Journal of Depression and Anxiety
Open Access
ISSN: 2167-1044
ISSN: 2167-1044
Research - (2023)Volume 12, Issue 1
Objectives: Extended-release (XL) bupropion hydrochloride (HCl) received FDA approval for Major Depressive Disorder (MDD) in 2003 and for Seasonal Affective Disorder (SAD) in 2006. XL bupropion hydrobromide (HBr) (Aplenzin®) received FDA approval in 2008 for both MDD and SAD following demonstration of pharmacokinetic bioequivalence to XL bupropion HCl. However, no human clinical efficacy trials of XL bupropion HBr have been completed and none comparing it to XL bupropion HCl. We wanted to compare equivalent FDA-approved dosing of XL bupropion HBr to generic XL bupropion HCl in patients with Treatment-Resistant Depression (TRD).
Methods: We obtained informed consent from each patient prior to conducting a retrospective chart review on 30 adult patients with TRD (18 females, 12 males) who directly switched from their maximally tolerated dose of generic XL bupropion HCl, due to inadequate response and/or side effects, to an equivalent dose of XL bupropion HBr.
Results: Patients had a mean age of 38.7+14.7 years, had failed a mean of 4+2 antidepressants, and had a mean baseline PHQ-9 score of 20.9+4.3 prior to taking a mean XL bupropion HCl dose of 300 mg/day (range 150‒450 mg/day) for a mean duration of 88.8 weeks (range 4‒480 weeks). After 2 weeks on a mean XL bupropion HBr dose of 348 mg/day (range 174‒522 mg/day), these patients demonstrated a decrease in PHQ-9 scores from a mean of 15.3+5.3 on XL bupropion HCl to a mean of 6.4+5.0 on XL bupropion HBr (t=−8.63, p<0.00001). The most commonly encountered side effects with generic XL bupropion HCl were insomnia (21 patients), anxiety (19 patients), and gastrointestinal upset (2 patients), which resolved on XL bupropion HBr except for some residual insomnia (3 patients). 96.7% of patients (29 of 30) chose to continue on XL bupropion HBr rather than change back to generic XL bupropion HCl.
Conclusion: Adult patients suffering from Treatment-Resistant Depression experience rapid and significant improvement in mood with greater tolerability when switching from generic XL bupropion HCl to an equivalent dose of XL bupropion HBr.
Bupropion; Hydrochloride; Hydrobromide; Depression; Treatment-resistant
In the regular course of our general outpatient psychiatry practice, we see many patients who suffer from Major Depressive Disorder (MDD) and may not have responded to 2 or more oral antidepressant medications. We routinely administer the brief and commonly used Patient Health Questionnaire-9 (PHQ-9) scale before starting a new treatment, 2 weeks after starting treatment to measure initial response, and periodically thereafter. Once-daily, extended-release (XL) bupropion hydrochloride (HCl), a norepinephrine and dopamine reuptake inhibitor, is among the many antidepressants we commonly use because it is available as a generic, does not require insurance prior authorization, and does not typically cause the sexual dysfunction, fatigue, affective flattening, and/or weight gain commonly associated with serotonin reuptake inhibitors. Oftentimes, patients do not adequately respond to generic XL bupropion HCl either because of lack of efficacy or side effects, such as anxiety, agitation, or insomnia. One treatment strategy we have used successfully is switching patients from generic XL bupropion HCl to an equivalent dose of once-daily, branded XL bupropion hydrobromide (HBr), which we have noticed further improves patients’ mood with more favorable tolerability. Once-daily, XL bupropion HCl received FDA approval for the treatment of MDD in 2003 and for Seasonal Affective Disorder (SAD) in 2006 [1]. Once-daily, XL bupropion HBr (Aplenzin®) received FDA approval in 2008 for both MDD and SAD through the 505(b)(2) pathway following demonstration of pharmacokinetic bioequivalence to XL bupropion HCl. Compared to XL bupropion HCl, XL bupropion HBr was found to produce lower peak plasma concentrations and area under of the curve for both bupropion and its 3 active metabolites (hydroxybupropion, threohydrobupropion, and erythrohydrobupropion). Hence, higher dosing equivalents of XL bupropion HBr 174 mg, 348 mg, and 522 mg correspond to XL bupropion HCl 150 mg, 300 mg, and 450 mg [2].
However, no human clinical efficacy trials of XL bupropion HBr have been completed as of this report and there are no head-to-head studies comparing it to generic XL bupropion HCl. Bromide salts have been used in the past in humans to treat seizures, anxiety, and insomnia [3], three potentially limiting side effects of taking XL bupropion HCl [1]. A study of rodents found that bupropion HBr was associated with fewer seizures than bupropion HCl [4]. The mechanism of action of these anxiolytic and anti-epileptogenic effects can be possibly explained by greater bromide-mediated potentiation of inhibitory potentials through GABA-A receptors. When activated, these receptors increase chloride influx and cause inhibitory neuronal hyperpolarization, which was found to be greater in the presence of bromide than chloride [5]. Recent renewed interest around bupropion has been spurred by the 2022 FDA approval of a combination of shorter-acting, low dose bupropion HCl (105 mg) and high dose dextromethorphan HBr (45 mg) administered twice daily for non-treatment resistant Major Depressive Disorder in adults [6]. Presently, the only FDA-approved oral medication for adult Treatment-Resistant Depression (TRD), defined as failing two or more antidepressants of adequate dose and duration in the current episode, is the combination of fluoxetine and olanzapine, which carries side effects of sedation, extrapyramidal symptoms, weight gain, and diabetes due to the olanzapine component [7]. We wanted to compare equivalent FDA-approved dosing of once-daily XL bupropion HBr to once-daily generic XL bupropion HCl in patients with TRD to measure antidepressant efficacy and tolerability.
The following study protocol was approved by the WCG-IRB institutional ethics committee and conducted in accordance with principles set forth in the Helsinki Declaration. We obtained informed consent from each patient prior to conducting a retrospective chart review on 30 adult patients with TRD (18 females, 12 males) who directly switched from their maximally tolerated dose of generic XL bupropion HCl, due to inadequate response and/or side effects, to an equivalent dose of XL bupropion HBr, open-label, in the regular course of our general outpatient psychiatry practice. The Patient Health Questionnaire-9 (PHQ-9) scale (range 0‒27) was administered prior to starting treatment, after completing a trial of XL bupropion HCl, and after 2 weeks on XL bupropion HBr. The PHQ-9 is a reliable and valid clinical and research tool that measures functionality and depression severity, whereby scores of 5, 10, 15, and 20 correspond to mild, moderate, moderately severe, and severe depression and a 5-point change is considered clinically meaningful [8]. Statistical analysis was performed using a within-subject, 2-tailed t-test with a significant level of p<0.05.
Patients had a mean age of 38.7+14.7 years, had failed a mean of 4+2 antidepressants, and had a mean baseline PHQ-9 score of 20.9+4.3 prior to taking a mean XL bupropion HCl dose of 300 mg/day (range 150‒450 mg/day) for a mean duration of 88.8 weeks (range 4‒480 weeks). After 2 weeks on a mean XL bupropion HBr dose of 348 mg/day (range 174‒522 mg/day), these patients demonstrated a decrease in PHQ-9 scores from a mean of 15.3+5.3 on XL bupropion HCl to a mean of 6.4+5.0 on XL bupropion HBr (t=−8.63, p<0.00001). The most commonly encountered side effects with XL bupropion HCl were insomnia (21 patients), anxiety (19 patients), and gastrointestinal upset (2 patients), which resolved on XL bupropion HBr except for some residual insomnia (3 patients). 96.7% of patients (29 of 30) chose to continue on XL bupropion HBr rather than change back to XL bupropion HCl. The one patient who chose not to continue on XL bupropion HBr described it as “too powerful” and “too stimulating” compared to XL bupropion HCl, even at the lowest available dose of 174 mg (Figure 1).
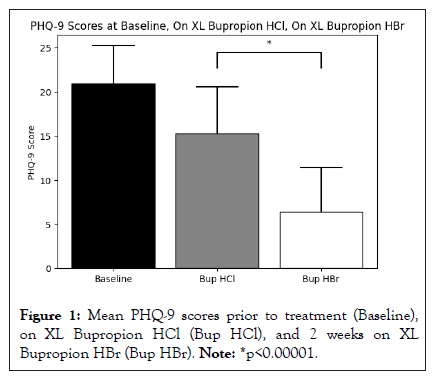
Figure 1: Mean PHQ-9 scores prior to treatment (Baseline), on XL Bupropion HCl (Bup HCl), and 2 weeks on XL Bupropion HBr (Bup HBr). Note: *p<0.00001.
This study found that adult patients with severe TRD experienced rapid and significantly improved mood with greater tolerability on XL bupropion HBr compared to generic XL bupropion HCl. Greater tolerability also affords capacity to increase XL bupropion HBr to its maximally approved dose, which is not always possible on XL bupropion HCl due to treatment-emergent side effects, such as insomnia, anxiety, and gastrointestinal upset. Based on mean PHQ-9 depression scores, patients improved from depression ratings of “severe” at baseline and “moderately severe” on XL bupropion HCl to “mild” after 2 weeks on XL bupropion HBr. Given that patients had been taking XL bupropion HCl well past the time frame required for it to take effect prior to switching to XL bupropion HBr, it is likely that improvements in PHQ-9 scores were attributable to the switch as all other concurrent treatments were held constant during this transition period. Among the many previously failed antidepressant trials prior to a maximally tolerated dose of XL bupropion HCl, administered before direct switch to XL bupropion HBr, 5 included the combination of bupropion HCl and dextromethorphan. All 5 patients in this subgroup preferred once-daily XL bupropion HBr over the combination of bupropion HCl plus dextromethorphan due to improved antidepressant efficacy on XL bupropion HBr. Superior efficacy may be explained by the ability to raise the bupropion component to 522 mg compared to a fixed, lower dose of bupropion 210 mg divided twice daily, and the once daily dosing of XL bupropion HBr that provides consistent antidepressant exposure over a 24-hour period, avoiding BID dose dumping and pharmacokinetic peaks and troughs.
Adult patients suffering from Treatment-Resistant Depression experience rapid and significant improvement in mood with greater tolerability when switching from generic XL bupropion HCl to an equivalent dose of XL bupropion HBr (Aplenzin®). Superior efficacy may be explained by the ability to raise the bupropion component to 522 mg compared to a fixed, lower dose of bupropion 210 mg divided twice daily, and the once-daily dosing of XL bupropion HBr that provides consistent antidepressant exposure over a 24-hour period, avoiding BID dose dumping and pharmacokinetic peaks and troughs. Limitations of this study were that it was an unblinded, retrospective analysis without a placebo control. A further limitation was that branded XL bupropion HBr (Aplenzin®) was compared to various available generic manufacturers of XL bupropion HCl, which may provide variable quality control and attenuated efficacy versus branded XL bupropion HCl.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
The authors received no financial support for the research.
Data availability statement
The authors confirm that data supporting findings of this study are available upon reasonable request.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Price MZ, Price RL (2023) Extended-Release of Bupropion Hydrobromide (Aplenzin®) Compared to Bupropion Hydrochloride in Treatment Resistant Major Depressive Disorder. J Dep Anxiety. 12:501.
Received: 27-Mar-2023, Manuscript No. JDA-23-22470; Editor assigned: 30-Mar-2023, Pre QC No. JDA-23-22470 (PQ); Reviewed: 13-Apr-2023, QC No. JDA-23-22470; Revised: 20-Apr-2023, Manuscript No. JDA-23-22470 (R); Published: 27-Apr-2023 , DOI: 10.35248/2167-1044.23.12.501
Copyright: © 2023 Price MZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.