | 0.101 |
| 0.0298 | 0.99833 | 0.667 | 54.6447 | 0.133 |
| 0.0449 | 0.99899 | 0.674 | 46.7675 | 0.159 |
| 0.0603 | 0.99985 | 0.681 | 39.6604 | 0.181 |
| 0.0754 | 1.00089 | 0.688 | 32.9022 | 0.201 |
| 0.0904 | 1.00199 | 0.695 | 27.6887 | 0.219 |
| Potassium bromide in aqueous mixture 0.05 M L-proline | ||||
| 298.15 K | ||||
| 0.0152 | 1.00061 | 0.935 | 108.4123 | 0.105 |
| 0.0304 | 1.00135 | 0.945 | 89.2875 | 0.137 |
| 0.0454 | 1.00251 | 0.954 | 73.4616 | 0.158 |
| 0.0605 | 1.00389 | 0.964 | 61.9252 | 0.181 |
| 0.0756 | 1.00556 | 0.974 | 51.1534 | 0.201 |
| 0.0907 | 1.00753 | 0.983 | 40.6693 | 0.216 |
| Potassium bromide in aqueous mixture 0.05 M L-proline | ||||
| 308.15 K | ||||
| 0.0152 | 0.99958 | 0.798 | 107.8545 | 0.124 |
| 0.0304 | 1.00046 | 0.809 | 84.4013 | 0.168 |
| 0.0454 | 1.00177 | 0.82 | 66.8686 | 0.203 |
| 0.0605 | 1.00345 | 0.83 | 52.0043 | 0.228 |
| 0.0756 | 1.00542 | 0.841 | 39.243 | 0.254 |
| 0.0907 | 1.00756 | 0.851 | 28.872 | 0.275 |
| Potassium bromide in aqueous mixture 0.05 M L-proline | ||||
| 318.15 K | ||||
| 0.0152 | 0.99789 | 0.664 | 108.0181 | 0.149 |
| 0.0304 | 0.99906 | 0.676 | 74.8907 | 0.211 |
| 0.0454 | 1.00072 | 0.687 | 52.7317 | 0.252 |
| 0.0605 | 1.00279 | 0.698 | 34.9094 | 0.287 |
| 0.0756 | 1.00533 | 0.709 | 18.0126 | 0.318 |
| 0.0907 | 1.00822 | 0.721 | 2.945 | 0.351 |
| Potassium iodide in aqueous mixture 0.05 M L-proline | ||||
| 298.15 K | ||||
| 0.0146 | 1.00065 | 0.941 | 152.2114 | 0.161 |
| 0.0303 | 1.00155 | 0.959 | 129.5148 | 0.224 |
| 0.0452 | 1.00301 | 0.976 | 109.0648 | 0.27 |
| 0.0607 | 1.00488 | 0.993 | 92.6019 | 0.308 |
| 0.0759 | 1.00713 | 1.009 | 77.4794 | 0.338 |
| 0.0908 | 1.00958 | 1.025 | 64.8759 | 0.367 |
| Potassium iodide in aqueous mixture 0.05 M L-proline | ||||
| 308.15 K | ||||
| 0.0146 | 0.99962 | 0.804 | 151.6684 | 0.19 |
| 0.0303 | 1.00072 | 0.823 | 122.6546 | 0.27 |
| 0.0452 | 1.00236 | 0.841 | 100.5065 | 0.329 |
| 0.0607 | 1.00433 | 0.859 | 84.5345 | 0.377 |
| 0.0759 | 1.00688 | 0.876 | 67.0643 | 0.416 |
| 0.0908 | 1.00963 | 0.893 | 52.8721 | 0.452 |
| Potassium iodide in aqueous mixture 0.05 M L-proline | ||||
| 318.15 K | ||||
| 0.0146 | 0.99794 | 0.67 | 151.2114 | 0.228 |
| 0.0303 | 0.99944 | 0.69 | 109.1685 | 0.335 |
| 0.0453 | 1.00165 | 0.709 | 78.8492 | 0.411 |
| 0.0607 | 1.00446 | 0.728 | 54.4682 | 0.473 |
| 0.0759 | 1.00769 | 0.746 | 34.0835 | 0.523 |
| 0.0908 | 1.01154 | 0.764 | 13.2989 | 0.57 |
Table 2: The concentration (c), density (ρ), viscosity (η), apparent molar volume 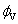 and
and 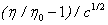 of potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01M L-proline , 0.03M L-proline, 0.05M L-proline at different temperatures.
of potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01M L-proline , 0.03M L-proline, 0.05M L-proline at different temperatures.
Density calculation
The apparent molar volumes (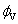 ) were determined from the solution densities using the following Equation [3]:
) were determined from the solution densities using the following Equation [3]:
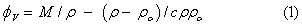
Where M is the molar mass of the solute, c is the molarity of the solution; ρ0 and ρ are the densities of the solvent and the solution respectively. The limiting apparent molar volumes 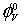 was calculated using a least-squares treatment to the plots of
was calculated using a least-squares treatment to the plots of 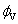 versus √c using the following Masson equation [11]:
versus √c using the following Masson equation [11]:
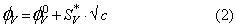
Where ( 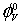 ) is the apparent molar volume at infinite dil ution and (
) is the apparent molar volume at infinite dil ution and ( 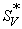 ) is the experimental slope. The plots of (
) is the experimental slope. The plots of (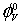 ) against square root of molar concentration (√c) were found to be linear as depicted graphically in Figures 1-9 with negative slopes. Values of
) against square root of molar concentration (√c) were found to be linear as depicted graphically in Figures 1-9 with negative slopes. Values of 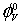 and
and 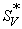 are reported in Table 3.
are reported in Table 3.
| Molarity of L-Proline | 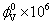 (m3/mol) (m3/mol) |
 (m2/mol3/2 L1/2) (m2/mol3/2 L1/2) |
||||
|---|---|---|---|---|---|---|
| 298.15 K | 308.15 K | 318.15 K | 298.15 K | 308.15 K | 318.15 K | |
| 0.01 | 46.56 | 52.19 | 56.59 | -46.89 | -68.46 | -84.87 |
| 0.03 | 71.52 | 81.51 | 85.63 | -73.76 | -102.01 | -167.33 |
| 0.05 | 77.16 | 89.67 | 90.59 | -90.67 | -196.92 | -208.7 |
| Potassium bromide | ||||||
| 0.01 | 60.6 | 75.84 | 87.98 | -56.09 | -113.71 | -151.83 |
| 0.03 | 141.42 | 160.01 | 173.02 | -288.91 | -388.12 | -477.11 |
| 0.05 | 155.11 | 162.3 | 178.49 | -379.72 | -446.42 | -584.88 |
| Potassium iodide | ||||||
| 0.01 | 84.48 | 113.9 | 131.59 | -58.75 | -168.01 | -227.4 |
| 0.03 | 203.81 | 218.21 | 237.49 | -460.91 | -521.5 | -634.07 |
| 0.05 | 212.82 | 217.51 | 241.73 | -489.42 | -545.32 | -758.61 |
Table 3: Limiting apparent molar volumes (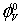 ) and experimental slopes (
) and experimental slopes (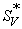 ) of potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01M L-proline , 0.03M L-proline, 0.05M L-proline at different temperatures.
) of potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01M L-proline , 0.03M L-proline, 0.05M L-proline at different temperatures.
In these systems the ion-solvent and ion-ion interactions can be interpreted in terms of structural changes between various components of the solvent and solution systems. 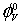 can be used to interpret ionsolvent interactions. A perusal of Table 3 reveals that the
can be used to interpret ionsolvent interactions. A perusal of Table 3 reveals that the 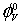 values are positive and increase with a rise in both the temperature and amount of L-Proline in the mixtures. This indicates the presence of strong ionsolvent interactions and these interactions are further strengthened at higher temperatures and higher molar mass of L-Proline in the mixtures, suggesting larger electrostriction at higher temperatures and in enhanced amount of L-Proline.
values are positive and increase with a rise in both the temperature and amount of L-Proline in the mixtures. This indicates the presence of strong ionsolvent interactions and these interactions are further strengthened at higher temperatures and higher molar mass of L-Proline in the mixtures, suggesting larger electrostriction at higher temperatures and in enhanced amount of L-Proline.
A perusal of Table 3 also reveals that the 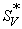 values are negative for all the solutions at all the experimental temperatures and
values are negative for all the solutions at all the experimental temperatures and 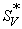 values decrease as the experimental temperature and amount of L-Proline in the mixtures increases. Since
values decrease as the experimental temperature and amount of L-Proline in the mixtures increases. Since 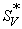 is a measure of ion-ion interactions, the results indicate the presence of weak ion-ion interactions in the solutions at all the experimental temperatures and these interactions further decrease with a rise in temperature and increase in molar mass L-Proline in the mixtures. In other words, it may be said that the solvation of electrolyte/ions increases with the increase of L-Proline content in water. This is probably due to more violent thermal agitation at higher temperatures, resulting in diminishing the force of ion-ion interactions (ionic-dissociation) [12]. This suggests that ion-solvent interactions dominate over ion-ion interactions in all the solutions and at all experimental temperatures.
is a measure of ion-ion interactions, the results indicate the presence of weak ion-ion interactions in the solutions at all the experimental temperatures and these interactions further decrease with a rise in temperature and increase in molar mass L-Proline in the mixtures. In other words, it may be said that the solvation of electrolyte/ions increases with the increase of L-Proline content in water. This is probably due to more violent thermal agitation at higher temperatures, resulting in diminishing the force of ion-ion interactions (ionic-dissociation) [12]. This suggests that ion-solvent interactions dominate over ion-ion interactions in all the solutions and at all experimental temperatures.
The variation of 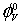 with temperature of potassium chloride, potassium bromide and potassium iodide in aqueous mixture of LProline follows the polynomial,
with temperature of potassium chloride, potassium bromide and potassium iodide in aqueous mixture of LProline follows the polynomial,
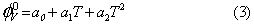
Over the temperature range under study where T is the temperature in K. Values of coefficients of the above equation for potassium chloride, potassium bromide and potassium iodide in aqueous mixture of L- Proline are reported in Table 4.
| Molarity of L-proline | a0( m3/mol) | a1( m3/mol/K) | a2(m3/mol/K) |
|---|---|---|---|
| Potassium chloride | |||
| 0.01 | -686.321 | 4.2917 | -0.0062 |
| 0.03 | -2922.861 | 18.7939 | -0.0293 |
| 0.05 | -5619.977 | 36.3861 | -0.0579 |
| Potassium bromide | |||
| 0.01 | -1817.842 | 10.9216 | -0.0155 |
| 0.03 | -2976.151 | 18.7747 | -0.0279 |
| 0.05 | 4075.112 | -26.5645 | 0.0451 |
| Potassium iodide | |||
| 0.01 | -6181.141 | 38.5014 | -0.0586 |
| 0.03 | 2016.222 | -13.3537 | 0.0244 |
| 0.05 | 9044.573 | -58.7362 | 0.0976 |
Table 4: Values of the coefficients of Eq. (4) for potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01 M L-proline, 0.03 M L-proline, 0.05M L-proline at different temperatures.
The apparent molar expansibilities (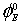 ) can be obtained by the following equation:
) can be obtained by the following equation:
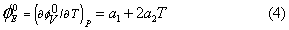
The values of 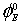 for different solutions of the studied electrolytes at 298.15, 308.15 and 318.15 K are reported in Table 5. From the table it is evident that the values of
for different solutions of the studied electrolytes at 298.15, 308.15 and 318.15 K are reported in Table 5. From the table it is evident that the values of 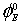 for potassium chloride increases with the increase in the amount of L- Proline in the mixture. However, for potassium bromide and potassium iodide the
for potassium chloride increases with the increase in the amount of L- Proline in the mixture. However, for potassium bromide and potassium iodide the 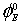 values were found to be rather complicated to explain. During the past few years it has been emphasized by a number of workers that
values were found to be rather complicated to explain. During the past few years it has been emphasized by a number of workers that 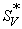 is not the sole criterion for determining the structure making or breaking tendency of any solute. Hepler [13] developed a technique of examining the sign of
is not the sole criterion for determining the structure making or breaking tendency of any solute. Hepler [13] developed a technique of examining the sign of 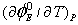 for the solute in terms of long-range structure-making and breaking capacity of the electrolytes in the mixed solvent systems. The general thermodynamic expression used is as follows
for the solute in terms of long-range structure-making and breaking capacity of the electrolytes in the mixed solvent systems. The general thermodynamic expression used is as follows
| Molarity of L-Proline | 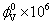 (m3/mol) (m3/mol) |
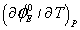 (m3/mol/k) (m3/mol/k) |
||
|---|---|---|---|---|
| 298.15 K | 308.15 K | 308.15 K | ||
| Potassium chloride | ||||
| 0.01 | 0.0595 | 0.47064 | 0.34664 | -0.0124 |
| 0.03 | 1.3223 | 1.7363 | 0.34664 | -0.0586 |
| 0.05 | 1.8603 | 0.7023 | 0.4556 | -0.1158 |
| Potassium bromide | ||||
| 0.01 | 1.6789 | 1.3690 | 1.0589 | -0.0310 |
| 0.03 | 2.1379 | 1.5799 | 1.0220 | -0.0558 |
| 0.05 | 0.3286 | 1.2306 | 2.1326 | 0.0902 |
| Potassium iodide | ||||
| 0.01 | 3.5582 | 2.3862 | 1.2142 | -0.1172 |
| 0.03 | 1.1960 | 1.6840 | 2.1720 | 0.0488 |
| 0.05 | -0.5373 | 1.4146 | 3.3666 | 0.1952 |
Table 5: Limiting partial molar expansibilities for potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01 M L-proline , 0.03 M L-proline, 0.05 M L-proline at different temperatures.

If the sign of 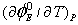 is positive or small negative [14,15] the electrolyte is a structure maker and when the sign of
is positive or small negative [14,15] the electrolyte is a structure maker and when the sign of 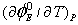 is negative, it is a structure breaker. As is evident from Table 5, the electrolyte under investigation generally acts as a structure breaker.
is negative, it is a structure breaker. As is evident from Table 5, the electrolyte under investigation generally acts as a structure breaker.
The viscosity data of solutions for the electrolytes in 0.01 M, 0.03M, 0.05M L-Proline have been analyzed using Jones-Dole [16] equation:
Where η0 and η are the viscosities of the solvent and solution respectively. A and B are the constants estimated by least square method and are reported in Table 6. From the table it is evident that the values of the A-coefficient are very small for all the solutions under investigation at all experimental temperatures. These results indicate the presence of weak ion-ion interactions, and these interactions further decrease with both rise of experimental temperatures and amount of L-Proline suggesting an increase in ion-solvation. Interestingly, values are found to be smallest for Potassium iodide and hence it may be concluded that solubility in aqueous L-Proline solutions is maximum for Potassium iodide and minimum for Potassium chloride.
| Molarity of L-proline | B-coefficient(dm3/2/mol1/2) | A-coefficient( dm3/mol) | ||||
|---|---|---|---|---|---|---|
| 298.15 K | 308.15 K | 318.15 K | 298.15 K | 308.15 K | 318.15 K | |
| Potassium chloride | ||||||
| 0.01 | 0.149 | 0.283 | 0.488 | 0.062 | 0.048 | 0.042 |
| 0.03 | 0.179 | 0.308 | 0.496 | 0.039 | 0.035 | 0.029 |
| 0.05 | 0.278 | 0.403 | 0.658 | 0.038 | 0.034 | 0.02 |
| Potassium bromide | ||||||
| 0.01 | 0.511 | 0.669 | 0.938 | 0.05 | 0.041 | 0.035 |
| 0.03 | 0.588 | 0.81 | 1.103 | 0.034 | 0.031 | 0.019 |
| 0.05 | 0.625 | 0.849 | 1.116 | 0.027 | 0.019 | 0.013 |
| Potassium iodide | ||||||
| 0.01 | 0.903 | 1.177 | 1.525 | 0.043 | 0.035 | 0.031 |
| 0.03 | 1.037 | 1.325 | 1.706 | 0.032 | 0.026 | 0.016 |
| 0.05 | 1.137 | 1.451 | 1.889 | 0.025 | 0.016 | 0.004 |
Table 6: Values of A and B coefficients for potassium chloride, potassium bromide and potassium iodide in different aqueous mixtures 0.01M L-proline , 0.03M L-proline, 0.05M L-proline at different temperatures.
The effects of ion-solvent interactions on the solution viscosity can be inferred from the B-coefficient [17,18]. The viscosity B-coefficient is a valuable tool to provide information concerning the solvation of the solutes and their effects on the structure of the solvent. From Table 6 it is evident that the values of the B-coefficient of potassium chloride, potassium bromide and potassium iodide in the studied solvent systems are more positive than A-coefficients, thereby suggesting the presence of strong ion-solvent interactions, and these types of interactions are strengthened with a rise in both temperature and amount of L-Proline in solutions. These conclusions are in excellent agreement with those drawn from values 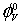 discussed earlier. It has been reported in a number of studies [19,20] that dB/dT is a better criterion for determining the structure-making/breaking nature of any solute rather than simply the value of the B-coefficient. It is found from Table 6 that the values of the B-coefficient increase with a rise in temperature (positive dB/dT) suggesting the structure-breaking tendency of potassium chloride, potassium bromide and potassium iodide in the solvent systems.
discussed earlier. It has been reported in a number of studies [19,20] that dB/dT is a better criterion for determining the structure-making/breaking nature of any solute rather than simply the value of the B-coefficient. It is found from Table 6 that the values of the B-coefficient increase with a rise in temperature (positive dB/dT) suggesting the structure-breaking tendency of potassium chloride, potassium bromide and potassium iodide in the solvent systems.
Extensive study of potassium chloride, potassium bromide and potassium iodide in aqueous mixture of L- Proline reveals that potassium iodide is more associated in L-Proline than the other two halides. The ion-association is found minimum in the case of potassium chloride in L- Proline. The said interaction of potassium bromide arises in the intermediacy of potassium iodide and potassium chloride. The present study reveals the predominance of ion-solvent interaction over the ion-ion interaction in all the solution under investigation.
The authors are grateful to the UGC supported Major research project, Ref. No. RP/5032/FCS/2011 New Delhi for financial support in order to continue this research work.
One of the authors, Prof. M. N. Roy is thankful to University Grant Commission, New Delhi, Government of India for being awarded one time grant under Basic Scientific Research via the grant-in-Aid No. F.4-10/2010 (BSR) regarding his active service for augmenting of research facilities to facilitate further research work.