
Journal of Clinical Toxicology
Open Access
ISSN: 2161-0495

ISSN: 2161-0495
Research Article - (2024)Volume 14, Issue 4
Tri-Ortho-Cresyl Phosphate (TOCP), an organophosphorus compound, is highly toxic and can induce, for instance, neurotoxicity, reproductive toxicity, and immunotoxicity. In particular, TOCP is known to cause a delayed neurodegenerative condition known as organophosphate-induced delayed neurotoxicity, which affects both the central and peripheral nerves, resulting in permanent damage to the neurological system. This review focuses on the neurotoxic effects of TOCP and the research that has been conducted to elucidate pertinent underlying mechanisms, including inhibition of NTE activity, alterations of cytoskeletal proteins, calcium imbalance, mitochondrial dysfunction, oxidative stress, cell cycle arrest, apoptosis and autophagy, etc., and provides an overview of some interventions for OPIDN and other strategies such as adrenocortical hormones and vitamin B as well as clinical drugs and protein factors.
ortho-cresyl phosphate; Organophosphate-induced delayed neurotoxicity; Intervention treatment; Organophosphorus compounds; Neurodegenerative diseases
Tri-Ortho-Cresyl Phosphate (TOCP), an Organo Phosphorus compound (OP), is one of the three isomers of tricresyl phosphate and the most neurotoxic [1]. The molecular formula of TOCP is C21H21O4P and its molecular weight is 368.36. The molecular structure data of TOCP are as follows: Its molar refractive index is 102.14, molar volume is 306.6 m3/mol, parachor (90.2 K) is 793.2, and polarization (10–24 cm3) is 40.49 (Figure 1).
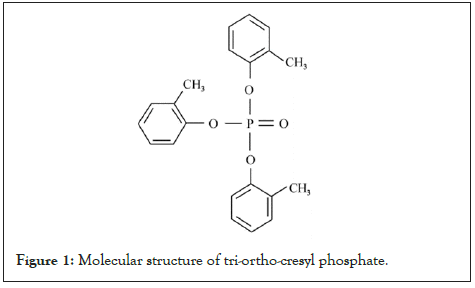
Figure 1: Molecular structure of tri-ortho-cresyl phosphate.
TOCP is a colorless or pale yellow transparent viscous liquid at room temperature; it is tasteless, water insoluble, and dissoluble in alcohol, benzene, dimethyl sulfoxide, amongst others. It is widely used as plasticizers, plastic softeners, lubricant and flame retardants in industry. Furthermore, TOCP is used as a solvent and is a component of jet engine oil (Figure 2) [2]. As with other plasticizers, TOCP has deleterious effects on the nervous system, male reproductive system, liver, and immune system [3-9]. Several countries have reported numerous cases of mass poisoning caused by eating or drinking TOCP-contaminated products. For example, in the 1930s, thousands of people in the United States of America were reported to be paralyzed on ingesting Jamaica ginger extract contaminated with TOCP. Subsequently, countries such as South Africa, Morocco, Sri Lanka, and India also reported cases of paralysis caused upon consuming TOCP-contaminated edible oil. Since the early 1990s, many similar events have occurred in Beijing, Xi'an, and Shenzhen in China. In recent years, exposure to TOCP via contaminated cabin air has been suggested to be associated with aerotoxic syndrome, causing serious concerns among airline crew members as well as passengers (Table 1). Therefore, the neurotoxic events caused by TOCP have received more and more attention with time.

Figure 1: Molecular structure of tri-ortho-cresyl phosphate.
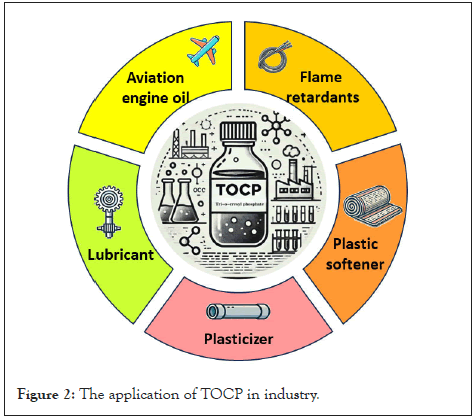
Figure 2: The application of TOCP in industry.
| Time period | Location | Incident description |
|---|---|---|
| 1930s | United States of America | Jamaica ginger paralysis: Approximately 15,000 people were affected, with 10 deaths due to contaminated “Jake” alcohol. |
| 1940s | Merseyside, Great Britain | Contaminated cottonseed cooking oil led to 17 cases of polyneuritis, paralysis, and foot drop. |
| 1942 | Italy | Tins and drums with residual military engine oil containing TOCP may have reached the farm and been emptied for recycling, leading to manure and ground contamination and consequently to contamination of animal fodder and of vegetables lying on the surrounding ground. |
| 1955 | South Africa | Poisoning from drinking water contaminated with TOCP |
| 1959 | Morocco | Outbreaks of TOCP poisoning from contaminated cooking oil. |
| 1960 | India | Poisoning due to TOCP-contaminated oil, resulting in severe health effects among the population. |
| 1960s | Romania | Toxic neuropathy due to ingestion of TOCP-contaminated alcohol |
| 1970s | Sri Lanka | Poisoned by ingesting gingili oil contaminated with TOCP. |
| 1995 | Xi’an, China | Mass poisoning from cooking oil adulterated with TOCP. |
Table 1: Human poisonings due to TOCP around the world.
Toxic effects of TOCP
TOCP has been proven to lead to toxicity in various organ systems, and it can cause irreversible damage. Its neurotoxic effects, in particular, are the most significant. TOCP exposure can lead to three different outcomes: A: Acute poisoning-induced inhibition of cholinesterases; B: Short or long-term multiple exposures can induce neurodegenerative diseases; and C: Organo Phosphate Induced Delayed Neurotoxicity (OPIDN), which is caused by single or multiple exposures. OPIDN is the most common neurodegenerative disease caused by TOCP [10].
Acute poisoning due to TOCP is relatively infrequent. On exposure to a large dose of TOCP for several hours, signs of cholinergic intoxication (e.g., muscle tension, hyperreflexia, clonus, pyramidal tract injury, and upper motor neuron syndrome) become evident owing to the anticholinergic effects of TOCP, with such signs and symptoms disappearing within 2 days. Chronic poisoning involves exposure to low doses of TOCP, and similar symptoms as those listed above are observed [11]. In a large majority of cases, OPIDN, characterized by distal axonal degeneration, is caused upon exposure to TOCP, becoming apparent within 1-3 weeks of poison ingestion. The clinical course of OPIDN involves paresthesia in the distal extremities, sensory loss, ataxia, flaccid paralysis and, ultimately, spastic paralysis (Figure 3).
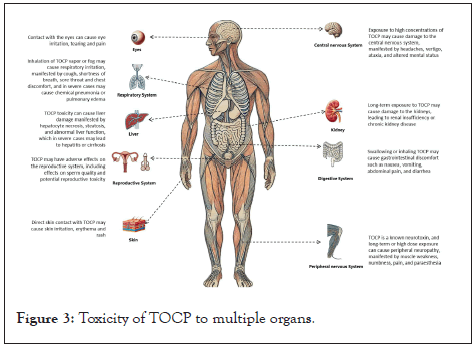
Figure 3: Toxicity of TOCP to multiple organs.
Mechanisms underlying the neurotoxicity of TOCP
Not much is definitively known about the pathogenesis of neurotoxicity induced by TOCP. The main effects include inhibition of Neuropathy Target Esterase (NTE) activity, alterations of cytoskeletal proteins, calcium imbalance, mitochondrial dysfunction due to oxidative stress, apoptosis and autophagy, amongst others (Figure 4).
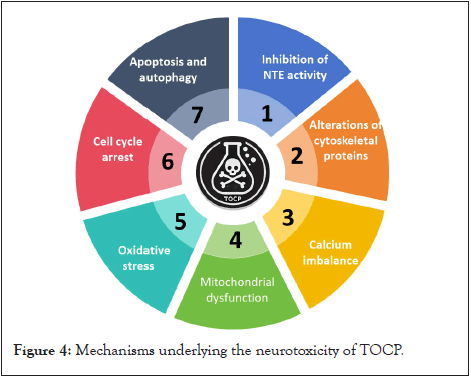
Figure 4: Mechanisms underlying the neurotoxicity of TOCP.
Inhibition of NTE activity
As early as the 1970s, OPIDN poisoning was suggested to stem from NTE phosphorylation caused by OP’s and inhibition of NTE activity [12]. TOCP can evidently reduce the activity of NTE and Acetyl Cholinesterase (AChE) in differentiated rat PC12 and C6 cells [13]. The role of NTE in OPIDN involves maintaining neurological functions and neural integrity at the same time. The effects of treating hens with 500 mg/kg TOCP for 24 h were assessed, and it was found that 80% NTE activity and approximately 20% AChE activity were inhibited [14]. NTE activity inhibition has been speculated to involve two main steps: NTE phosphorylation and NTE “aging.” NTE aging reportedly occurs due to the molecular rearrangement of proteins. As per some studies, non-OP’s can also inhibit NTE activity, but they do not cause OPIDN. Non-OP’s first interact with the NTE active site and then inhibit NTE activity, but because they do not cause NTE aging, no clinical symptoms are observed [12]. The inhibition of TOCP-induced neurites and cell processes growth might be related to the cholinergic system or NTE-related OPIDN target (one or more).
A multistep hypothesis can be formulated based on the existing literature: (1) The inhibitory effect of OPIDN’s OP inducer on NTE is much higher than that of AChE; (2) Neuropathological effects can be observed only once 70%–90% NTE activity is inhibited; (3) NTE is phosphorylated at the serine residue in the catalytic center; (4) Loss of an alkoxy group (referred to as aging) leaves a negatively charged phosphate at the active site; (5) Increase of toxic function causes neurodegeneration [15-19].
Furthermore, NTE has been found to be related to phospholipid homeostasis in cell membranes: NTE is located in the Endoplasmic Reticulum (ER), and its activity is inversely related to phosphatidylcholine and lysophosphatidylcholine levels. It catalyzes their deacylation to produce glycerophosphocholine, which suggests that disruption of ER phospholipid homeostasis plays a role in OPIDN initiation [20].
Alterations of cytoskeletal proteins
In OPIDN animal models, disruptions in the arrangement and distribution of microtubules and microfilaments have been reported, which seem to be related to the initiation of OPIDN [21]. Microfilaments, microtubules, and intermediate filaments are involved in the formation of three-dimensional cytoskeletal structures, and they support and maintain vital biological functions of neurons. Any factor that influences the polymerization and depolymerization of microfilaments can thus cause aberrations in the structure and function of the entire cytoskeleton. TOCP has been found to markedly increase the content of microtubuleassociated protein 2 in the chicken brain, which might be associated with OPIDN. Microtubule-associated protein 2 is localized in neuronal cell bodies and dendrites and has been used as a marker to identify dendritic processes. Microtubules are the main components of the cytoskeleton of neurons and play a key role in maintaining the normal structure and function of neurons. Axons substance acquisition of TOCP-damaged is an important cause of axonal degeneration. Swelling is typically observed upon axonal degeneration. On knocking out the gene encoding NTE, cultured NTE-deficient neurons were found to display modestly impaired secretion, consistent with neuronal viability and damage in vivo initially restricted to distal parts of the longest axons [22]. In addition, in an in vitro model of neurotoxicity and neurodegenerative disease, TOCP was observed to inhibit the growth of cell protrusions in differentiated mouse neuroblastoma N2a cells, affect the expression of cytoskeletal proteins, and destroy the integrity of the neurofilament network [23].
The delayed neurotoxic organophosphate [3H]Diisopropyl Fluoro Phosphate (DFP) binds with high affinity to membrane-bound proteins from the chicken spinal cord. In response to a neurotoxic dose of TOCP, the amount of [3H]DFP binding sites were observed to markedly decrease in membrane preparations from the chicken spinal cord, but the amount of [3H]DFP binding sites between the spinal nerve cell membranes of each segment was changed little [24]. Therefore, it is speculated that TOCP first combines with a specific protein on the spinal nerve cell membrane, and [3H]DFP binding is subsequently inhibited due to competition between the specific protein and [3H]DFP. On exposing adult chickens to TOCP, OPIDN was found to occur, and neurofilament content in the spinal cord was disturbed. Treatment with Phenyl Methane Sulfonyl Fluoride (PMSF) suppressed OPIDN and inhibited neurofilament content-related abnormalities in the spinal cord [25]. Further, TOCP has been reported to not only reduce the expression of F-actin stress fibers in undifferentiated SH-SY5Y cells and alter the structure of SH-SY5Y cell microfilaments but also inhibit the growth of cell protuberances in differentiated mouse neuroblastoma N2a cells, change the expression of cytoskeletal proteins, and destroy the integrity of the neurofilament network. Chen, et al. [26], Chang, et al. [27], found that TOCP caused microtubule-associated protein-related changes and cytoskeletal component loss during the early stages of toxicity. Collectively, these findings indicate that cytoskeletal proteins play a pivotal role in the development of TOCP-induced OPIDN.
Calcium imbalance
Ca2+, a multifunctional second messenger, is closely associated with pathophysiological processes and nerve injuries caused by the nerve poison. At present, it is generally accepted that intracellular calcium influx is involved in the pathophysiological process of nerve damage caused by chemical nerve agents. TOCP can induce calcium dyshomeostasis, which indirectly affects the activity of several protein kinases. For instance, according to some in vivo (hens) and in vitro (SH-SY5Y cells) studies, axonal degeneration after exposure to OP’s is associated to excessive calcium influx and calcium-dependent protease activation. Calpain activity in the brain was found to increase at 4, 7, 10, and 37 days after TOCP exposure [28]. Studies have also shown that on TOCP treatment for 12 hours, plasma Ca2+ levels begin to decrease, whereas calpain activity significantly increases in sciatic nerves. This indicates that in response to TOCP poisoning, Ca2+ enters the cell from the extracellular environment, which reduces the extracellular and increases the intracellular concentrations of Ca2+; this results in the activation of calpain. In this manner, Ca2+-activated calpain is associated with the occurrence of OPIDN. It has also been reported that in the hen brain, calpain activity increased by 40% on exposure to 500 mg/kg TOCP for 24 hours [14]. Altogether, these data suggest that OPIDN occurrence is related to an imbalance of intracellular calcium homeostasis.
On TOCP exposure, followed by an imbalance of calcium homeostasis, there is activation of calcium-activated neutral protease and Ca2+/CaMII, which results in the abnormal phosphorylation of cytoskeletal proteins, namely tubulin and tubulin-associated proteins [29]. Abnormal axonal transport further inhibits the activity of calpain in the sciatic nerves of hens. This series of events leads to axonal degeneration and subsequent demyelination.
Mitochondrial dysfunction
Mitochondrial dysfunction evidently plays a pivotal role in several neurodegenerative diseases, such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease [30-32]. Besides, in vitro biochemical and pathological studies have reported that mitochondrial dysfunction is involved in OP-induced neurotoxicity. In hens, TOCP was found to alter the structure of mitochondria in the nerve tissue, cause edema, vacuolation and mitochondrial crista reduction, fracture, and disappearance. Further, TOCP exposure has been reported to markedly increase mitochondrial membrane permeability in the nervous tissue of hens and alter mitochondrial respiration and respiratory chain enzyme activities. TOCP administration also evidently induces neuron apoptosis in hen spinal cords, which is apparently mediated by the activation of mitochondrial apoptotic pathway [33]. In the OPIDN hen model, different doses of TOCP were found to induce mitochondrial dysfunction in the nervous tissue before clinical symptoms appeared. The disease manifested as obvious pathological changes; structural changes of mitochondria were observed in the nervous tissues of hens, including vacuolation and fission, and mitochondrial permeability transition increased in both the cerebrum and spinal cord. These results suggested that mitochondrial dysfunction is partly responsible for OPIDN development induced by TOCP [34].
A study investigated the role of mitophagy in axon degeneration after TOCP administration in an in vitro model. P62, a major autophagic receptor, was found to be accumulated on the mitochondria, suggesting that P62 plays an essential role in facilitating mitophagy under TOCP-induced axonal degeneration. Moreover, TOCP exposure reportedly activated PINK1-Parkin-dependent mitophagy in N2a cells [35]. Thus, it appears that mitophagy acts as a positively reactive mode in eliminating dysfunctional mitochondria and protecting neurons against TOCP neurotoxicity.
Oxidative stress
Oxidative stress has a direct impact on many diseases, including neurodegenerative diseases, autoimmune skin diseases, cardiovascular disorders, and chronic renal failure [36-39]. This can be attributed to the attack by reactive oxygen species and defects in the intracellular antioxidant defense system. TOCP has been found to cause lipid peroxidation in the nervous tissue of adult hens [40]. Moreover, Zhang, et al. [41] observed time-dependent and tissue-specific changes in lipid peroxidation and antioxidant status in the cerebrum, spinal cord, and sciatic nerve of hens treated with TOCP, and suggested that reactive oxygen species and concomitant lipid peroxidation, at least in part, are involved in the toxic effects of TOCP and that oxidative stress seems to participate in OPIDN occurrence and development. Our previous studies showed that TOCP induced changes in the antioxidant system of hens with OPIDN. Over time, TOCP was found to increase the level of lipid peroxides in the nervous tissue and serum of hens, which eventually damaged the antioxidant defense system. In vitro experiments revealed that TOCP induced oxidative damage in SHSY5Y cells. With an increase in TOCP concentration, the content of malondialdehyde increased and that of catalase decreased. Further, with an increase in exposure time, the effects on malondialdehyde and catalase content became very obvious. To prevent the accumulation of oxidation-modified proteins and other damaged proteins in the cell and to avoid consequent cytotoxicity, protective mechanisms are activated and hydrolysis of abnormal proteins is accelerated. The proteolytic system plays a fundamental role in the response of cells to toxicity as well as environmental stresses. High TOCP concentrations markedly increase the aggregation of ubiquitination proteins, and there exists a significant dose-response relationship. Besides, high concentrations of TOCP can reportedly block or inhibit the ubiquitin-proteasome pathway.
Cell cycle arrest
Long, et al. [41], reported that TOCP downregulates the mRNA and protein expression levels of the cell cycle arrest-associated protein cyclin D1 and upregulates those of p21. Further, TOCP blocked cell cycle progression by arresting the cell cycle at the G1 phase in SHSY5Y cells. TOCP also suppressed cell proliferation and reduced cell viability in a time- and dose-dependent manner. Based on these findings, they suggested that TOCP induces neurodevelopmental toxicity, and a possible mechanism underlying this toxicity involves the disturbance of cell proliferation by disrupting cyclin D1 and p21 expression.
Besides SH-SY5Y cells, few recent studies have reported that OP’s even target astrocytes. Liu treated rat C6 astroglial cells with TOCP to report that the number of G1 phase cells increased with an increase in the concentration of TOCP. Astrocytes regulate the microenvironment around neurons by synthesizing and secreting some neurotrophic factors and cytokines, and TOCP blocks the cell cycle of astrocytes [42,43].
Apoptosis and autophagy
Apoptosis, or programmed cell death, is generally characterized by distinct morphological changes and energy-dependent biochemical mechanisms, and it involves the activation, expression, and regulation of a series of genes [44]. Aberrations in the regulation of apoptosis may lead to the occurrence of, for example, cancer, autoimmune diseases, and neurodegenerative diseases [45]. A study reported TOCP induced neuronal apoptosis in the anterior horn of the spinal cord of hens, suggesting that cellular apoptosis plays a vital role in the pathogenesis of OPIDN [46,47]. Another study on TOCP-induced neuronal apoptosis suggested that TOCP mediates apoptosis of motor neurons in the spinal cord of hens via FasL/ Fas [47].
Autophagy is an evolutionary conserved lysosomal degradation process [48]. It involves processing cell materials (e.g., longevity proteins, macromolecules, and organelles) that cannot be degraded by the ubiquitin–proteasome system [49]. Autophagy or type II programmed cell death is a newly discovered form of programmed cell death. Many studies have proven that autophagy plays an important role in TOCP-induced neurotoxicity. Further, the occurrence of abnormal autophagy is closely related to cancer and neurodegenerative diseases [50-52]. The regulatory mechanism of autophagy and the role of autophagy in neurodegenerative diseases have attracted much attention [53]. Autophagy is regulated by diverse factors, particularly calcium. Hausherr, et al. [53], reported that glutamate signaling was impaired in mouse central nervous system neurons in vitro by TOCP at noncytotoxic concentrations; moreover, they speculated that intracellular calcium contributes to the reduction of overall neuronal responsiveness by depolarizationdependent inactivation of voltage-gated ion channels. It has also been reported that TOCP induces strong, delayed neurotoxicity in humans and other animals and that its effects are associated with autophagy [55,56]. For example, TOCP causes autophagy in SH-SY5Y cells. Long, et al. [54], reported that with an increase in the concentration of TOCP, the level of autophagy-related protein light chain-3 also increased; in addition, the number of autophagic vacuoles significantly increased. TOCP poisoning is also reportedly associated with a significant decrease in the level of beclin-1, a key molecule in the process of autophagy, in hen nerve tissues [57]. Some studies suggest that autophagy plays a pivotal role in axonal damage initiation and progression during TOCP-induced neurotoxicity. Xu, et al. [57] ,investigated whether dysfunctional autophagy was associated with TOCP-induced delayed neuropathy in hens, and they found that the number of autophagosomes was considerably increased in the myelinated and unmyelinated axons of hen spinal cords; furthermore, the levels of microtubule-associated protein light chain-3 were decreased and those of p62 were increased. They also found a significant reduction in the expression of autophagyregulated proteins, including ULK1, AMBRA1, ATG5, ATG7, ATG12, and VPS34. Based on these results, they concluded that TOCP inhibits autophagy activity in neurons, which is possibly related to the pathogenesis of OPIDN [58,59].
Intervention treatment for OPIDN
It is currently believed that this lesion is not caused by the inhibition of Cholinesterase (ChE), even some serious patients cannot be fully recovered and can be disabled for life.
Although some experimental studies have reported that the symptoms of OPIDN can be relieved by restoring calcium balance or using neurotoxic esterase activity inhibitors, there is no single specific drug in clinical practice that can prevent or mitigate serious effects. For the recovery of nerve function, the early and timely use of glucocorticoid, vitamin B, and nerve growth factor can prove helpful.
NTE activity inhibitor
NTE “aging” is vital for the occurrence of OPIDN, but NTE activity inhibition alone cannot induce OPIDN; this is significantly different from cholinergic toxicity observed on the inhibition of AChE. NTE activity inhibitors avoid the occurrence of aging by reversibly binding with the NTE active site (no negatively charged terminal group is formed). If NTE activity inhibitor is administered in advance before TOCP exposure, then its protective effects on OPIDN become observable.
Through preventive use, inhibitors of carbamates, thiocarbamates, sulfonyl fluorides, and phosphates can prevent the development of OPIDN [60]. Most studies have used PMSF as the test agent, probably because its protection time limit is approximately the same as the recovery time of NTE activity [29]. For example, carbamate provides only temporary protection, while PMSF, which belongs to the serine esterase family, inhibits NTE activity for a long time.
It is notable that if PMSF is administered after exposure to OP’s, the clinical signs, neuropathic function, and morphological indicators are significantly heavier than in the presence of OP’s alone [61]. The different capabilities of PMSF remain to be well explained, probably because PMSF interacts with targets other than NTE; in other words, NTE might not be the only potential molecular target of OPIDN. Sunderhaus, et al. [61], proposed that the activation of cytoprotective ER stress pathways may provide a therapeutic approach to alleviate neurodegeneration and motor symptoms associated with NTE-related diseases.
At present, there is a lack of clinical evidence for the effective use of NTE activity inhibitors and also the ideal treatment duration and dosages; thus, more studies need to be conducted to elucidate the pathological mechanism of OPIDN. Further research on NTE can facilitate the development of therapeutic strategies to better manage patients with TOCP-induced OPIDN.
Regulating calcium homeostasis
Calcium ions play an integral role in regulating nerve cell excitation, neurotransmitter release, membrane integrity, and muscle contraction. Further, calcium-triggered signaling pathways have a fundamental role in neuron survival, plasticity, and nerve transmission. Some studies have found that neuronal cell death in OPIDN is related to calpain, the activation of which is triggered by an increase in intracellular calcium levels. Neurotoxins significantly increase intracellular calcium concentration by enhancing intracellular calcium influx or decreasing intracellular calcium excretion. In other words, OPIDN seems to be associated with an increase in intracellular calcium ions and a decrease in serum calcium ions.
Piao, et al. [62], examined if OPIDN is prevented or alleviated when calcium (Calcicol®) is administered to experimental animals before or after OP administration and found that calcium improved the symptoms of polyneuropathy but could not completely prevent the occurrence of OPIDN. In addition, it has been reported that nifedipine, nimodipine and verapamil, which are L-type calcium channel blockers, are useful in cases of OPIDN, but little is known regarding the neuroprotective effects of T-type calcium channel blockers on neurotoxicity induced by Op’s [63-67]. Fernandes, et al. [66], observed that the T-type calcium channel blocker amiloride was able to improve SH-SY5Y cell differentiation and axon length by inhibiting calcium overload and calpain activation. However, nimodipine may be more effective than amiloride in protecting neuroplastic proteins. Emerick, et al. [66], used a combination of calcium gluconate and calcium channel blockers to regulate calcium levels. Calcium channel blockers prevented calcium from entering the cytoplasm, and calcium gluconate restored the dynamic balance of calcium in the extracellular matrix. The specific treatment strategies were as follows: Nimodipine was given 12, 18, and 24 h after TOCP exposure, and calcium gluconate was given 30 minutes later. No clinical symptoms of neurotoxicity were observed [67-69].
For OPIDN, there is insufficient evidence pertaining to the use of the aforementioned agents in clinical practice. According to a meta-analysis, both preclinical and clinical data suggested that magnesium sulfate and calcium channel blocking drugs are promising adjunct treatments for acute organophosphorus insecticide poisoning; however, their application to treat OPIDN was not mentioned [70].
Large multicenter randomized controlled trials need to be conducted to test calcium channel blocking drugs to generate sufficient evidence to prove their effectiveness for treating OPIDN. The method of restoring calcium homeostasis has a significant advantage: the administration of such drugs after exposure to neurotoxic OP’s can still inhibit calpain activation and relieve clinical symptoms. This indicates that the pharmacological inhibition of calpain can prove to be an effective strategy to manage OPIDN, and further confirms that calpain activation plays a key role in triggering neuronal degeneration.
Other strategies
The dose and time of exposure to OP’s determine if OPIDNrelated symptoms improve and if there will be recovery. Although substantial progress has been made in treatment strategies, there is still a lack of preventive measures against OPIDN. As the mechanism remains unclear, many researchers have started exploring intervention strategies that can bring good news to patients with OPIDN by blocking a certain link of OPIDN progress and repairing peripheral nerve injuries (such as myelin sheath and Schwann cells).
Adrenocortical hormones and vitamin B
Neurohistopathological examination of the OPIDN hen model revealed, for instance, axonal swelling, axonal fragments, and lysosomal infiltration, indicating that the development of OPIDN may involve an inflammatory reaction. Adrenocortical hormones have anti-inflammatory effects; at the same time, they can indirectly modulate calcium balance by reducing calcium absorption. Their timely application can facilitate the recovery of nerve function.
Vitamin B plays an important role in maintaining nerve health and normal brain function. It also serves as a coenzyme, helping enzymes to chemically react with other substances and participate in energy production. Mecobalamin (a derivative of vitamin B12) can reportedly repair damaged nerve tissue by promoting nucleic acid and protein synthesis; moreover, it can help improve motor and sensory disorders.
Prednisolone can prevent the occurrence of OPIDN by propofol [71]. Emerick, et al. [72], investigated the effects of glucocorticoid triamcinolone and mineralocorticoid deoxycorticosterone on OPIDN in chickens and found that both synthetic glucocorticoids and endogenous corticosteroids relieved clinical symptoms to a certain extent, but attention should be given to dosage (Table 2).
| Clinical drugs | Drugs type | Mechanism |
|---|---|---|
| Prednisolone | Glucocorticoid | Reduces inflammation, suppresses immune response, stabilizes neuronal cell membranes |
| Glucocorticoid triamcinolone | Glucocorticoid | Reduces inflammation, suppresses immune response, stabilizes neuronal cell membranes |
| Mineralocorticoid deoxycorticosterone | Mineralocorticoid | Primarily regulates electrolyte and water balance |
| Liraglutide | GLP-1 Receptor Agonist | Neuroprotection, anti-inflammatory effects, reduction of oxidative stress |
| Miconazole | Antifungal | Inhibits ergosterol synthesis |
| Duloxetine | SNRI (Serotonin-Norepinephrine Reuptake Inhibitor) | Inhibits reuptake of serotonin and norepinephrine, enhances descending pain inhibition pathways |
| Apelin-13 | Peptide (Endogenous ligand) | Neuroprotection, anti-inflammatory effects, improvement of blood-brain barrier integrity |
Table 2: Clinical drugs use of TOCP poisoning.
The combined use of multivitamin B and dexamethasone or their individual use has been reported to significantly inhibit the toxic and side effects of DFP [73,74]. Prednisolone and vitamin B complex are evidently the best for relieving the clinical manifestations of OPIDN in chickens, particularly when they are used during the early stage of OPIDN.
Clinical drugs and protein factors
Liraglutide is a long-acting glucagon-like peptide-1 analog and is clinically used to treat diabetes. Its neurotrophic and neuroprotective effects have been confirmed in vitro and in Parkinson animal models; clinical trials for Parkinson’s disease are currently underway. Axonal degeneration and synaptic plasticity destruction are common characteristics of neurological dysfunction, including chemically induced peripheral neuropathy (such as OPIDN). It has been speculated that liraglutide has a protective effect on neurotoxicity caused by OP’s. Furthermore, liraglutide reportedly protects SH-SY5Y cells from the neurotoxic effects of mipafox by restoring glucose homeostasis, axon cytoskeleton protein levels, and synaptic plasticity [75].
Miconazole is a synthetic imidazole antifungal drug with a high curative effect and high safety. On administering miconazole to hens every day from the seventh day after exposure to TOCP, miconazole was found to significantly improve neurotoxic symptoms and histopathological damage of the spinal cord and sciatic nerve [76]. Further, they speculated that miconazole promotes glial cell differentiation and migration and enhances myelination by inhibiting the activation of the ErbB/Akt signaling pathway. Therefore, miconazole appears promising for the clinical treatment of OPIDN. It has also been proven that the epidermal growth factor inhibitor lapatinib alleviates TOCP-induced delayed neurotoxicity [77].
TRPA1, a non-selective cation channel that permeates calcium ions, is activated, for example, by low temperatures or on mechanical stimulation. It participates in various physiological and pathological processes, and it is a potential drug target for treating neuropathic pain and allergic asthma. Duloxetine, an antidepressant, and ketotifen, an antihistamine for asthma, have been proven to be similar to HC030031, a TRPA1 channel antagonist; they inhibit TRPA1 channel at a micromolar level, significantly relieving OPIDN symptoms in hens induced by TOCP [78]. Both drugs have been clinically used for a long time and are safe; therefore, they appear promising for treating nerve injury caused by OP poisoning. Thus, for effectively inhibiting OP-induced nerve injury, further studies are warranted on TRPA1.
The neuroprotective factor apelin has been proven to regulate autophagy in both in vivo and in vitro models. Apelin-13 shows higher biological efficacy than other forms of apelin. Apelin-13 evidently prevents TOCP-induced OPIDN in hens by reducing the accumulation of autophagosomes in neural tissues, which could be attributed to autophagic flux regulation by apelin-13 [79]. As an important medium involved in nerve regeneration and functional repair, nerve growth factor can directly act on injured neurons and significantly relieve the neurological symptoms of OPIDN, which is related to the recovery of nerve growth factor or an obvious improvement in nerve cell function. These data provide novel ideas and strategies for the intervention therapy of OPIDN [78-83].
The study of TOCP has gradually evolved from morphological, physiological, and biochemical properties to molecular biology, and knowledge pertaining to pertinent toxicity mechanisms, particularly neurotoxicity, has improved. Research on the neurotoxicity of TOCP is important as this issue needs to be urgently addressed. However, not much has been reported regarding its detoxification mechanism. On TOCP poisoning, there is no effective drug treatment; clinical measures are taken according to clinical symptoms. Therefore, comprehensively understanding the mechanism responsible for the neurotoxicity of TOCP is pivotal to adequately treat patients. Furthermore, similar studies are warranted to better understand toxicity mechanisms of other OP’s.
The authors gratefully acknowledge the financial support from National Natural Science Foundation of China (Grant No. 81673227; 81172712).
The authors declare that they have no conflicts of interests.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Jiang L, Yang Y, Tian X, Hu F, Long D (2024) Exploring Tri-Ortho-Cresyl Phosphate Neurotoxicity and Underlying Mechanisms. J Clin Toxicol. 14:569.
Received: 17-Jun-2024, Manuscript No. JCT-24-32918; Editor assigned: 20-Jun-2024, Pre QC No. JCT-24-32918 (PQ); Reviewed: 04-Jul-2024, QC No. JCT-24-32918; Revised: 11-Jul-2024, Manuscript No. JCT-24-32918 (R); Published: 18-Jul-2024 , DOI: 10.35841/2161-1017.24.14.569
Copyright: © 2024 Jiang L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.