
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2014) Volume 4, Issue 1
Objective: 1. To determine
a. Statistically, the Knowledge, Attitude and Practice (KAP) related variables responsible for underreporting of ADRs in
India, using Logistic Regression Analysis (LRA) Model,
b. Extent of under reporting of ADRs at the current KAP levels of Medical Practitioners (MPs).
2. To recommend measures at National level to reduce underreporting of ADRs
Methods: The results of survey on KAPs of MPs in India towards underreporting of ADRs were published. In the survey, the information was provided on number of ADRs observed during medical practice and number of ADRs reported to ADR monitoring center by 116 MPs. MPs reported less than 25% of ADRs was assumed to contribute to underreporting. Thus, dependent variable ‘underreporting’ was measured on binary scale as ‘Yes’ or ‘No’. Similarly, six independent variables were also measured on a binary scale as ‘Yes’ or ‘No’. The six 2×2 contingency tables were prepared with ‘underreporting’ as dependent variable and each of the 6 independent variables. However, contingency table assumes the levels of all other independent variables to be the same, which is unrealistic and thus fails to estimate the true odds ratio. Hence, Logistic Regression Analysis was used to analyze the data.
Results: 2×2 contingency tables revealed that each independent variable was significantly associated with ‘underreporting’. The odds ratio was statistically significant with all six variables. Stepwise LRA applied to data of 116 MPs, picked up 4 variables as statistically significant (P<0.05).
Conclusion: At the current level of KAP of MPs, there is high probability of continuing the problem of underreporting of ADRs. To reduce the rate of underreporting it is recommended to develop appropriate training modules at National level to create awareness among all healthcare professionals and design simple ADR forms and procedures for ADR reporting.
Keywords: Adverse drug reactions; Pharmacovigilance; ADR reporting; Logistic regression analysis; Odds ratios
Voluntary Adverse Drug Reaction (ADR) reporting schemes are in operation since the early sixties in many Western countries [1]. These surveillance systems enable physicians and pharmacists to report suspected ADRs and thus act as a tool to identify new ADRs and risk factors predisposing to recognized ADRs. Monitoring of adverse drug reactions started in India about two decades ago (1982) [2]. Five centers were established with the idea of starting ADR monitoring under the chairmanship of the Drug Controller of India. In 1986, a formal ADR monitoring system consisting of 12 regional centers, each covering a population of 50 million was proposed for India [3]. In 1997, India joined the WHO Adverse Drug Reaction Monitoring Programme based in Uppsala, Sweden [3]. However, the progress was not as expected. A voluntary reporting system of ADRs is fundamental to drug safety surveillance but under-reporting was its major limitation [4]. Other studies [5] also identified, a significant and widespread under-reporting of ADRs to spontaneous reporting systems including serious or severe ADRs. Hence, National Pharmacovigilance Program for India - sponsored by World Health Organization (WHO) and funded by World Bank was made operational from 1 January 2005.
In spite of such efforts to strengthen ADR monitoring in India, only a small proportion of ADRs were actually reported to national reporting centers and pharmaceutical companies. Study by Aagaard et al. [6] showed that high-income countries had the highest ADR reporting rates and low-income countries the lowest, with large variations across countries in each group. The median under-reporting rate across the 37 studies, as reported by Hazell and Shakir [5] was 94% (inter-quartile range 82-98%). Further, the median under-reporting rate for 19 studies [5] investigating specific serious/severe ADR-drug combinations was less than that for 37 studies but was still high at 85%. Thus, there has been a considerable degree of underreporting of ADRs.
Many studies carried out from time to time have tried to understand the reason for such under reporting of ADRs. Almost all such studies, with some minor variations identified the same variables responsible for under reporting of ADRs. While personal and professional factors display a weak influence, the knowledge and attitudes of health professionals appear to be strongly related with reporting in a high proportion of studies [4]. Similar reasons were expected to be prevalent in India contributing to under reporting of ADRs in spite of the efforts by Government regulatory authorities in setting up PV/ADR monitoring centers in India. In order to assess the latest/most current situation in India, the survey of KAPs of Medical Practitioners was carried out by the authors to gather latest information from a representative sample of MPs from all over India and the results of the same have been Published in the Perspectives in Clinical Research (PICR) [1].
The results of the survey [1] of KAPs of MPs towards the ADR reporting revealed that MPs in the country have reasonably good knowledge, awareness and attitude towards ADR reporting. However, the percentage of MPs reporting ADRs to PV/Govt ADR Monitoring centers was just 18.5% [1].
In the same survey, a provision was made in the questionnaire to record information on number of ADRs noticed by MPs during their medical practice along with the number of ADRs reported to ADR monitoring centers. Only 116 out of 870 who participated in the survey could provide this information. The complete data of these 116 MPs was further analyzed to find out statistically significant variables responsible for under reporting by using Logistic Regression Analysis (LRA) Model. Based on the findings of statistical analysis, recommendations in terms of possible corrective measures have been provided for implementation at the National level to resolve successfully the issue of under reporting of ADRs.
1. To determine statistically, the Knowledge, Awareness/Attitude and Perception/Practice (KAP) related risk factors/variables significantly responsible for under reporting of ADRs in India, using Logistic Regression Analysis (LRA) Model,
2. Use the LRA model to estimate the extent of under reporting of ADRs if the current levels of KAPs of MPs in India continue to prevail and
3. To recommend corrective measures for implementation at National level to improve KAP related risk variables in order to reduce the risk of underreporting of ADRs.
The data collected from 870 MPs who participated in the survey conducted by the same authors [1] for assessing KAP of MPs towards ADR reporting in India was reviewed for assessing the information on number of ADRs reported to PV/ADR monitoring centers and total number of ADRs observed in their respective clinics. Percentage of ADRs reported to PV/ADR monitoring centers by each MP who provided these data, was computed as follows:
ADR reported (%)=(Number of ADRs reportedx100)/(Number ADRs observed).
All MPs who reported less than 25% of ADRs were assumed to contribute to the under reporting of ADRs prevailing in India. Hence, in respect of these MPs the status of “underreporting” was categorized as “Yes” and in the others as “No”. Thus, dependent or outcome variable namely status of underreporting of ADRs was measured on binary/ dichotomous scale as Yes (0)/No (1). Similarly six independent/ predictor variables were also measured on a binary/dichotomous scale as “Yes” or “No”. The new database for 116 MPs who provided information on percentage ADR reported in addition to other variables was used for further statistical analysis.
2×2 contingency tables
Six, 2×2 Contingency tables with “Under Reporting of ADRs (Yes/No)” as one of the variables and each of the six variables as second variable were used to assess which of the six variables are responsible for increased risk of “Under reporting”. However, 2×2 contingency tables has a major limitation as it assumes the levels of all other independent variables except the one used in preparing 2×2 contingency tables, to be the same in all other respondents. This assumption is not realistic hence, 2×2 contingency tables fail to estimate the true odds ratio.
When a non-causal association is observed between a given exposure (independent variable) and outcome because of the influence of the other independent variable, it is termed confounding. This other independent variable is termed as a confounding variable. A confounding variable is causally associated with the outcome of interest and non-causally or causally associated with the other exposure/ independent variable [7]. Logistic regression [8] is the most widely used method for adjustment of confounding in epidemiologic studies. The method simultaneously adjusts for confounders measured on different scales. Hence, the data were also analyzed using Logistic Regression Analysis (LRA) model to find out statistically significant independent variables adjusted for confounders.
Logistic regression analysis (LRA) model
Logistic regression analysis (LRA) model uses the experience to estimate the odds of an outcome by mathematically modeling or simulating that experience and describing it by means of a regression equation. The method of calculation for the regression coefficients takes into consideration all possible combinations of the independent variables [9]. Logistic regression analysis (LRA) extends the techniques of multiple regression analysis to research situations in which the outcome variable is categorical. In practice, situations involving categorical outcomes are quite common. In the current project, an outcome variable “underreporting of ADRs” measured on dichotomous/binary scale has two categories as “Yes” and “No”. However, the extension of the techniques of LRA to outcomes with three or more categories (e.g., improved, same, or worse) is possible.
Logistic regression analysis is a powerful tool for assessing the relative importance of factors that determine outcome [7].
Thus, logistic regression is the
• Most important model for categorical response (yi) data with
• 2 levels (that is dichotomous or binary)
• 3 or more levels (nominal or ordinal) and
• Independent or Predictor variables (xi) can take on any form: binary, categorical, and/or continuous
LRA model application
LRA model was applied to data extracted in a database for 116 MPs who provided data on following parameters
1. number of ADRs observed and
2. number of ADRs reported to Government ADR monitoring/ PV centers
In addition to the data on responses to questions used to assess KAP of MPs towards ADR reporting. Logistic regression was useful in determination of the impact of multiple independent variables presented simultaneously to predict membership of each respondent to one or other of the two categories (Yes or No) of dependent variable – Underreporting of ADR.
The objective of LRA model was
1. To identify statistically valid/significant risk factors and
2. To estimate probability of underreporting ADRs with the current level of KAPs of MPs towards ADR reporting
Formulae
Y=logit pi=log {odds}=log=α+ β1X1+ β2X2+ …+ βkXk+ ε=α + Σ βiXi + ε
Where; 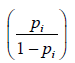
pi=probability that event of underreporting occurs in ith case
Dependent variable – Categorical (binary or dichotomous):
• Event=underreporting (UR)=Yes (0)/No (1)
Underreporting for this study was defined as % ADRs reported <25%
Predictor/independent variables (all categorical and binary) – all from MP survey):
1. X1=Awareness=No (0)/Yes (1)
2. X2=Proc_diff=Difficult Procedure=Yes (0)/No (1)
3. X3=Proc_knowledge=Knowledge of procedure of reporting=No (0)/Yes (1)
4. X4=ADR attributable to Med=No (0)/Yes (1)
5. X5=ADR centers needed=No (0)/Yes (1)
6. X6=Govt ADR centers useful=No (0)/Yes (1)
Stepwise logistic regression method was used. Stepwise logistic regression method uses all predictors/independent variables in the model to start with. Then at each step it checks each independent/ predictor variable for its statistically significance and removes independent/predictor variable from the model for which P 0.05 and retains only those which are statistically significant, that is for which P<0.05.
2×2 Contingency tables
Variables/factors responsible for increasing the risk of underreporting of ADRs: The Odds Ratios (ORs) of underreporting of ADRs with respect to each of the six variables were worked out using 2 by 2 contingency tables. As can be seen from Table 1, all variables were detected as statistically significant ORs.
| Predictors / Levels | UNDER REPORTING | Total | Odds-Ratio | Chi-Square | Stat. Sign. (P-value) | |
|---|---|---|---|---|---|---|
| 1.AWARENESS (X1) | Yes = 0 | No = 1 | ||||
| No = 0 | 48 | 2 | 50 | 17.7 | 21.9 | P<0.001 |
| Yes = 1 | 38 | 28 | 66 | |||
| Total | 86 | 30 | 116 | |||
| 2.PROC_KNOWN (X2) | Yes = 0 | No = 1 | 18.6 | 29.4 | P<0.001 | |
| No = 0 | 58 | 3 | 61 | |||
| Yes = 1 | 28 | 27 | 55 | |||
| Total | 86 | 30 | 116 | |||
| 3.ADR_C_NEEDED (X3) | Yes = 0 | No = 1 | 20.1 | 35.8 | P<0.001 | |
| No = 0 | 65 | 4 | 69 | |||
| Yes = 1 | 21 | 26 | 47 | |||
| Total | 86 | 30 | 116 | |||
| 4.GOVT._Cs_USEFUL (X4) | Yes = 0 | No = 1 | 12.9 | 28 | P<0.001 | |
| No = 0 | 62 | 5 | 67 | |||
| Yes = 1 | 24 | 25 | 49 | |||
| Total | 86 | 30 | 116 | |||
| 5.ADR_ATTRIBUTED to MEDICINE (X5) | Yes = 0 | No = 1 | 55.6 | 28 | P<0.001 | |
| No = 0 | 77 | 4 | 81 | |||
| Yes = 1 | 9 | 26 | 35 | |||
| Total | 86 | 30 | 116 | |||
| 6.PROC_DIFFICULT (X6) | Yes = 0 | No = 1 | 55.6 | 60.8 | P<0.001 | |
| Yes = 0 | 77 | 4 | 81 | |||
| No = 1 | 9 | 26 | 35 | |||
| Total | 86 | 30 | 116 | |||
Table 1: 2×2 Contingency tables and Odds-Ratios.
Stepwise logistic regression
The same data were then analyzed by stepwise logistic regression using above six variables as contributing to “underreporting of ADRs” observed in India. The results of this analysis are presented in Table 2.
| Independent/Predictor Variable@ | Coefficient | Std.Error | P |
|---|---|---|---|
| Diff_Proc | 3.0906 | 1.0079 | 0.0022 |
| Proc_Kn | 2.3330 | 1.0802 | 0.0308 |
| ADRs_attribted_to_med | 3.3681 | 1.0021 | 0.0008 |
| Govt_Cs_Useful | 2.9766 | 1.2417 | 0.0165 |
| Constant | -7.5289 |
Table 2: Coefficients and Standard Errors.
Equation [1] presents the results in Table 2 in the form of equation:
Y=log {odds} =log=-7.53 + 3.09xProc_Diff + 2.33xProc_Kn + 3.36x ADRs_attributed to med + 2.97xGovt_Cs_Useful …. …… …… ….. Equation I
where, 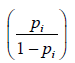
1. Proc_Diff=Difficult Procedure of Reporting ADR (Yes/No)
2. Proc_Kn=Know the Procedure of Reporting ADR (Yes/No)
3. ADRs_attributed_to_med=ADRs attributed to medicine (Yes/ No) and
4. Govt_Cs_Useful=Government ADR centers are useful (Yes/ No)
The odds ratios for ith predictor variable (Xi), defined as exp(βi) are presented along with respective 95% Confidence Intervals (95%CI) in the Table 3.
| Independent/Predictor Variable | Odds ratio | 95% CI |
|---|---|---|
| Diff_Proc | 22.00 | 3.05 to 158.53 |
| Proc_Kn | 10.31 | 1.24 to 85.65 |
| ADRs_attributed_to_med | 29.02 | 4.07 to 206.91 |
| Govt_Cs_Useful | 19.62 | 1.72 to 223.71 |
Table 3: Odds Ratios and 95% Confidence Intervals.
ORs in Table 3 show that the risk of under reporting is significantly (p<0.05) high when
1. the procedure for ADR reporting is perceived as difficult by MPs
2. the procedure for ADR reporting is not known to MPs
3. the ADRs are not perceived by MPs as attributable to medicine and
4. the MPs feel that Government ADR monitoring centers are NOT useful
Substituting the actual values of respective predictor variables for 116 responders used in equation I. the category “Yes”/“No” of dependent variable (Under reporting) was estimated for each MP and compared with actual category “Yes”/“No” of “underreporting” for respective MPs . The result of this comparison between actual category and predicted category using equation I is summarized in Table 4.
| Actual group | Predicted group | Percent correct Classification | |
|---|---|---|---|
| 0 | 1 | ||
| Y = 0 | 83 | 3 | 96.51 % |
| Y = 1 | 2 | 28 | 93.33 % |
| Percent of cases correctly classified = [(83+28)x100]/116 | 95.69 % | ||
Table 4: Classification table.
Table 4 shows that the categories “Yes” or “No” of “dependent or outcome” variable matched in respect of 111 out of 116 MPs leading to 95.69 % of correct classification.
Thus, the LRA model has correctly predicted 96% of instances of “under reporting” and “not under reporting”. This indicates that LRA model was the best fit and is in agreement with the “Overall model fit” tested using “full – 2log likelihood” as well as “null – 2 log likelihood” estimates given in Table 5.
| Null model -2 Log Likelihood | 132.613 |
| Full model -2 Log Likelihood | 33.120 |
| Chi-square | 99.493 |
| DF | 4 |
| Significance level | P < 0.0001 |
Table 5: Overall Model Fit.
Probability of underreporting at the “current level” of KAP
Current levels of four KAP related variables, identified as significantly (P<0.05) responsible for high risk of underreporting of representative sample of 870 Indian MPs were substituted in the LRA model (Equation 1) to estimate
• Log of odds of probability of “Underreporting” (UR)
• odds of UR and then
• the probability of “UR”
The probability of underreporting (UR) at current levels of KAPs of 870 MPs worked out to be as high as 70%.
Under reporting of ADRs is a universal phenomenon and is attributed to inherent weakness of adverse-reaction particularly with the current voluntary reporting schemes.
Many Published results [4,5] of surveys conducted in the past have identified following variables/factors as responsible factors for “under reporting of ADRs” observed in the world in general and in India in particular.
1. diffidence (fear of appearing ridiculous for reporting merely suspected ADRs)
2. lethargy (an amalgam of procrastination, lack of interest or time to find a report card, and other excuses)
3. indifference (the one case that an individual doctor might see could not contribute to medical knowledge) and
4. insecurity (it is nearly impossible to determine whether or not a drug is responsible for a particular adverse reaction)
5. Perception that reporting process is tedious, lack of time, poor knowledge of reporting mechanism and inadequate expertise
Kamatane and Jayavardhini [10] reported busy schedule, lack of knowledge about the exact authority to report ADRs, unavailability of ADR reporting forms, lack of incentives as some of the reasons for under-reporting of ADRs. The major reasons for not reporting ADR as identified by Amrita and Singh [11] were:
1. not aware of reporting centers,
2. non-availability of ADR reporting form,
3. adverse drug reaction already well known,
4. uncertainty about drug causing it and
5. lack of set procedure for ADR reporting.
According to Irujo et al. [12], the most frequently mentioned reasons for not reporting ADRs were the ADR is not serious, the ADR is already known, uncertainty concerning the causal relationship between the ADR and the drug, forgetting to report the ADR and a lack of time. In an attitudinal survey conducted by Eland et al. [13] it is reported that over 35% of medical practitioners in Netherlands, were of the opinion that reporting of ADRs takes too much time and that it is too bureaucratic. They also reported other reasons for not reporting ADRs as lack of knowledge like not knowing how to report, not knowing which ADRs to report and even unawareness of the existence of a reporting scheme. Others have also reported similar findings with little variation [14,15].
Our findings are in agreement with those reported by many others. However, in our study after conducting survey to identify factors currently responsible for underreporting of ADRs in India [1], applied LRA model to find out the statistically significant predictor variables/ factors from among those identified as responsible for under reporting of ADRs in India.
Some of the international as well as Indian publications [16,17] have suggested involving patients who are ultimate consumers of the medicines, in ADR reporting directly to ADR monitoring centers and pharmacovigillance centers. Ahmed et al. [18], concluded that consumer reporting of suspected ADRs could add many benefits to drug monitoring system, overcome under reporting, promote consumer rights, improve the public quality of life and can be an important information source for clinical practice. This is expected to increase the percentage of ADR reporting as it may eliminate an intermediary step of reporting ADR experienced by patient to health care professional/ MPs. Secondly, it is uncertain in the light of some findings that the ADRs reported by patients to healthcare professionals or MPs will be, in turn, reported to the concerned authorities.
1. The probability of “underreporting of ADRs” will continue to remain as high as 70% at the current levels of the following four KAP related variables identified using LRA as significantly (P<0.05) responsible for high risk of underreporting:
X2=Proc_diff=Difficult Procedure
X3=Proc_knowledge=Knowledge of procedure for ADR reporting
X4=ADR attributable to Med
X6=Govt ADR centers useful
2. The issue of underreporting of ADRs in India can be resolved successfully if the current levels of KAPs of MPs are corrected/improved by implementing certain measures at National Level. The increased level of KAPs of MPs after implementation of measures recommended at National level will reduce the probability of existing underreporting as per LRA model.
Various studies in the past have suggested certain measures to get rid of issue of underreporting of ADRs like
1. internet reporting,
2. pharmacist/nurse reporting,
3. direct patient reporting,
4. improved education and training of healthcare professionals [5],
5. educating and increasing awareness about reporting of ADRs among the healthcare professionals [19],
6. incorporating education on pharmacovigilance issues and
7. the importance of ADR reporting more extensively in medical training [13],
8. Sending regular communication to health care workers explaining ADR reporting procedures [13].
Pillans [20] has suggested improvement in current methods of communication like writing Patient information leaflets, in lay language in more user-friendly format, highlighting risk-benefit issues and key safety information. Pillans [20] also suggested increased emphasis on education on drug safety and pharmacovigilance at all levels by including it in undergraduate medical and pharmacy curricula and postgraduate educational programmes. Agard et al. [6] concluded that there is a need for more research on the impact of organizational structures and economic resources of national pharmacovigilance centers in order to strengthen ADR reporting rates, especially in lowincome countries.
Many local cultural factors influence the ratio of publications on adverse reactions to all drug effects. Strong local initiatives may substantially increase publication rates [21]. This deduction about publications on ADRs can be assumed applicable to initiatives planned to improve ADR reporting in various parts of the world. Rishi et al. [22] suggested that the PVPI should take strong steps to motivate physicians for ADR reporting in order to increase the numbers. According to Rishi et al. [22], despite improvement of ADR reporting systems in India by launching PVPI, we still have to do lots of work to improve ADR reporting rate. This includes encouraging medical staff for spontaneous reporting of ADRs, distributing ADR reporting forms to the medical practitioners, conducting workshops and conferences with continuous medical education for increasing motivation for better learning about ADRs.
Based on above-mentioned suggestions described in the literature and our interaction with various groups with many experts besides MPs during the conduct of survey, the following measures suitable to the cultural scenario in India are recommended in order to improve KAPs of MPs
a) Develop appropriate training modules on awareness for different types of health care professionals and common people,
b) Encourage people from different disciplines in the healthcare industry like pharmacist, pharmaceutical companies, nurses to assist/help reporting of all ADRs to appropriate authorities
c) Provide hands on training on ADR reporting not only to health care professionals but also to general public who will be patient/ relative of patient sometime during the lifetime and be true consumers
d) Encourage more and more patients to report ADRs after imparting appropriate hands on training on “ADR Reporting”
e) Equip ADR monitoring/PV centers with sufficient number of user friendly blank ADR forms to ensure availability on continuous basis
f) Provide incentive for timely reporting of appropriate ADR
g) Simplify the procedures of ADR reporting
h) Government/appropriate regulatory body to decide about
a. Appointing a brand ambassador/s to create awareness about ADRs and its reporting
b. Developing system to disseminate ADR reporting information to public create awareness among common people.
i) Encourage Non-Government Organizations (NGOs) to take up ADR reporting as the social responsibility
j) Involve Gram Panchayats, Zilla Parishads, Municipal Corporations to devise schemes like quiz competitions and honour the winner with best awareness medal for creating interest in this field amongst school and college students as well as teachers and professors.
k) Periodically assess the impact of these measures on the ADR reporting and appropriately modify the concerned initiatives.